Abstract
Cyclin E is an important regulator of cell cycle progression. Various studies examined the relationship between cyclin E overexpression with the clinical outcome in patients with breast cancer but yielded conflicting results. Electronic databases updated to May 2013 were searched to find relevant studies. A meta-analysis was conducted with eligible studies which quantitatively evaluated the relationship between cyclin E overexpression and survival of patients with breast cancer. Survival data were aggregated and quantitatively analyzed. We conducted a final analysis of 7,759 patients from 23 eligible studies and evaluated the correlation between cyclin E overexpression and survival in patients with breast cancer. Combined hazard ratios suggested that cyclin E overexpression had an unfavorable impact on overall survival (OS) (hazard ratio (HR) = 1.30, 95 % confidence interval (CI), 1.12–1.49) and breast cancer-specific survival (BCSS) (HR = 1.48, 95 % CI, 1.03–1.93), but not disease-free survival (HR = 1.11; 95 % CI, 0.96–1.27) in patients with breast cancer. Significantly, risks were found among stage I–II breast cancer for (HR = 1.75; 95 % CI, 1.30–2.19). Cyclin E overexpression is associated with poor OS and BCSS in breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death in females worldwide, accounting for 23 % of the total new cancer cases and 14 % of the total cancer deaths in 2008 [1]. The significant improvements in both disease-free survival (DFS) and overall survival (OS) following breast cancer diagnosis are due to the extensive use of adjuvant systematic therapies such as mastectomy, radiotherapy, chemotherapy, hormone treatment, and biological therapy. Prognostic factors are frequently helpful in the clinical management of breast cancer patients and have the potential to improve the quality of individual care for these patients [2]. Several independent prognostic factors for patient survival have been identified, including tumor size, histological grade, nodal status, hormone receptor status, HER-2 status, and patient age [3–5]. However, the discriminant value of most potential prognostic biological markers is insufficient to predict the optimal therapeutic course for an individual.
Cell cycle checkpoints are critical episodes in controlling cell proliferation paradigm [6, 7]. Indeed, cell cycle is regulated by the multiple actions of cyclins, cyclin-dependent kinase (CDK), and CDK inhibitors [8]. Many tumors, such as colon, breast, and gastric carcinomas appear to deregulate or amplify cyclin expression, especially cyclin E. This crucially exerts its action during the transition of G1 into S phase of the cell cycle. Cyclin E activity is also needed for the initiation of DNA replication and regulating genes essential for proliferation and progression through S phase [9–11].
Many studies have evaluated whether the overexpression of cyclin E may be a prognostic factor for survival in patients with breast cancer. However, the results of the studies are inconclusive, and no consensus has been reached. It is unknown whether differences in these investigations have been mostly due to their limited sample size or genuine heterogeneity. We therefore carried out a meta-analysis of published studies to quantitatively review the effects of cyclin E overexpression in tumor tissue on survival in patients with breast cancer.
Materials and methods
Search strategy and study selection
The electronic databases PubMed, Embase, and China National Knowledge Infrastructure were searched for studies to include in the present meta-analysis. An upper date limit of May 01, 2013 was applied; we used no lower date limit. Searches included the terms “breast,” “cancer or carcinoma or tumor or neoplasm,” “Cyclin E,” and “prognosis.” We also reviewed the Cochrane Library for relevant articles. The references reported in the identified studies were also used to complete the search.
Studies eligible for inclusion in this meta-analysis met the following criteria: (1) measure cyclin E expression in the primary breast cancer tissue with immunohistochemistry (IHC) or reverse transcription-polymerase chain reaction (RT-PCR); (2) provide information on survival (i.e., DFS and/or OS, and/or breast cancer-specific survival (BCSS), studies investigating response rates only were excluded); (3) When the same author reported results obtained from the same patient population in more than one publication, only the most recent report, or the most complete one, was included in the analysis. Two reviewers (S.G. and J.M.) independently determined study eligibility. If these two authors could not reach a consensus, another author (C. L.) was consulted to resolve the dispute and a final decision was made by the majority of the votes.
Data extraction and quality assessment
The final articles included were assessed independently by two reviewers (S.G. and J.M.). Data retrieved from the reports included author, publication year, patient source, test method, definition of positivity (cutoff value), follow-up, and survival data (Table 1). If data from any of the above categories were not reported in the primary study, items were treated as “not applicable.” We did no contact the author of the primary study to request the information. We did not use prespecified quality-related inclusion or exclusion criteria and did not weigh each study by a quality score, because the quality score has not received general agreement for use in a meta-analysis, especially observational studies [12].
Statistical methods
Included studies were divided into two groups for analysis: those with data regarding OS/BCSS and those regarding DFS. For the quantitative aggregation of the survival results, we measured the impact of cyclin E overexpression on survival by hazard ratio (HR) between the two survival distributions. HRs and 95 % confidence intervals (CIs) were used to combine as the effective value. If the HRs and their 95 % CIs were given explicitly in the articles, we used crude ones. When these variables were not given explicitly, they were calculated from the available numerical data using methods reported by Parmar et al. [13].
Heterogeneity of the individual HRs was calculated with χ 2 tests according to Peto’s method [14]. Heterogeneity test with inconsistency index (Ι 2) statistic and Q statistic was performed. If HRs were found to have fine homogeneity, a fixed effect model was used for secondary analysis; if not, a random-effect model was used. DerSimonian–Laird random-effects analysis [15] was used to estimate the effect of cyclin E overexpression on survival. By convention, an observed HR >1 implies worse survival for the group with cyclin E overexpression. The impact of VEGF on survival was considered to be statistically significant if the 95 % CI did not overlap with 1. Horizontal lines represent 95 % CIs. Each box represents the HR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (HR =1.0).
Evidence of publication bias was sought using the methods of Egger et al. [16] and of Begg et al. [17]. Intercept significance was determined by the t test suggested by Egger (P < 0.05 was considered representative of statistically significant publication bias). All of the calculations were performed by STATA version 11.0 (STATA Corporation, College Station, TX).
Results
Study selection and characteristics
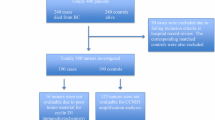
Twenty-three studies [18–40] published between 1997 and 2010 were eligible for this meta-analysis. All reported the prognostic value of cyclin E status for survival in breast cancer patients. The total number of patients included was 7,759, ranging from 56 to 2,032 patients per study (median sample size, 337 patients). The major characteristics of the 23 eligible publications are reported in Table 1. The studies were conducted in 14 countries (USA, Norway, South Africa, Korea, Germany, Netherlands, Sweden, Argentina, Canada, Poland, Belgium, Italy, and France). Among the 23 studies, two studies were performed in Asian populations, and the remaining 21 studies followed non-Asian patients. All patients in the eligible studies were determined by pathological stage.
All of the studies reported the prognostic value of cyclin E status for survival in patients with breast cancer. Of the 23 studies, 19 directly reported HRs (multivariate analysis), while the other four studies provided survival curves. Estimation using survival curves were segregated according to either DFS or OS/BCSS. A HR on DFS and OS/BCSS could be extracted for nine and 20 publications of studies, respectively. Four of the nine studies identified cyclin E overexpression as an indicator of poor DFS, and the other five studies showed no statistically significant impact of cyclin E overexpression on DFS. Thirteen of the 20 studies identified cyclin E overexpression as an indicator of poor OS/BCSS, and the other 7 studies showed no statistically significant impact of cyclin E overexpression on OS/BCSS. Of the 23 studies, 4 studies detected the cyclin E expression by RT-PCR, other 19 studies performed by IHC.
Meta-analysis
The results of the meta-analysis were shown in Table 2. Overall, the combined HR for nine eligible studies evaluating cyclin E overexpression on DFS was 1.11 (95 % CI, 0.96–1.27), suggesting that cyclin E overexpression was not associated with poor prognosis of DFS for breast cancer (Fig. 1). However, significant heterogeneity was observed among the studies (Q = 7.39, I 2 = 76 %, P = 0.000). When grouped according to the methods for detecting cyclin E expression, the combined HRs for IHC and RT-PCR were 1.14 (95 % CI, 0.96–1.31) and 1.03 (95 % CI, 0.69–1.37), respectively, indicating cyclin E was not an indicator of poor prognosis of DFS in all different methods for detecting cyclin E expression
However, statistically significant effect on OS and BCSS for cyclin E overexpression in patients with breast cancer was observed (for OS: HR = 1.30; 95 % CI, 1.12–1.49; for BCSS: HR = 1.48; 95 % CI, 1.03–1.93), suggesting that cyclin E overexpression was an indicator of poor prognosis for OS and BCSS of breast cancer (Fig. 2). No significant heterogeneity was observed among the studies on cyclin E overexpression on BCSS (Q = 3.58, I 2 = 56.6 %, P = 0.059) and significant heterogeneity was observed among the studies on OS (Q = 4.18, I 2 = 57.6 %, P = 0.003). When we aggregated five studies that reported results for stage I–II breast cancer, the combined HR for DFS and OS was statistically significant: HR 1.75 (95 % CI, 1.30–2.19; Q = 16.7; I 2 =76.1 %; P = 0.002 for heterogeneity) (Fig. 3).
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias in the literature. All nine eligible studies investigating cyclin E overexpression on DFS yielded a Begg’s test score of P = 0.835 and an Egger’s test score of P = 0.293, meanwhile according to the funnel plot (Fig. 4), the absence of publication bias was found. Similar results were found for investigating cyclin E overexpression on OS (a Begg’s test score of P = 0.456 and an Egger’s test score of P = 0.828) (Fig. 5). These results suggested that there were no publication biases in these subgroup analyses.
Discussion
Cyclin E is an important cell cycle regulator, which promotes G1/S transition by activation of CDK2 kinase activity [41, 42]. Cyclin E expression in normal dividing cells is upregulated at late G1 phase by transcription activation through E2F family transactivators [43]. The accumulated cyclin E at G1/S boundary simultaneously forms complex with CDK2 and subsequently promotes initiation of DNA replication and centrosome duplication. The abundant cyclin E eventually becomes phosphorylated and destroyed by ubiquitin-mediated proteolysis that allows normal cell cycle progression [44, 45]. However, the level of cyclin E and activity of cyclin E–CDK2 can be aberrantly regulated and this excessive activity of the cyclin E–CDK2 complex, in turn, drives cells to replicate their DNA prematurely, resulting in genome instability [46] and tumorigenesis [8].
The present meta-analysis has combined 23 publications including 7,759 patients with breast cancer to yield statistics, indicating a statistically significant role of cyclin E on OS and BCSS in breast cancer, but not on disease-free survival. In subgroup analysis according to the different test methods on cyclin E for DFS, statistically significant detrimental effect of VEGF-C was not found for IHC or RT-PCR. When analysis was restricted to stage I–II breast cancer, we found that the combined HR (1.75) was larger than the combined HR for all 20 eligible studies of stages I–III (1.33), suggesting that cyclin E expression could be an important prognostic factor for early-stage breast cancer.
Our data were consistent with the results of a previous meta-analysis [47] published in 2006 that showed an association between cyclin E overexpression and poor overall survival of patients with breast cancer. That analysis [47] included only 12 studies, and the data were insufficient to determine the prognostic value of cyclin E in different test methods and disease stage. We have improved upon that previous meta-analysis by including more recent related studies and by generally using a more comprehensive search strategy. Screening, study selection, and quality assessment were performed independently and reproducibly by two reviewers. We also explored heterogeneity and potential publication bias in accordance with published guidelines. In addition, we performed the combined HR for subgroups divided according to disease stage and method of cyclin E detection.
Diversity within the test methods used to identify alteration of the cyclin E status is a potential source of bias. The test methods with IHC varied considerably among the 19 studies in our analysis. The primary antibodies used were not identical, and many different cutoffs for cyclin E positive tissues (2–50 %, different scores) were used. To exclude technique bias, subgroup analyses were performed for the most frequently used methods: IHC and RT-PCR. The results were consistent within all methods, with poorer survival in cases where cyclin E was overexpressed, suggesting that the techniques are unlikely to be a source of bias. However, it is still important to use well-defined, standardized methods to reproducibly evaluate biological markers.
The heterogeneity issue was complicated in the systematic review and meta-analysis. We found the significant heterogeneity among all studies included and subgroup analysis. Another potential source of bias is related to the method of HR and 95 % CI extrapolation. If these statistics were not reported by the authors, we calculated them from the data available in the article. If this was not possible, we extrapolated them from the survival curves, necessarily making assumptions about the censoring process. Data for multivariate survival analysis reported in the article were included in the present systematic review with meta-analysis; if these data were not available, data calculated from survival curves by univariate analysis were included. These results should be confirmed by an adequately designed prospective study. Furthermore, the exact value of cyclin E overexpression status needs to be determined by appropriate multivariate analysis. Unfortunately, few prospectively designed prognostic studies concerning biomarkers have been reported; thus, our collection of many retrospective studies revealed more significance.
Publication bias [48] is a major concern for all forms of meta-analysis; positive results tend to be accepted by journals, while negative results are often rejected or not even submitted. The present analysis does not support publication bias; the obtained summary statistics likely approximate the actual average. However, it should be noted that our meta-analysis could not completely exclude biases. For example, the study was restricted to papers published in English and Chinese, which probably introduced bias.
In conclusion, despite the limitations described above, we concluded that cyclin E overexpression was associated with poor overall OS and BCSS in breast cancer, but not DFS. To strengthen our findings, well-designed prospective studies with better standardized assessment of prognostic markers should help to explore the relation between cyclin E overexpression and survival of breast cancer.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Saurel CA, Patel TA, Perez EA. Changes to adjuvant systemic therapy in breast cancer: a decade in review. Clin Breast Cancer. 2010;10:196–208.
Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16:1723–39.
Hayes DF. Prognostic and predictive factors revisited. Breast. 2008;14:493–9.
Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153.
Tandis SH, Murray T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1998;48:6–29.
Qu Z, Weiss JN, MacLellan WR. Regulation of the mammalian cell cycle: a model of the G1-to-S transition. Am J Physiol Cell Physiol. 2003;284:349–64.
Bortner DM, Rosenberg MP. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol. 1997;17:453–9.
Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–24.
Ekholm-Reed S, Mendez J, Tedesco D, Zetterberg A, Stillman B, Reed SI. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol. 2004;165:789–800.
Berglund P, Landberg G. Cyclin E overexpression reduces infiltrative growth in breast cancer. Cell Cycle. 2006;5(6):606–9.
Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323(7306):224–8.
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34.
Yusuf S, Peto R, Lewis J, et al. Blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–71.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Egger M, Smith GD, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, et al. Expression of cell cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3(2):222–5.
Donnellan R, Kleinschmidt I, Chetty R. Cyclin E immunoexpression in breast ductal carcinoma: pathologic correlations and prognostic implications. Hum Pathol. 2001;32(1):89–94.
Kim HK, Park IA, Heo DS, Noh DY, Choe KJ, et al. Cyclin E overexpression as an independent risk factor of visceral relapse in breast cancer. Eur J Surg Oncol. 2001;27(5):464–71.
Bukholm IR, Bukholm G, Nesland JM. Overexpression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int J Cancer. 2001;93(2):283–7.
Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347(20):1566–75.
Span PN, Tjan-Heijnen V, Manders P, Beex LV, Sweep CG. Cyclin-E is a strong predictor of endocrine therapy failure in human breast cancer. Oncogene. 2003;22(31):4898–904.
Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim YD, et al. Prognostic implication of cyclin E expression and its relationship with cyclin D1 and p27Kip1 expression on tissue microarrays of node negative breast cancer. J Surg Oncol. 2003;83(4):241–7.
Rudolph P, Kuhling H, Alm P, Ferno M, Baldetorp B, et al. Differential prognostic impact of the cyclins E and B in premenopausal and postmenopausal women with lymph node-negative breast cancer. Int J Cancer. 2003;105(5):674–80.
Peters MG, Vidal Mdel C, Gimenez L, Mauro L, Armanasco E, et al. Prognostic value of cell cycle regulator molecules in surgically resected stage I and II breast cancer. Oncol Rep. 2004;12(5):1143–50.
Foulkes WD, Brunet JS, Stefansson IM, Straume O, Chappuis PO, et al. The prognostic implication of the basal-like (cyclin E high/p27low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 2004;64(3):830–5.
Lindahl T, Landberg G, Ahlgren J, Nordgren H, Norberg T, et al. Overexpression of cyclin E protein is associated with specific mutation types in the p53 gene and poor survival in human breast cancer. Carcinogenesis. 2004;25(3):375–80.
Chappuis PO, Donato E, Goffin JR, Wong N, Bégin LR, Kapusta LR, et al. Cyclin E expression in breast cancer: predicting germline BRCA1 mutations, prognosis and response to treatment. Ann Oncol. 2005;16(5):735–42.
Arnes JB, Brunet JS, Stefansson I, Bégin LR, Wong N, Chappuis PO, et al. Placental cadherin and the basal epithelial phenotype of BRCA1-related breast cancer. Clin Cancer Res. 2005;11(11):4003–11.
Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, Duffy MJ, et al. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res. 2006;12(21):6421–31.
Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12(8):2468–75.
Desmedt C, Ouriaghli FE, Durbecq V, Soree A, Colozza MA, Azambuja E, et al. Impact of cyclins E, neutrophil elastase and proteinase 3 expression levels on clinical outcome in primary breast cancer patients. Int J Cancer. 2006;119(11):2539–45.
Sieuwerts AM, Look MP, Meijer-van Gelder ME, Timmermans M, Trapman AM, Garcia RR, et al. Which cyclin E prevails as prognostic marker for breast cancer? Results from a retrospective study involving 635 lymph node-negative breast cancer patients. Clin Cancer Res. 2006;12(11 Pt 1):3319–28.
Porter PL, Barlow WE, Yeh IT, Lin MG, Yuan XP, Donato E, et al. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J Natl Cancer Inst. 2006;98(23):1723–31.
Potemski P, Pluciennik E, Bednarek AK, Kusinska R, Jesionek-Kupnicka D, Pasz-Walczak G, et al. Cyclin E expression in operable breast cancer quantified using real-time RT-PCR: a comparative study with immunostaining. Jpn J Clin Oncol. 2006;36(3):142–9.
Somlo G, Chu P, Frankel P, Ye W, Groshen S, Doroshow JH, et al. Molecular profiling including epidermal growth factor receptor and p21 expression in high-risk breast cancer patients as indicators of outcome. Ann Oncol. 2008;19(11):1853–9.
Potemski P, Kusińska R, Pasz-Walczak G, Piekarski JH, Watała C, Płuciennik E, et al. Prognostic relevance of cyclin E expression in operable breast cancer. Med Sci Monit. 2009;15(2):MT34–40.
Sgambato A, Camerini A, Collecchi P, Graziani C, Bevilacqua G, Capodanno A, et al. Cyclin E correlates with manganese superoxide dismutase expression and predicts survival in early breast cancer patients receiving adjuvant epirubicin-based chemotherapy. Cancer Sci. 2009;100(6):1026–33.
Lemée F, Bergoglio V, Fernandez-Vidal A, Machado-Silva A, Pillaire MJ, Bieth A, et al. DNA polymerase theta up-regulation is associated with poor survival in breast cancer, perturbs DNA replication, and promotes genetic instability. Proc Natl Acad Sci U S A. 2010;107(30):13390–5.
Koff A, Giordano A, Desai D, Yamashita K, Harper JW, et al. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–94.
Sauer K, Lehner CF. The role of cyclin E in the regulation of entry into S phase. Prog Cell Cycle Res. 1995;1:125–39.
Malumbres M, Barbacid M. To cycle or not cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–31.
Clurman BE, Sheaff RJ, Thress K, Groudine M, Roberts JM. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–90.
Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature. 2001;413:311–6.
Wingate H, Puskas A, Duong M, Bui T, Richardson D, et al. Low molecular weight cyclin E is specific in breast cancer and is associated with mechanisms of tumor progression. Cell Cycle. 2009;8:1062–8.
Wang L, Shao ZM. Cyclin E expression and prognosis in breast cancer patients: a meta-analysis of published studies. Cancer Invest. 2006;24:581–7.
Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J R Stat Soc A. 1988;151:419–63.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81172501).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Sheng Gao and Jing-Jing Ma contributed equally to this work and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Gao, S., Ma, JJ. & Lu, C. Prognostic value of cyclin E expression in breast cancer: a meta-analysis. Tumor Biol. 34, 3423–3430 (2013). https://doi.org/10.1007/s13277-013-0915-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0915-8