Abstract
We assessed the expression of M3 receptor in non-small cell lung cancer (NSCLC) and determined its relationship with clinicopathological features and its impact on patient outcome. Specimens from 192 patients with NSCLC were investigated by immunohistochemistry for M3 receptor and Ki67 expression. Correlation between the expression of M3 receptor and Ki67 and various clinicopathological features of NSCLC patients was analyzed. We found that M3 receptor expression was gradually elevated from normal to metaplasia/dysplasia tissues to cancer tissues. Furthermore, there was a similar trend for Ki67 expression. Statistical analysis revealed that M3 receptor expression in tumor cells were correlated significantly with stage (P < 0.0001), histology type (P = 0.0003), Ki67 expression (P < 0.0001), tumor size (P < 0.0001), lymph node status (P < 0.0001), LVS invasion (P = 0.0002), and histology grade (P < 0.0001). Patients with M3 receptor high expression showed far lower disease-free survival (DFS) and overall survival (OS) rates than those with M3 receptor low expression. Multivariate Cox regression analysis demonstrated that high M3 receptor expression was an independent prognostic factor for both DFS and OS. High M3 receptor expression correlates with poor survival in NSCLC patients. M3 receptor expression may be related with tumor progression in NSCLC, indicating that M3 receptor may be a novel antineoplastic target in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the leading causes of cancer deaths worldwide [1]. Of all the lung cancer cases, approximately 80 % of lung cancers are non-small cell lung cancer (NSCLC). NSCLC is classified into three types: adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma based on histologic and immunophenotypic characteristics [2]. Despite major advances in surgical techniques, lung cancer patients have an overall 5-year survival rate of 15 % [3]. With regard to this, novel therapeutic approaches are urgently needed in patients with NSCLC.
Acetylcholine is an important neurotransmitter in the central and peripheral nervous systems. In addition to neuronal production and release at nerve synapses, acetylcholine can be produced and released by nonneuronal cells to modulate cell function. Furthermore, recent researches support the importance of muscarinic acetylcholine receptor (M1–M5R) expressions and activation in cancers. Muscarinic stimulation of cancer growth has been reported in colon [4], lung [5], glial [6], and prostate [7] cancer. In ovarian cancer, expression of muscarinic receptors correlates with a poor prognosis [8]. In breast cancer, Espanol et al. [9] demonstrated that M1R and M2R expression levels are associated with angiogenesis, whereas M1R, M2R, and M3R are involved in cell proliferation. Proliferation of breast cancer cells is regulated by postmuscarinic receptor activation of extracellular signal-regulated kinas (ERK) signaling [10]. In colon cancer, Cheng et al. concluded that luminal bile acids stimulate colon cancer cell proliferation by interacting with M3R and inducing transactivation of epidermal growth factor receptor (EGFR). In colon cancer cells, agonist binding to M3R results in MMP-7 activation which cleaves pro-heparin-binding epidermal growth factor (HB-EGF), thereby releasing HB-EGF, an EGFR ligand [11]. Post-EGFR signaling, mediated by ERK activation, stimulates cell proliferation [4]. Strong evidence demonstrates that lung cancer cell lines synthesize and secrete Ach to act as an autocrine growth factor, which provides a basis for understanding the effects of nicotine in cigarette smoke on lung cancer growth [5]. ACh stimulates lung cancer cell proliferation by interacting with either nicotinic acetylcholine receptors or muscarinic receptors. In SCLC, acetylcholine stimulates cell growth via M3 muscarinic mechanisms that involve increased [Ca2+]I and increased phosphorylation of mitogen-activated protein kinase (MAPK) [12]. In NSCLC, nicotine stimulates cell growth through AKT- and MAPK-dependent mechanisms [13] and that modulation of Akt signaling pathways may provide a target for directed therapy [14].
In this study, we investigated M3 receptor expression in cancer and adjacent normal tissues from 192 patients with NSCLC by immunohistochemical staining. We further investigated the relationship between M3 receptor expression of tumor cells and some important clinicopathologic characteristics, such as sex, age, smoking, lymph node state, histologic type, and Ki67 expression in NSCLC. We discussed whether M3 receptor expression in NSCLC could negatively correlate with survival of patients.
Materials and methods
Patients and tumor sample
Diagnostic tumor samples were obtained from patients who were diagnosed between the period 2003 and 2005 after prior approval of the local Institutional Review Board. They received care and regularly follow up at the Affiliated Tumor Hospital of Harbin Medical University for 5 years. None of the patients had received chemotherapy or radiation therapy prior to surgery or died in the peri- or postoperative time period (within 45 days after surgery). The tumor sample was histologically classified according to the criteria of the World Health Organization. Postsurgical pathologic stage was determined by the current tumor-node metastasis (TNM) classification.
Immunohistochemical staining
The following primary antibodies were used for immunohistochemical staining: rabbit polyclonal immunoglobulin (Ig) G specific for M3 receptor (dilution, 1:250; Abcam, USA) and rabbit polyclonal IgG specific for Ki67 (dilution, 1:200; Santa Cruz Biotechnology, Carpinteria, CA). Formalin-fixed, paraffin-embedded tissue samples (4-μm thick) were deparaffinized with xylene and hydrated through graded alcohol and rinsed in phosphate-buffered saline (PBS). Antigen retrieval was done by heating samples in a steamer for 10 min with 10 mmol/L sodium citrate (pH 6.0). We performed protein blocking by incubating samples for 30 min in 10 % bovine serum albumin in TBS with 0.5 % Tween 20. Primary antibody incubation was done overnight at 4 °C. Samples were next washed with PBS and incubated for 30 min with secondary antibody and then stained with 0.02 % DAB and 0.02 % H2O2 in 0.05 M Tris–HCl buffer for10 min. Finally, samples were lightly counterstained with 10 % Mayer’s hematoxylin, dehydrated, mounted, and observed. The negative controls were substituting mouse (for mAb) or rabbit (for poly Ab) nonimmune IgG for the primary antibody and omitting the primary antibody in the staining protocol.
Immunohistochemical staining assessment
M3 receptor staining was mainly localized in the cell membrane in the vast majority of cancer tissues. Staining for M3 receptor was assessed in a series of randomly selected ten high-power fields, and they were believed to be representative of the average in tumors at ×400 magnification. The sections were scored by combining the proportion and intensity of positively stained tumor cells. The proportion of positively stained tumor cells was scored as follows: 0 (no positive tumor cells), 1 (<10 % positive tumor cells), 2 (10–50 % positive tumor cells), and 3 (>50 % positive tumor cells). Staining intensity was classified according to the following criteria: 0 (no staining), 1 (weak staining: light yellow), 2 (moderate staining: yellow brown), and 3 (strong staining: brown). Staining index (SI) was calculated as the staining intensity score × the proportion score. Using this method, we evaluated the expressions of M3 receptor in NSCLC samples by determining the SI, with scores 0, 1, 2, 3, 4, 6, or 9. M3 receptor cutoff values were based on measuring heterogeneity by the log-rank test with regard to overall survival (OS). The SI score of 4 (a cutoff point) was used to distinguish between low and high expression of M3 receptor. Assessment of the staining was scored independently by two investigators (Di Wang and Yanying Wang) without knowledge of the clinicopathological findings. The scoring staining and allocation of tumors by the two investigators were similar. Cases with discrepancies were rereviewed simultaneously by the original two pathologists and a senior pathologist until a consensus was reached.
Ki67 staining is mainly located in the nuclear of colorectal proliferative cells. Only cells with a distinct nuclear Ki67 staining were considered as positive, and the percentage of immunoreactive nuclei was calculated by counting in a series of randomly selected ten microscopic fields (corresponding to a total of at least 100 tumor cells) under high-power magnification (×400). Finally, the patients were divided into two groups including Ki67-negative groups (≤10 %) and Ki67-positive groups (>10 %).
Statistical analysis
All data were analyzed by statistics software (SPSS 13.0 for Windows; SPSS, Inc., Chicago, IL). Analyzed variables included age, tumor size, histological type, depth of invasion, lymph node status, venous and lymphatic invasion, TNM stage, and expressions of M3 receptor and Ki67. The correlation between expressions of M3 receptor and Ki67 and the other variables was assessed by the chi-square and Fisher’s exact tests. Bivariate correlations between two independent variables were analyzed by calculating the Spearman’s correlation coefficients. Survival analysis was performed using the Kaplan–Meier method and compared by the log-rank test. Prognostic relevance was evaluated by multivariate Cox regression analysis. P < 0.05 was considered as significant.
Results
Clinicopathologic features of NSCLC
One hundred ninety-two patients with NSCLC included in this study ranged from 24 to 74 years, and the mean age at the time of surgery was 59 years. One hundred four were women and 88 were men. At the time of diagnosis, 77 were stage I and 115 stages II–III. Patients data were analyzed after a 5-year follow-up, and the information were obtained in 92.8 % (192/207) of patients. The median overall survival was 41.1 months, and median disease-free survival was 38.6 months.
Immunohistochemical expression of M3 receptor and Ki67 in NSCLC
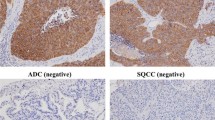
M3 receptor was mainly detected in the cell membrane and Ki67 in the nucleus of NSCLC and some inflammatory cells. Overall, the frequency and intensity of M3 receptor expression was gradually elevated from normal to metaplasia/dysplasia tissues to cancer tissues. M3 receptor high expression was observed in 103 cases (53.65 %) and low in 89 cases (46.35 %) including samples without M3 receptor expression (Table 1). Furthermore, there was a similar trend for Ki67 expression (as a proliferative index) from normal to metaplasia/dysplasia tissues to cancer tissues. Ki67 positive expression was found in 120 cases (62.5 %) and negative expression was found in 72 cases (37.5 %) (Table 1).
Expression of M3 receptor in NSCLC and correlation with clinicopathologic features
The association of M3 receptor expression with clinicopathologic factors was shown in Table 2. The M3 receptor expression in tumor cells were correlated significantly with stage (P < 0.0001), histology type (P = 0.0003), Ki67 expression (P < 0.0001), tumor size (P < 0.0001), lymph node status (P < 0.0001), lymphatic vessel size (LVS), invasion (P = 0.0002), and histology grade(P < 0.0001). It was not correlated with age, sex, and smoking.
Univariate and multivariate analysis for prognosis of patients with NSCLC
Both univariate and multivariate survival analysis was used to evaluate the effect of M3 receptor, Ki67 expression, smoking, and clinicopathological characteristics (including age, histology type, histology grade, stage, tumor size, lymph node status, LVS invasion, and so on) on prognosis. Univariate Cox regression analysis identified that stage, tumor size, lymph node status, LVS invasion, M3 receptor, Ki67 expression, and smoking were prognostic factors influencing both 5-year disease-free survival (DFS) and OS (Table 3). By multivariate analysis, we further examined prognostic parameters of NSCLC that were significant in univariate analysis. LVS invasion, lymph node status, TNM stage, M3 receptor, and Ki67 expression were independent prognostic factors influencing both 5-year DFS and OS (Table 4).
Effect of M3 muscarinic acetylcholine receptor on patients’ survival
The Kaplan–Meier 5-year survival curves stratified for M3 receptor expression were provided in our study. High-expression M3 receptor was clearly associated with poor 5-year DFS (28.20 % vs. 44.44 %) and 5-year OS (35.56 % vs. >50 %) rate. The median DFS was 38.6 months, and the median OS was 41.1 months. In adenocarcinoma, the 5-year DFS of M3 receptor high expression patients was 25.64 %, and the 5-year OS was 32.05 %. However, the 5-year DFS of M3 receptor low expression patients was 46.67 %, and the 5-year OS was >50 %. In non-adenocarcinoma, the 5-year DFS of M3 receptor high expression patients was 26.67 %, and the 5-year OS was 33.33 %. Nevertheless, the 5-year DFS of M3 receptor low expression patients was 43.75 %, and the 5-year OS was >50 %.
Discussion
Lung cancer account for more than 25 % of cancer-related deaths and about 15 % of new cancer cases annually [1]. The discovery of novel molecular targets for its diagnosis and treatment has the potential to improve the clinical strategy and to predict patient’s outcome. Our research has showed that M3 receptor expression may play an important role in the carcinogenesis and progression of NSCLC. M3 receptor high expression may be associated with unfavorable clinical outcomes in NSCLC patients.
The importance of acetylcholine signaling via muscarinic receptors has long been recognized in neuronal tissue. However, the role of muscarinic signaling in cancer has not received enough attention until recently. Muscarinic receptor expression is reported in tissues and cell lines derived from cancers of the brain [15], breast, colon, lung, ovary, pancreas, prostate, skin [16, 17], stomach [18], and uterus. Depending on the cell types involved, muscarinic signaling can activate ERK, NF-κB [19], and phosphatidylinositol 3-kinase/Akt [20] pathways which stimulate various aspects of tumor malignancy such as proliferation, angiogenesis, cell survival, and so on. This suggests that inhibition of M3 receptor may be a novel therapeutic approach.
We investigated the status of M3 receptor expression in a large number of NSCLC tissues, and we showed that M3 receptor was highly expressed in patients with NSCLC. Only weak or none expression of M3 receptor was observed in normal tissues. M3 receptor expression was significantly correlated with the expression of Ki67. We observed that 103 of 192 NSCLC patients were high M3 receptor expression, and 120 of 192 NSCLC patients were positive Ki67 expression. These results suggested that M3 receptor high expression may be involved in the NSCLC carcinogenesis. Overexpression of M3 receptor may represent an independent prognostic factor for NSCLC patients influencing both 5-year DFS and OS. Patients with higher M3 receptor expression had a shorter overall survival time, whereas patients with lower M3 receptor expression had better survival. These results are consistent with the previous reports concerning the roles of muscarinic receptors in the carcinogenesis and progression of cancer. Moreover, the prognostic impact of the M3 receptor was statistically higher than the LVS invasion, lymph node state, TNM stage, and Ki67 expression in the multivariate analysis. The biological function of M3 receptor in the aggressiveness of NSCLC was supported by significant correlations between N and M classification and M3 receptor expression in NSCLC. From these results, we can see that M3 receptor may become a valuable predictor for prognosis among NSCLC patients. Further studies are still needed on the molecular mechanism underlying M3 receptor involvement in the progression of NSCLC.
The upregulation of M3 receptor combined with smoking by most lung cancer patients provides not only a proliferative stimulus but also a pathway to target for new therapeutic intervention. In SCLC cells, augmented cell proliferation is observed following addition of eserine, and attenuated proliferation is observed with hemicholinium-3 [5]. In colon cancer, using eserine to inhibit Ach degradation results in potentiation of cell proliferation, whereas reducing ACh production with a choline transport inhibitor, hemicholinium-3, attenuates proliferation [21]. In addition, an M3R-selective inhibitor reduced the size of small cell lung cancer xenografts in nude mice [12], and genetic ablation of M3R reduces murine colon tumor number and size [22]. This raises the possibility that M3 receptor may be a novel antineoplastic therapy in the future. Inhibition of muscarinic receptor is practicable because oral muscarinic antagonists are well tolerated and in wide clinical use for overactive bladder [23], and inhaled muscarinic antagonists are widely used for chronic obstructive pulmonary disease [24]. Anti-M3 receptor and its mechanism in the treatment of different cancers were also studied [25–28].
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
World Health Organization. Classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004.
Cancer Facts & Figures. Atlanta: American Cancer Society; 2009
Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003;63:6744–50.
Song P, Sekhon HS, Jia Y, et al. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–21.
Yagle K, Lu H, Guizzetti M, Moller T, Costa LG. Activation of mitogen-activated protein kinase by muscarinic receptors in astroglial cells: role in DNA synthesis and effect of ethanol. Glia. 2001;35:111–20.
Rayford W, Noble MJ, Austenfeld MA, Weigel J, Mebust WK, Shah GV. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. Prostate. 1997;30:160–6.
Oppitz M, Mobus V, Brock S, Drews U. Muscarinic receptors in cell lines from ovarian carcinoma: negative correlation with survival of patients. Gynecol Oncol. 2002;85:159–64.
Espanol AJ, de la Torre E, Fiszman GL, Sales ME. Role of non-neuronal cholinergic system in breast cancer progression. Life Sci. 2007;80:2281–5.
Jimenez E, Montiel M. Activation of MAP kinase by muscarinic cholinergic receptors induces cell proliferation and protein synthesis in human breast cancer cells. J Cell Physiol. 2005;204:678–86.
Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol. 2007;73:1001–12.
Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, et al. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–44.
West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90.
Castillo SS, Brognard J, Petukhov PA, et al. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64:2782–92.
Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen:muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–73.
Boss A, Oppitz M, Drews U. Muscarinic cholinergic receptors in the human melanoma cell line SK-Mel 28: modulation of chemotaxis. Clin Exp Dermatol. 2005;30:557–64.
Bowers JW, Schlauder SM, Calder KB, Morgan MB. Acetylcholine receptor expression in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:340–3.
Kodaira M, Kajimura M, Takeuchi K, Lin S, Hanai H, Kaneko E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J Gastroenterol. 1999;34:163–71.
Shant J, Cheng K, Marasa B, Wang JY, Raufman JP. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp Cell Res. 2009;315(3):432–50.
Raufman JP, Shant J, Guo CY, Roy S, Cheng K. Deoxycholyltaurine rescues human colon cancer cells from apoptosis by activating EGFR-dependent PI3K/Akt signaling. J Cell Physiol. 2008;215:538–49.
Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70:1035–47.
Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, et al. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 2008;68:3573–8.
Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol. 2006;147 Suppl 2:S80–87.
Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol. 2006;533:36–9.
Li C, Cai J, Geng J, Li Y, Wang Z, Li R. Purification, characterization and anticancer activity of a polysaccharide from Panax ginseng. Int J Biol Macromol. 2012;51:968–73.
Peng Z, Heath J, Drachenberg C, Raufman JP, Xie G. Cholinergic muscarinic receptor activation augments murine intestinal epithelial cell proliferation and tumorigenesis. BMC Cancer. 2013;13:204.
Kramer-Marek G, Capala J. The role of nuclear medicine in modern therapy of cancer. Tumour Biol. 2012;33:629–40. doi:10.1007/s13277-012-0373-8.
Hua N, Wei X, Liu X, Ma X, He X, Zhuo R, et al. A novel muscarinic antagonist R2HBJJ inhibits non-small cell lung cancer cell growth and arrests the cell cycle in G0/G1. PLoS One. 2012;7:e53170.
Acknowledgments
This paper was supported by the natural science foundation of Heilongjiang province (no: D201049) and HeiLongjiang Science and Technology foundation (no: WB07C08).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, J., Zhou, J., Yao, L. et al. High expression of M3 muscarinic acetylcholine receptor is a novel biomarker of poor prognostic in patients with non-small cell lung cancer. Tumor Biol. 34, 3939–3944 (2013). https://doi.org/10.1007/s13277-013-0982-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0982-x