Abstract
The well-known classification of neuroendocrine neoplasms of the lung into four major subtypes (including typical and atypical carcinoids and small- and large-cell neuroendocrine carcinomas) has a proven prognostic validity but only partially helps to predict the response to specific therapies. Therapeutic biomarkers are incompletely known and include morphological, immunophenotypic, and molecular markers. Morphology alone has no specific predictive role, nor has any immunophenotypic marker been proven to bear predictive implications. Ki67 is a relevant prognostic marker and can indirectly predict response to chemotherapy, when levels are extremely high in high-grade neuroendocrine (NE) carcinomas. The expression of somatostatin receptors, especially of the type 2A, has been shown to predict response to somatostatin analog treatments, paralleling the information derived from octreotide scintigraphy. mTOR pathway is targeted by specific inhibitors, but the exact cellular molecules predicting response are still to be defined. It seems that high levels of phosphorylated forms of mTOR and of its downstream factor S6K are associated to a better response to rapalogs in experimental models. Data from gene expression profiling and mutational analyses are currently emerging, providing a more detailed map of different molecular activation pathways, potentially leading to a more accurate molecular classification of lung NE tumors as well as to the discovery of new therapeutic targets. The combination of mutational profiles with those of upregulated or downregulated genes also by gene gains or losses may ultimately provide a better characterization of NE tumor histological types in terms of response to specific chemotherapy or biotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Therapeutic strategies for lung neuroendocrine neoplasms are not completely defined and greatly vary from carcinoids to high-grade carcinomas. In the former group, surgery is the mainstay for the therapy of resectable tumors, whereas adjuvant strategies are not well established yet. In recurrent or metastatic diseases, biotherapies (i.e., somatostatin analogs and mTOR inhibitors) have been proposed, but no clinical evidence is currently available on their real impact to increase patient survival, and large prospective trials are still ongoing [1]. Chemotherapeutic strategies are the only effective treatment in small-cell lung carcinoma (SCLC) and large-cell neuroendocrine carcinoma (LCNEC), although the high response rates (especially for SCLC) are paralleled by a very high likelihood of recurrence/progression and by a generally unfavorable outcome. Chemotherapeutic strategies are also considered in moderately proliferating carcinoids [2] generalizing to the lung the experience in gastrointestinal and pancreatic neuroendocrine neoplasms, but the evidence on the efficacy of chemotherapeutic agents derives from small retrospective series rather than from specifically designed prospective clinical trials.
The present paper is aimed at summarizing the pathologist’s role in determining predictive biomarkers of response to treatment in lung neuroendocrine neoplasms, with special reference to carcinoids (see Table 1).
Morphological and Phenotypic Markers
Morphology
Neuroendocrine (NE) neoplasms of the lung have been variably labeled for the purpose of better defining the different histotypes and differentiation grades [3]. The current WHO classification [4] recognizes four entities, defined by the term “carcinoid” for low/intermediate-grade tumors and “large- or small-cell carcinoma” for high-grade tumors [5, 6].
Typical carcinoids (TCs) account for about 1–2 % of lung tumors and exhibit a classical organoid (acinar, trabecular) pattern with polygonal, minimally atypical cells. Necrosis is absent and mitoses are <2/2 mm2. Similar tumors having a size of <5 mm are labeled neuroendocrine tumorlets [7, 8].
Atypical carcinoids (ACs) are extremely rare, cigarette-smoking-related tumors, though often associated with regional and distant metastases. Their morphology overlaps that of TC, except that necrosis is present and/or the mitotic count is 2–10/2 mm2 [9, 10].
Large-cell NE carcinoma (LCNEC) partly resembles the organoid architecture of AC but is made of larger cells, necrosis is extensive and the mitotic index exceeds by far 10 in 2 mm2. In the 2004 WHO scheme [4], LCNEC is classified among non-NE large-cell carcinomas, from which it should be distinguished based on the recognition of a NE phenotype, by either morphology or immunohistochemistry for neuroendocrine markers [11]. A subgroup of undifferentiated large-cell carcinomas with NE morphology but no NE marker expression has been also described [12].
Small-cell lung carcinoma (SCLC) is the most common lung NE neoplasm and classically is characterized by small cells with scant cytoplasm and condensed chromatin, a diffuse growth pattern, extensive necrosis, and a very high mitotic index (largely exceeding 10/2 mm2).
Combined NE carcinomas are the result of a relatively uncommon association of SCLC or LCNEC with conventional squamous cell or adeno-carcinoma component. Focal NE differentiation in conventional lung carcinomas is excluded from this definition, because its relevance to clinics and tumor behavior is unclear.
Immunophenotype
Chromogranin A and synaptophysin expression, in the absence of high molecular weight cytokeratins [13] or p40 [14], are the most reliable NE markers, whereas PGP9.5, NSE, and CD56 are less specific. In high-grade NECs (but not in carcinoids), hASH-1 (a transcription factor driving NE differentiation during human development) expression has been reported [15]. NE markers are mandatory for recognizing the NE nature of a lesion, although none of them has been demonstrated to be superior to morphology in terms of prognostication or response-to-therapy prediction. Lung (and thyroid) specific marker TTF-1 is mostly expressed by high-grade NE carcinomas of both pulmonary and extra-pulmonary origins, while carcinoid tumors are either unreactive or may show variable positivity especially in peripheral lesions [4, 16, 17]. A non-NE marker has been recently proposed to help in identifying mitotic figures, namely phosphohistone 3 [18].
Prognostic and Predictive Molecular Markers
Ki67 index
The proliferation index detected by Ki67 is also a useful tool to better classify a lung neuroendocrine tumor (NET), although not included in the WHO classification criteria [4], at variance with the digestive tract NETs [19]. The reason of this difference may be related to the current lack of validation of Ki67 index in lung NETs [20]. Indeed, the proliferative activity of pulmonary NE tumors has been extensively investigated (reviewed by Pelosi et al. 2014 [21]). The diagnostic role of Ki67 is so far well established in small biopsy or cytology samples, in which artifacts may hamper the differential diagnosis between small-cell lung cancer and carcinoid tumor in individual cases [22]. Analyzing the Ki67 indexes in over 1,800 reported cases, it appears that the mean proliferation values for TC, AC, LCNEC, and SCLC are 1.6, 7.3, 48.5, and 58.9 %, respectively, and these figures are paralleled by the different survival rates reported for the single histotypes [23]. There may be, however, significant overlap of Ki67 indexes between biologically adjacent tumor categories (TC vs. AC; AC vs. LCNEC; LCNEC vs. SCLC), thus preventing its reliable diagnostic use in individual cases (Fig. 1). Some authors found a Ki67 performance equal or higher than that of mitotic count for both diagnostic (cutoff between TC and AC proposed at 4 %) and prognostic purposes [24–26].
In a recent study on 399 NE tumors of the lung [27], Ki67 index was incorporated into a newly proposed grading system, which also considered two conventional morphological parameters (mitotic count and necrosis). Adapting cutoff values for mitoses and Ki67 at 4 and 25 %, it was found that a three-grade system can be reliably obtained when at least two of the three parameters were identified which allows the stratification of NE tumors into three subgroups with significantly different survivals. In terms of prediction, Ki-67 has not been associated with the response to any specific treatment in carcinoids. By contrast, in high-grade carcinomas, the higher Ki67 values detected in SCLC (90 %) as compared to those in LCNEC (50–60 %) have been postulated to be associated with different chemotherapy responses [28].
Molecular Profile
The molecular profile of lung NE tumors has been extensively investigated to identify diagnostic, prognostic, and predictive factors, and to possibly lead to a “molecular classification” of NETs [29]. Specific chromosomal alterations (e.g., 11q22.3-q25 losses) [30], oncogene mutations, and cell cycle deregulation [31] were documented in lung NETs [32]. The mutational profile of lung NETs is still largely unknown. MEN1 gene is the most largely investigated and found to be mutated in approximately 13 % of carcinoids but very rarely in high-grade NE carcinomas [33]. In term of prognosis, MEN1 gene mutation or loss was significantly related to shorter survival in carcinoid patients, together with tumor stage [34], in keeping with its function as a tumor suppressor gene. Novel data are coming from next-generation sequencing analysis, showing a genetic similarity between pulmonary and pancreatic well-differentiated neuroendocrine tumors, but surprisingly not between TC and AC in the lung [35].
Gene and protein expression profiles were also extensively investigated. The latter was analyzed by proteomics and immunohistochemistry in carcinoid and SCLC, ultimately leading to the identification of over 300 differentially expressed proteins in each tumor subtype [36]. Gene expression profiling studies identified also novel prognostic markers in the group of lung carcinoids, independent of the histological type. Among others, three genes were found to bear prognostic implications, namely orthopedia homeobox (OTP), CD44, and RET. In particular, significant associations with reduced 20-year survival were observed in the case of low messenger RNA (mRNA) levels of CD44 (p = 0.000018) and OTP (p = 0.00054), and high RET levels (p = 0.025) [37]. The same authors also found a different gene expression profile in a small series of 10 bronchial carcinoids having a favorable or a poor outcome (five cases each). The latter had a significantly higher number of downregulated genes at chromosome 11q, a region frequently lost in carcinoids (p = 0.00017). Upregulated genes are involved in the mitotic spindle checkpoint, the chromosomal passenger complex (CPC), mitotic kinase CDC2 activity, and BRCA-Fanconi anemia pathway. The above mentioned CD44 and OTP genes, as well as others, including BIRC5 (survivin), BUB1, IL20RA, and KLK12, were found to be independent predictors of patient outcome [38].
Predictive Markers
Predictors of response to chemotherapy or biotherapy are increasingly being evaluated [39–41], but investigational studies specifically designed in lung neuroendocrine neoplasms are meagre.
Thymidylate synthase (TS) is the target of antifolate drugs and intratumoral expression levels may predict response to an antifolate-based regimen. A differential expression of TS mRNA and protein in the spectrum of pulmonary NE neoplasms was observed. TS levels were higher in poorly differentiated NE carcinomas, thus supporting the extremely poor activity of these drugs in small-cell lung cancer [42].
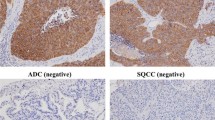
The expression of specific receptors or enzymes implicated in the response to biotherapies has been demonstrated [16]. Somatostatin receptors (SSTRs) have been identified in NE tumors by different techniques [43]. The immunohistochemical expression of SSTR types 2 and 3 was investigated in 218 aggressive lung NE tumors (metastatic TC, AC, LCNEC, and resected SCLC). The expression of SSTRs was progressively reduced in poorly differentiated forms and correlated with octreotide scintigraphy in 70 % of cases [44] (Fig. 2). The mTOR pathway has been explored in lung NET [45]. and a lower expression of active forms of mTOR and S6K was detected in high-grade carcinomas (of either large or small cell types) [46] (Fig. 3). Indeed, the mTOR pathway is a complex network of factors, and the potential role of its players in predicting response to mTOR inhibitors (rapalogs) is unknown. Several proteins belonging to the mTOR complexes, or downstream and upstream to mTOR, interplay in regulating such central intracellular signaling pathway, ideally all being candidate biomarkers of prediction of response to mTOR inhibition. Moreover, additional players interact with mTOR, such as nutrient transporters or somatostatin receptors themselves. Preliminary data from our laboratory (Rapa and Volante, unpublished results) indicate, among others, a significant inverse correlation between the expression of glucose transporter GLUT-1 and mTOR signaling. In addition, the expression of some of the above molecules, in particular p-mTOR and GLUT-1, as well as of the amino acid transporter LAT-1, was strongly associated with SSTR2A expression, suggesting that somatostatin receptor inhibitor effects may result from mTOR pathway control and that synergies can be obtained by combined treatments, as suggested in intestinal NETs [47]. Indeed, in in vitro models, bronchial carcinoid cells of patients responding to mTOR inhibitors were shown to have higher levels of phosphorylated mTOR [48].
Other synergistic combination effects (i.e., apoptosis induction) were recently reported, involving erlotinib (targeting EGFR) combined with everolimus (targeting mTOR) in AC and LCNEC [49].
In another study, significant differences in c-KIT and HER2 expression were seen between LCNEC and AC, while EGFR mutations were more common in AC than in LCNEC. A potential role for VEGF and c-KIT (and possibly HER2) targeting agents in the treatment of LCNEC was therefore suggested [50, 51].
Finally, c-MET oncogene has been investigated in pulmonary NE neoplasms. PAX5 was shown to upregulate c-MET in small-cell lung carcinoma, and PAX5 and c-MET co-inhibition produced a synergistic effect in killing tumor cells, probably related to paxillin inactivation (a downstream target of activated c-Met involved in cell motility and tumor spread) [52]. However, regardless of TKI treatment, c-Met activation (phosphorylation) was not influenced by the mutational status, which was detected in 25 % of SCLC cell lines, in 8.3 % of NETs, and in 6.5 % of SCLC cases [53].
References
Oberg K, Hellman P, Ferolla P, Papotti M Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23 Suppl 7: vii120-123, 2012.
Noel-Savina E, Descourt R Focus on treatment of lung carcinoid tumor. Onco Targets Ther 6: 1533–1537, 2013.
Moran CA, Suster S, Coppola D, Wick MR Neuroendocrine carcinomas of the lung: a critical analysis. Am J Clin Pathol 131: 206–221, 2009.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC: Tumours of the Lung, Pleura, Thymus and Heart, Lyon: IARC Press, 2004.
Travis WD, Giroux DJ, Chansky K et al. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 3: 1213–1223, 2008.
Sobin LH: TNM classification of malignant tumors, Oxford, 2009.
Travis WD Advances in neuroendocrine lung tumors. Ann Oncol 21 Suppl 7: vii65-71, 2010
Tsuta K, Raso MG, Kalhor N, Liu DD, Wistuba, II, Moran CA Histologic features of low- and intermediate-grade neuroendocrine carcinoma (typical and atypical carcinoid tumors) of the lung. Lung Cancer 71: 34–41, 2011.
Arrigoni MG, Woolner LB, Bernatz PE Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg 64: 413–421, 1972.
Travis WD, Rush W, Flieder DB et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol 22: 934–944, 1998.
Travis WD, Linnoila RI, Tsokos MG et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol 15: 529–553, 1991.
Iyoda A, Hiroshima K, Toyozaki T, Haga Y, Fujisawa T, Ohwada H Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 91: 1992–2000, 2001.
Sturm N, Rossi G, Lantuejoul S et al. 34BetaE12 expression along the whole spectrum of neuroendocrine proliferations of the lung, from neuroendocrine cell hyperplasia to small cell carcinoma. Histopathology 42: 156–166, 2003.
Pelosi G, Rossi G, Cavazza A et al. DeltaNp63 (p40) distribution inside lung cancer: a driver biomarker approach to tumor characterization. Int J Surg Pathol 21: 229–239, 2013.
Jiang SX, Kameya T, Asamura H et al. hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol 17: 222–229, 2004.
Rekhtman N Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 134: 1628–1638, 2010.
La Rosa S, Chiaravalli AM, Placidi C, Papanikolaou N, Cerati M, Capella C TTF1 expression in normal lung neuroendocrine cells and related tumors: immunohistochemical study comparing two different monoclonal antibodies. Virchows Arch 457: 497–507, 2010.
Tsuta K, Liu DC, Kalhor N, Wistuba, II, Moran CA Using the mitosis-specific marker anti-phosphohistone H3 to assess mitosis in pulmonary neuroendocrine carcinomas. Am J Clin Pathol 136: 252–259, 2011.
Bosman F: Tumours of the Digestive Tract, Lyon: IARC Press, 2010.
Walts AE, Ines D, Marchevsky AM Limited role of Ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod Pathol 25: 1258–1264, 2012.
Pelosi G, Rindi G, Travis WD, Papotti M Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 9: 273–284, 2014.
Pelosi G, Rodriguez J, Viale G, Rosai J Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 29: 179–187, 2005.
Asamura H, Kameya T, Matsuno Y et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 24: 70–76, 2006.
Costes V, Marty-Ane C, Picot MC et al. Typical and atypical bronchopulmonary carcinoid tumors: a clinicopathologic and KI-67-labeling study. Hum Pathol 26: 740–745, 1995.
Skov BG, Holm B, Erreboe A, Skov T, Mellemgaard A ERCC1 and Ki67 in small cell lung carcinoma and other neuroendocrine tumors of the lung: distribution and impact on survival. J Thorac Oncol 5: 453–459, 2010.
Grimaldi F, Muser D, Beltrami CA et al. Partitioning of bronchopulmonary carcinoids in two different prognostic categories by ki-67 score. Front Endocrinol (Lausanne) 2: 20, 2011.
Rindi G, Klersy C, Inzani F et al. Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocr Relat Cancer 21: 1–16, 2014.
Travis WD Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol 25 Suppl 1: S18-30, 2012.
Pelosi G, Papotti M, Rindi G, Scarpa A Unraveling tumor grading and genomic landscape in lung neuroendocrine tumors. Endocrine Pathology, 2014.
Swarts DR, Claessen SM, Jonkers YM et al. Deletions of 11q22.3-q25 are associated with atypical lung carcinoids and poor clinical outcome. Am J Pathol 179: 1129–1137, 2011.
Beasley MB, Lantuejoul S, Abbondanzo S et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol 34: 136–142, 2003.
Swarts DR, Ramaekers FC, Speel EJ Molecular and cellular biology of neuroendocrine lung tumors: evidence for separate biological entities. Biochim Biophys Acta 1826: 255–271, 2012.
Debelenko LV, Swalwell JI, Kelley MJ et al. MEN1 gene mutation analysis of high-grade neuroendocrine lung carcinoma. Genes Chromosomes Cancer 28: 58–65, 2000.
Swarts DR, Scarpa A, Corbo V et al. MEN1 gene mutation and reduced expression are associated with poor prognosis in pulmonary carcinoids. J Clin Endocrinol Metab 99: E374–378, 2014.
Fernandez-Cuesta L, Peifer M, Lu X et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 5: 3518, 2014.
Tanca A, Addis MF, Pagnozzi D et al. Proteomic analysis of formalin-fixed, paraffin-embedded lung neuroendocrine tumor samples from hospital archives. J Proteomics 74: 359–370, 2011.
Swarts DR, Henfling ME, Van Neste L et al. CD44 and OTP are strong prognostic markers for pulmonary carcinoids. Clin Cancer Res 19: 2197–2207, 2013.
Swarts DR, Van Neste L, Henfling ME et al. An exploration of pathways involved in lung carcinoid progression using gene expression profiling. Carcinogenesis 34: 2726–2737, 2013.
Rudin CM, Durinck S, Stawiski EW et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 44: 1111–1116, 2012.
Nakamura H, Tsuta K, Yoshida A et al. Aberrant anaplastic lymphoma kinase expression in high-grade pulmonary neuroendocrine carcinoma. J Clin Pathol 66: 705–707, 2013.
Odate S, Nakamura K, Onishi H et al. TrkB/BDNF signaling pathway is a potential therapeutic target for pulmonary large cell neuroendocrine carcinoma. Lung Cancer 79: 205–214, 2013.
Ceppi P, Volante M, Ferrero A et al. Thymidylate synthase expression in gastroenteropancreatic and pulmonary neuroendocrine tumors. Clin Cancer Res 14: 1059–1064, 2008.
Tsuta K, Wistuba, II, Moran CA Differential expression of somatostatin receptors 1–5 in neuroendocrine carcinoma of the lung. Pathol Res Pract 208: 470–474, 2012.
Righi L, Volante M, Tavaglione V et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 'clinically aggressive' cases. Ann Oncol 21: 548–555, 2010.
Ali G, Boldrini L, Capodanno A et al. Expression of p-AKT and p-mTOR in a large series of bronchopulmonary neuroendocrine tumors. Exp Ther Med 2: 787–792, 2011.
Righi L, Volante M, Rapa I et al. Mammalian target of rapamycin signaling activation patterns in neuroendocrine tumors of the lung. Endocr Relat Cancer 17: 977–987, 2010.
Pavel ME, Hainsworth JD, Baudin E et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 378: 2005–2012, 2011.
Gagliano T, Bellio M, Gentilin E et al. mTOR, p70S6K, AKT, and ERK1/2 levels predict sensitivity to mTOR and PI3K/mTOR inhibitors in human bronchial carcinoids. Endocr Relat Cancer 20: 463–475, 2013.
Bago-Horvath Z, Sieghart W, Grusch M et al. Synergistic effects of erlotinib and everolimus on bronchial carcinoids and large-cell neuroendocrine carcinomas with activated EGFR/AKT/mTOR pathway. Neuroendocrinology 96: 228–237, 2012.
Rossi G, Cavazza A, Marchioni A et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 23: 8774–8785, 2005.
Iyoda A, Travis WD, Sarkaria IS et al. Expression profiling and identification of potential molecular targets for therapy in pulmonary large-cell neuroendocrine carcinoma. Exp Ther Med 2: 1041–1045, 2011.
Song J, Li M, Tretiakova M, Salgia R, Cagle PT, Husain AN Expression patterns of PAX5, c-Met, and paxillin in neuroendocrine tumors of the lung. Arch Pathol Lab Med 134: 1702–1705, 2010.
Voortman J, Harada T, Chang RP et al. Detection and therapeutic implications of c-Met mutations in small cell lung cancer and neuroendocrine tumors. Curr Pharm Des 19: 833–840, 2013.
Volante M, Brizzi MP, Faggiano A et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 20: 1172–1182, 2007.
Miederer M, Seidl S, Buck A et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging 36: 48–52, 2009.
Kulke MH, Hornick JL, Frauenhoffer C et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 15: 338–345, 2009.
Sodja E, Knez L, Kern I, Ovcaricek T, Sadikov A, Cufer T Impact of ERCC1 expression on treatment outcome in small-cell lung cancer patients treated with platinum-based chemotherapy. Eur J Cancer 48: 3378–3385, 2012.
Ceppi P, Longo M, Volante M et al. Excision repair cross complementing-1 and topoisomerase IIalpha gene expression in small-cell lung cancer patients treated with platinum and etoposide: a retrospective study. J Thorac Oncol 3: 583–589, 2008.
Acknowledgments
This work is partially supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan) (IG number 13567 to MV). IR and SV are PhD fellows at the University of Turin, Doctorate School of Biomedical Sciences and Oncology. Presented in part at the Companion Meeting of the Endocrine Pathology Society st the USCAP annual meeting in San Diego, March 1, 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Righi, L., Volante, M., Rapa, I. et al. Therapeutic Biomarkers in Lung Neuroendocrine Neoplasia. Endocr Pathol 25, 371–377 (2014). https://doi.org/10.1007/s12022-014-9335-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-014-9335-6