Abstract
Accumulating evidence suggests that cancer-associated stromal fibroblasts (CAFs) contribute to tumor growth by actively communicating with cancer cells. Our aim was to identify the signaling pathways that are involved in tumor–stromal cell interactions in human papillary thyroid carcinoma (PTC). Immunohistochemical analyses were performed with 127 archived formalin-fixed and paraffin-embedded thyroid tissue samples that included 70 cases of PTC, 35 cases of nodular goiter (NG), and 22 cases of normal thyroid tissues. The results showed that the expression levels of Notch1, transforming growth factor β (TGF-β1), and p-Smad3 in PTC cells and α-smooth muscle actin (α-SMA) in the stroma of PTC were all significantly higher than in NG and normal thyroid tissues. Further analysis showed that in PTC, higher expression levels of Notch1 and TGF-β1 were closely related with lymph node metastasis (P < 0.05), whereas for α-SMA and p-Smad3, the percent expression increased significantly with advanced tumor stages (P < 0.05). Correlation analysis revealed that TGF-β1 expression increased with increased Notch1 and p-Smad3 levels in PTC cells (P < 0.05). Moreover, a significant correlation was found between higher TGF-β1 expression in PTC cells and increased α-SMA levels in the fibroblasts surrounding the cancer cells (P < 0.05). We identified TGF-β1 as an important factor from PTC cells that act in a paracrine manner to influence the activation of stromal fibroblasts. These data suggest that the activation of Notch and TGF-β/Smad3 pathways in cancer cells influence tumor growth. Moreover, cancer cell-derived-TGF-β ligands also affect stromal cells in a paracrine fashion and enhance tumor growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy, accounting for 70 to 80 % of cases. There is increasing awareness that solid malignancies do not contain transformed neoplastic cells only but comprise a mixed population of cells and an extracellular matrix that collectively constitute the tumor microenvironment, also known as the tumor stroma [1, 2]. Although the molecular background of cancer cells in PTC has been comprehensively investigated in many recent studies [3–8], relatively little is known about the molecular mechanisms mediating resident stromal cell activation and the crosstalk between adjacent tumor cells and its microenvironment.
Fibroblasts within the tumor stroma, known as carcinoma-associated fibroblasts (CAFs), which include both fibroblasts and myofibroblasts [9], play a critical role in the regulation of tumor growth [10–13]. Myofibroblasts, which are distinct from fibroblasts in their expression of α-smooth muscle actin (α-SMA), have recently been implicated in important aspects of solid tumor progression [14–18] as they produce a number of important factors that can directly promote growth in the adjacent epithelium [19]. Immunohistochemical analysis has shown that α-SMA, the CAF marker, is absent in normal ovarian tissue, whereas CAFs are abundant in the stroma of metastatic epithelial ovarian cancer [20]. It has been reported that PTC is frequently associated with a variable degree of fibrosis. Inaba et al. found that PTC was characterized by an extensive stromal component accounting for 40–60 % of the tumor [21]. In the study by Isarangkul, 33 (89.2 %) of 37 cases of papillary carcinoma showed dense fibrosis within the tumor masses, giving an odds ratio of 37.95 compared with the follicular type, indicating a very strong and highly significant association. It was concluded that dense fibrosis within a malignant tumor of the thyroid can be used as another diagnostic criterion of papillary carcinoma, especially when other classic criteria are absent or inconspicuous [22]. However, the phenotype of fibroblasts and its regulatory mechanisms in PTC have not been fully investigated.
Recent studies provide evidence that transforming growth factor β (TGF-β) signaling in fibroblasts plays a major role in the initiation of carcinomas [1]. TGF-β/Smad signaling is involved in tissue homeostasis and cancer development [23]. TGF-β1 remains one of the key factors responsible for the development of a myofibroblastic phenotype from a variety of precursor cells, including fibroblasts [24]. It has been reported that the cancer cell-derived TGF-β1 modulates myofibroblast differentiation in colon cancer [25], breast cancer [26], and squamous cancer [27]. TGF-β signaling is also required to induce CAF differentiation of mouse BM-MSCs in vivo and can induce expression of some CAF markers in human BM-MSCs in vitro [28]. Previous studies have reported that TGF-β1 induction of a myofibroblast phenotype was partly mediated through Notch signaling [29].
The Notch signaling pathway plays a critical role in the development and homeostasis of tissues by regulating cell-fate decisions, proliferation, differentiation, and apoptosis [30, 31]. Notch signaling also contributes to carcinogenesis in many cancers [32, 33]. Studies have demonstrated that Notch signaling promotes smooth muscle cell differentiation [34, 35]. Recently, FIZZ1 (found in inflammatory zone 1; also known as hypoxia-induced mitogenic factor) was described as inducing myofibroblast differentiation through Notch signaling [36]. Furthermore, emerging evidence indicates a role for Notch1 in the EMT during development and oncogenesis [37, 38]. Notch1-induced tumor development and progression may involve modulation of the TGF-β signaling pathway [38]. The data of Aoyagi-Ikeda et al. indicated that Notch induces myofibroblast differentiation through a TGF-β–Smad3 pathway that activates SMA gene transcription in a CArG-dependent and TCE-dependent manner in alveolar epithelial cells [29]. It was found that pathways regulated by TGF-β and Notch proteins are both important for PTC invasion [39, 40]. However, the role of the Notch and TGF-β pathways following the interaction between cancer cells and stromal cells is not well understood.
Here, we examined the immunohistochemical profiles of Notch1, TGF-β1, p-Smad3, and α-SMA in PTC. We explored whether Notch and TGF-β/Smad signaling play roles in the metabolic reprogramming of both cancer cells and their tumor microenvironment.
Materials and methods
Tissue samples
One hundred and twenty-seven formalin-fixed and paraffin-embedded thyroid resection samples were obtained from the archives of the Department of Pathology of the Second Hospital of Hebei Medical University during October 2009 and May 2012. The average age of the patients was 43 years. Among the cases, 70 were PTC, 35 were nodular goiter (NG), and 22 were normal thyroid tissues free from inflammation, metaplasia, dysplasia, or carcinoma, which were used for comparison. All patients selected for analysis had classical PTC histopathology.
Immunohistochemistry
Sections (4 μm thick) were prepared from paraffin blocks. After deparaffinization, antigen retrieval was performed with citrate buffer for 15 min. Endogenous peroxidase activity was blocked with 3 % hydrogen peroxide in methanol for 10 min. Incubation with primary antibodies against α-SMA, TGF-β1 (Maixin-Bio Fujian, China), p-Smad3, and Notch1 (Epitomics, CA, USA) was performed overnight at 4 °C in a humidified chamber. After washing with phosphate-buffered saline, the sections were stained according to the instructions of ElivisionTM Plus Kit (Maixin-Bio Fujian, China). Counterstaining was performed with hematoxylin. Parallel staining was performed in the absence of the primary antibody to serve as negative controls.
Immunohistochemical staining evaluation
To rule out the possibility of an interpersonal bias, the results were interpreted by two pathologists who were blind to the clinical outcome. Tumors with moderate or strong immunostaining of Notch1 were classified as having positive (+) cytoplasmic expression, whereas tumors with absent or weak immunostaining were classified as having negative (−) expression [41]. Samples were regarded as TGF-β1 or p-Smad3 positive when more than 10 % of the cytoplasm was stained [42]. Evaluation of α-SMA immunoreactivity was performed according to Graham et al. [43]. The degree of staining for α-SMA was evaluated based on a four-grade scale: − (negative), + (10 to 40 %), ++ (40 to 70 %), or +++ (70 to 100 %). The grade of (++ ~ +++) was defined as high expression of α-SMA, and the grade of (− ~ +) was defined as low expression.
Statistical analysis
The experimental data were statistically analyzed with Chi-square test and Fisher's exact test and correlated with the SPSS13.0 edition of statistics software. P < 0.05 was considered statistically significant.
Results
The expressions of Notch1, TGF-β1, p-Smad3, and α-SMA were all significantly higher in PTC when compared with NG and normal thyroid tissues
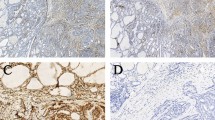
Among the 70 PTC in the retrospective cohort, 36 (51.4 %) were positive for Notch1 expression, whereas 34 (48.6 %) were negative for Notch1 expression. The positive expression of Notch1 in PTC was significantly higher than that in NG (8.6 %) and normal thyroid tissues (13.6 %, P < 0.05). Positive cytoplasmic immunoreactivity for TGF-β1 was identified in 40 cases of PTC (57.1 %), 5 cases of nodular goiters (14.3 %), and 4 cases of normal thyroid tissues (18.2 %), thereby indicating that the percentage of positive expression in PTC was significantly higher than that in the latter two groups (P < 0.05). PTC also showed a higher percentage of p-Smad3-positive expression (78.6 %) compared with NG (22.9 %) and normal thyroid tissues (18.2 %, P < 0.05). In addition, compared with NG (11.4 %) and normal thyroid (9.1 %) tissues, significantly higher levels of α-SMA (68.6 %) were observed in fibroblasts surrounding the cancer cells in PTC (Table 1, Fig. 1).
Expression patterns of α-SMA, p-Smad3, TGF-β1, and Notch1 in normal thyroid tissues, nodular goiter (NG), and papillary thyroid carcinoma (PTC). From left to right, a, d, g, and j show normal thyroid tissues; b, e, h, and k show NG tissues; c, f, i, and l show PTC. From top to bottom, tissues were stained for α-SMA (a, b, and c), p-Smad3 (d, e, and f), TGF-β1 (g, h, and i), and Notch1 (j, k, and l). Magnification, ×200
However, no significant differences were found in the positive expression patterns of Notch1, TGF-β1, p-Smad3, and α-SMA between NG and normal thyroid tissues (P > 0.05).
Clinicopathological significance of Notch1, TGF-β1, p-Smad3, and α-SMA in PTC
Further analysis showed that PTC cases with lymph node metastasis had a significantly higher proportion of Notch1-positive expression than those without lymph node metastasis (76.9 vs 45.6 %, P < 0.05). Similar results were found for TGF-β1-positive expression (84.6 % in metastatic group vs 50.9 % in nonmetastatic group; P < 0.05). The percentages of α-SMA- and p-Smad3-positive expressions were both closely related with tumor stages in PTC, being significantly higher in cases of advanced tumor stages (III/IV) than that in cases of early tumor stages (I/II) (87.0 vs 59.6 % for α-SMA and 95.6 vs 70.2 % for p-Smad3, P < 0.05) (Table 2).
Correlation among Notch1, TGF-β1, p-Smad3, and α-SMA expressions in PTC
The correlation among the expression patterns of Notch1, TGF-β1, p-Smad3, and α-SMA were analyzed. Table 3 shows that in PTC, TGF-β1 expression increased with increased Notch1, p-Smad3, and α-SMA expression levels (r = 0.487, r = 0.251, and r = 0.284, respectively, P < 0.05).
Discussion
There have been several studies on the expression of Notch1 in PTC, but the results remains controversial. Ferretti et al. demonstrated that PTC displayed decreased Notch1 protein expression compared with normal thyroid tissues [44]. By contrast, Geers et al. found that all types of PTC contained a higher number of strongly Notch1-positive cells compared with normal and hyperplastic tissues, whereas the expression of Notch1 in follicular thyroid carcinomas remained in the same range as in the benign tissues [39]. Our results indicated that the positive expression of Notch1 in PTC was significantly higher than that in NG and normal thyroid tissues, affirming the important role of the Notch pathway in thyroid carcinogenesis and angiogenesis.
TGF-β1 is secreted in large amounts from many cancer cells and acts as a tumor promoter by inducing tumor angiogenesis, immune escape, and metastasis [45, 46]. High expression of TGF-β1 correlates with cancer progression and clinical prognosis [47]. However, the role of TGF-β1 in the progression of PTC is still somewhat uncertain. Zurawa-Janicka et al. demonstrated that the TGF-β1 levels in thyroid tumor tissues were not significantly altered compared with control tissues [48]. While Eloy et al. found that increased cytoplasmic expression of TGF-β at the periphery of poorly circumscribed PTC was associated with morphological features of invasiveness, featuring epithelial-to-mesenchymal transition, and the presence of nodal metastases [49]. TGF-β is known to signal through phosphorylation-mediated activation of Sma- and MAD-related family (Smad) of transcription factors [50]. Our own results demonstrate that the positive expression of TGF-β1 was significantly higher in PTC than that in NG and normal thyroid tissues, and there was a significant positive correlation between TGF-β1 and p-Smad3 expressions in PTC, thus confirming the role of TGF-β/Smad pathway in the carcinogenesis and progression of PTC.
Different modes of cross talk between the TGF-β and Notch signaling pathways of both synergistic and antagonistic nature have been reported in various cellular contexts [51–53]. Kennard et al. demonstrated that the overexpression of activated Notch3 caused repression of TGF-β-induced smooth muscle-specific genes [54], whereas in clear cell renal carcinoma, characterized by high activity of both pathways, Notch signaling seems superimposed on TGF-β signaling [55]. In the present study, we demonstrated that there was a significant positive correlation between Notch1 and TGF-β1 expression in PTC, and both Notch1 and TGF-β1 expression were related with lymph node metastasis (P < 0.05). These results indicate that the two proteins may be predictors of lymph node metastasis and may be related to poor prognostic markers in patients with PTC.
Current understanding of mechanisms underlying tumor growth and progression assigns critical functions to cells constituting the tumor microenvironment, such as endothelial cells and pericytes, tumor-infiltrating immune cells, and CAFs [56]. Whereas the tumor growth-promoting ability of CAFs has been extensively studied, it is not clear whether CAFs are reciprocally controlled by developmental pathways that are activated in tumor cells. In the present study, we found that compared with NG and normal thyroid tissues, significantly higher levels of α-SMA were observed in fibroblasts surrounding cancer cells in PTC. More importantly, we found that α-SMA-positive expression was closely related with higher tumor stages (III/IV) in PTC. It should be noted that in contrast to studies by Na et al., which showed cytoplasmic accumulation of TGF-β expression in the stromal cell of PTC [57], in this study, we found that TGF-β1 was only expressed in the cancer cells of PTC. Moreover, our correlation analysis revealed that there was a significant positive correlation between TGF-β1 expression in PTC cells and α-SMA in the fibroblasts surrounding the cancer cells. A possible explanation for this phenomenon may be that TGF-β1 could exert its effect on tumor growth via a paracrine loop. The elevated TGF-β1 has been proposed to promote tumor progression through paracrine effects on stromal elements [58]. Paracrine secretion of TGF-β may modulate the tumor microenvironment for the benefit of melanoma growth, invasion, and metastasis, especially through its ability to activate stromal fibroblasts and its immunosuppressive effects and pro-angiogenic properties [59]. Through paracrine signaling molecules, TGF-beta and IL-1beta, cancer cells activate stromal fibroblasts and induce the expression of fibroblast activation protein (FAP). FAP, in turn, affects the proliferation, invasion, and migration of the cancer cells. Our result was confirmed by Chen, who reported that TGF-β was an important factor in inducing differentiation of myofibroblasts and expression of functional markers, notably α-SMA [60].
In summary, our study suggests that the activation of the Notch and TGF-β/Smad3 pathways in cancer cells not only influence tumor growth, but cancer cell-derived-TGF-β ligands also affect stromal cells in a paracrine fashion and enhance tumor growth.
References
Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7.
Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18:27–34.
Aldred MA, Huang Y, Liyanarachchi S, Pellegata NS, Gimm O, Jhiang S, et al. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol. 2004;22:3531–9.
Frattini M, Ferrario C, Bressan P, Balestra D, De Cecco L, Mon-dellini P, et al. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 2004;23:7436–40.
Jarzab B, Wiench M, Fujarewicz K, Simek K, Jarzab M, Oczko-Wojciechowska M, et al. Gene expression profile of papillary thyroid cancer: sources of variability and diagnostic implications. Cancer Res. 2005;65:1587–97.
Yano Y, Uematsu N, Yashiro T, Hara H, Ueno E, Miwa M, et al. Gene expression profiling identifies platelet-derived growth factor as a diagnostic molecular marker for papillary thyroid carcinoma. Clin Cancer Res. 2004;10:2035–43.
Giordano TJ, Kuick R, Thomas DG, Misek DE, Vinco M, Sanders D, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–56.
Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115:1068–81.
Semba S, Kodama Y, Ohnuma K, Mizuuchi E, Masuda R, Yashiro M, et al. Direct cancer–stromal interaction increases fibroblast proliferation and enhances invasive properties of scirrhous-type gastric carcinoma cells. Br J Cancer. 2009;101:1365–73.
Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–71.
Noma K, Smalley KS, Lioni M, Naomoto Y, Tanaka N, El-Deiry W, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–93.
Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25.
Yashiro M, Hirakawa K. Cancer–stromal interactions in scirrhous gastric carcinoma. Cancer Microenviron. 2010;3:127–35.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11.
Hasebe T, Sasaki S, Imoto S, Ochiai A. Proliferative activity of intratumoral fibroblasts is closely correlated with lymph node and distant organ metastases of invasive ductal carcinoma of the breast. Am J Pathol. 2000;156:1701–10.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63.
Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–90.
Matsubara D, Morikawa T, Goto A, Nakajima J, Fukayama M, Niki T. Subepithelial myofibroblast in lung adenocarcinoma: a histological indicator of excellent prognosis. Mod Pathol. 2009;22:776–85.
Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Scholmerich J, et al. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol. 2009;15:1431–42.
Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303:47–55.
Inaba M, Umemura S, Satoh H, Ichikawa Y, Abe Y, Kirokawa K, et al. Papillary thyroid carcinoma with fibromatosis-like stroma: a report of two cases. Endocr Pathol. 2002;13:219–25.
Isarangkul W. Dense fibrosis. Another diagnostic criterion for papillary thyroid carcinoma. Arch Pathol Lab Med. 1993;117:645–6.
Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810.
Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30.
De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, et al. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016–8.
Casey TM, Eneman J, Crocker A, White J, Tessitore J, Stanley M, et al. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat. 2008;110:39–49.
Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–32.
Shangguan L, Ti X, Krause U, Hai B, Zhao Y, Yang Z, et al. Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells. 2012;30:2810–9.
Aoyagi-Ikeda K, Maeno T, Matsui H, Ueno M, Hara K, Aoki Y, et al. Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-{beta}-Smad3 pathway. Am J Respir Cell Mol Biol. 2011;45:136–44.
Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6.
Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–18.
Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, et al. Yes-associated protein upregulates jagged-1 and activates the NOTCH pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–42.
Ercan C, Vermeulen JF, Hoefnagel L, Bult P, van der Groep P, van der Wall E, et al. HIF-1α and NOTCH signaling in ductal and lobular carcinomas of the breast. Cell Oncol. 2012;35:435–42.
Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, et al. Jagged1-selective notch signaling induces smooth muscle differentiation via a RBP–Jkappa-dependent pathway. J Biol Chem. 2006;281:28555–64.
Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, et al. Smooth muscle alpha-actin is a direct target of Notch/CSL. Circ Res. 2006;98:1468–70.
Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, et al. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174:1745–55.
Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, et al. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115.
Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–33.
Geers C, Colin IM, Gérard AC. Delta-like4/Notch pathway is differentially regulated in benign and malignant thyroid tissues. Thyroid. 2011;21:1323–30.
Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007;104:2803–8.
Chu D, Zhou Y, Zhang Z, Li Y, Li J, Zheng J, et al. Notch1 expression, which is related to p65 status, is an independent predictor of prognosis in colorectal cancer. Clin Cancer Res. 2011;17:5686–94.
Koumoundourou D, Kassimatis T, Zolota V, Tzorakoeleftherakis E, Ravazoula P, Vassiliou V, et al. Prognostic significance of TGFbeta-1 and pSmad2/3 in breast cancer patients with T1–2, N0 tumours. Anticancer Res. 2007;27:2613–20.
Graham RP, Dry S, Li X, Binder S, Bahrami A, Raimondi SC, et al. Ossifying fibromyxoid tumor of soft part: a clinicopathologic, proteomic, and genomic study. Am J Surg Pathol. 2011;35:1615–25.
Ferretti E, Tosi E, Po A, Scipioni A, Morisi R, Espinola MS, et al. Notch signaling is involved in expression of thyrocyte differentiation markers and is down-regulated in thyroid tumors. J Clin Endocrinol Metab. 2008;93:4080–7.
Mumm JB, Oft M. Cytokine-based transformation of immune surveillance into tumor-promoting inflammation. Oncogene. 2008;27:5913–9.
Siegel PM, Shu W, Cardiff RD, Muller WJ, Massagué J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA. 2003;100:8430–5.
Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91:964–71.
Zurawa-Janicka D, Kobiela J, Galczynska N, Stefaniak T, Lipinska B, Lachinski A, et al. Changes in expression of human serine protease HtrA1, HtrA2 and HtrA3 genes in benign and malignant thyroid tumors. Oncol Rep. 2012;28:1838–44.
Eloy C, Santos J, Cameselle-Teijeiro J, Soares P, Sobrinho-Simões M. TGF-beta/Smad pathway and BRAF mutation play different roles in circumscribed and infiltrative papillary thyroid carcinoma. Virchows Arch. 2012;460:587–600.
Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. ThRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2–Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–85.
Asano N, Watanabe T, Kitani A, Fuss IJ, Strober W. Notch1 signaling and regulatory T cell function. J Immunol. 2008;180:2796–804.
Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, et al. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol. 2003;163:723–8.
Fu Y, Chang A, Chang L, Niessen K, Eapen S, Setiadi A, et al. Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells. J Biol Chem. 2009;284:19452–62.
Kennard S, Liu H, Lilly B. Transforming growth factor-beta (TGF- 1) down-regulates Notch3 in fibroblasts to promote smooth muscle gene expression. J Biol Chem. 2008;283:1324–33.
Sjölund J, Boström AK, Lindgren D, Manna S, Moustakas A, Ljungberg B, et al. The notch and TGF-β signaling pathways contribute to the aggressiveness of clear cell renal cell carcinoma. PLoS One. 2011; e23057.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Na KY, Kim HS, Sung JY, Park WS, Kim YW. Papillary carcinoma of the thyroid gland with nodular fasciitis-like stroma. Korean J Pathol. 2013;47:167–71.
Böttinger EP, Jakubczak JL, Roberts IS, Mumy M, Hemmati P, Bagnall K, et al. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–33.
Perrot CY, Javelaud D, Mauviel A. Insights into the transforming growth factor-β signaling pathway in cutaneous melanoma. Ann Dermatol. 2013;25:135–44.
Chen H, Yang WW, Wen QT, Xu L, Chen M. TGF-beta induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM [corrected]. Exp Mol Pathol. 2009;87:189–94.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, Y., Li, D. et al. Notch and TGF-β/Smad3 pathways are involved in the interaction between cancer cells and cancer-associated fibroblasts in papillary thyroid carcinoma. Tumor Biol. 35, 379–385 (2014). https://doi.org/10.1007/s13277-013-1053-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1053-z