Abstract
The aim of this study was to detect FOXC1 expression in human non-small cell lung cancer (NSCLC) and to analyze its association with prognosis of NSCLC patients. Expressional levels of FOXC1 mRNA and protein in 30 cases of NSCLC and corresponding non-tumor tissue samples were examined by quantitative real-time PCR and Western blotting. Immunohistochemistry was performed to detect the expression of FOXC1 in 125 NSCLC tissues. We found that the expression levels of FOXC1 mRNA and protein in NSCLC tissues were significantly higher than those in corresponding non-tumor tissues. High-level FOXC1 expression was correlated with poor tumor differentiation, tumor–node–metastasis stage, and lymph node metastasis. Patients with high expression levels of FOXC1 showed lower overall survival rate than those with low expression levels. Multivariate analysis showed that high FOXC1 protein expression was an independent prognostic factor for NSCLC patients. Our study suggests that over-expression of FOXC1 may play an important role in the progression of NSCLC, and FOXC1 expression may offer a valuable marker for predicting the outcome of patients with NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths, accounting for 15 % of all cancer diagnoses and with 1.1 million deaths annually worldwide. Non-small cell lung cancer (NSCLC) constitutes approximately 80–85 % of total lung malignancies [1]. Despite significant advances in multidisciplinary treatment approaches, such as surgery, chemotherapy, and radiotherapy, the 5-year survival in patients with NSCLC amenable to definitive treatment remains only about 15 % [1, 2]. Consequently, there is an urgent need to explore novel prognostic markers and therapeutic targets, both of which may be realized through a more sophisticated understanding of the molecular mechanisms involved in lung carcinogenesis.
Members of the forkhead box (FOX) family of transcription factors regulate a wide array of biological processes including development, differentiation, and invasion [3]. FOXC1 (Mf1, FKHL7, FREAC3) was originally identified as an important transcription factor that controls development of structures derived from the neural crest, and FOXC1 mutations have long been recognized as a primary cause of Axenfeld–Rieger syndrome [4–6]. In addition to its roles in normal function and development of the eye and meninges, FOXC1 has recently emerged as a possible master regulator of breast cancer. Although one report has demonstrated decreased invasion and metastasis of breast cancer cells in response to FOXC1 expression [7], other groups have reported that FOXC1 increases invasion and metastasis in endometrial and breast cancer models [8–11]. Stable over-expression of FOXC1 elicits changes in gene expression indicative of epithelial to mesenchymal transition and increases cellular invasion, migration, and proliferation [8–11]. In addition, FOXC1 expression predicts poor breast cancer patient outcome [9, 12, 13]. To the best of our knowledge, little has been uncovered regarding the involvement of FOXC1 genes in NSCLC. In this study, we investigated FOXC1 expression in NSCLC and its correlation with clinicopathological features, including the survival of patients with NSCLC.
Materials and methods
Patients and tissue samples
A total of 125 primary lung cancer specimens were collected from patients with NSCLC undergoing surgery at the Department of Respiratory Medicine, The Fourth Affiliated Hospital of China Medical University, Shenyang. These patients included 70 men and 55 women, ranging in age from 35 to 82 years, with a median age of 60 years. None of the patients received preoperative chemotherapy or radiotherapy, and all the patients were treated with routine chemotherapy after the operation. The mean follow-up period was 57.5 months (range, 2–110.5 months). Written informed consent was obtained from all patients before surgery, and the study protocol was approved by the Institutional Review Board for the Use of Human Subjects at China Medical University. The clinicopathological findings were determined according to the classification of malignant tumors by the World Health Organization and International Union against Cancer Tumor–Node–Metastasis (TNM) staging system [14]. All tumor tissues were diagnosed histopathologically by at least two trained pathologists. Surgically removed tumors and matched non-cancerous tissue samples used for mRNA detection were immediately frozen in liquid nitrogen and kept at −80 °C until extraction of RNA.
RNA isolation and quantitative real-time RT-PCR
Total RNA from frozen tissues was isolated using Trizol reagent (Life Technologies, Rockville, MD, USA) according to the manufacturer’s instructions. Reverse transcription was performed on 1 μg of total RNA from each sample. Quantitative real-time RT-PCR was performed using SYBR Green (Takara, Dalian, China) on a Real-Time Quantitative Thermal Block (Biometra, Germany). The PCR primer sequences were designed according to the human FOXC1 and β-actin gene sequences reported in GenBank and were chemically synthesized: FOXC1, forward 5′-TTACCGGTAAGCCTAGATTAGGCC-3′; reverse 5′-TTGAATTCGGTAACATTATTGGTT-3'; β-actin, forward 5′-GTGGGGCGCCCCAGGCACCA-3′; reverse 5′-CTCCTTAATGTCACGCACGATTTC-3′. The reactions were carried out at 95 °C for 30 s to activate the enzyme, then 40 cycles of 95 °C for 20 s, 55 °C for 15 s, and 72 °C for 20 s, and a final extension at 72 °C for 10 min. The specificity of the PCR was confirmed by examining the dissociation reaction plot subsequent to real-time RT-PCR. β-actin served as the constitutive control. PCR reactions of each sample were conducted in triplicate. Data were analyzed through the comparative threshold cycle method [15]. The relative FOXC1 mRNA expression was calculated by the 2−ΔCt method (ΔCt = Ct of FOXC1 − Ct of β-actin). The fold change of FOXC1 expression in each tissue was defined as the ratio of relative FOXC1 mRNA expression in tumor tissue to that in corresponding normal tissues.
Western blot analysis
Total proteins were extracted from frozen lung cancer tissues using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China), and protein concentrations were determined using a bovine serum albumin standard line. Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then electrotransferred to polyvinylidene fluoride membranes. Membranes were blocked with 5 % skimmed milk at room temperature for 2 h and then incubated overnight (4 °C) with anti-FOXC1 (1:500; SC-1971, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-GAPDH antibody (1:1,000, Sigma-Aldrich, St. Louis, MO, USA), followed by horseradish peroxidase-conjugated secondary antibodies. Protein bands were visualized with ECL plus chemiluminescence kit (Millipore, Bedford, MA, USA).
Immunohistochemistry analysis
FOXC1 protein expression in 125 tumor tissue samples was confirmed by immunohistochemistry analysis, which was performed on formalin-fixed, paraffin-embedded, 3-μm-thick tissue sections using the avidin–biotin–peroxidase complex method. The sections were deparaffinized and dehydrated using a graded series of ethanol solutions. Endogenous peroxidase activity was halted through the administration of 0.3 % hydrogen peroxidase and methanol for 20 min. After having been rinsed in phosphate-buffered saline, the tissue sections were processed in a 0.01 M citrate buffer (pH 6.0) inside a heat-resistant plastic container. Sections were then irradiated in a domestic microwave oven for 20 min. After microwave irradiation, the slides were allowed to cool at room temperature. The following antibody was applied as the primary antibody: rabbit antibody specific to FOXC1 (1:15, Atlas Antibodies, Sigma-Aldrich, USA). The sections were incubated with the primary antibody overnight at 4 °C followed by the secondary antibody. The results were visualized with diaminobenzidine. In each immunohistochemistry run, the negative controls were stained without primary antibody.
Two independent observers particularly experienced in immunohistochemistry evaluated the slides. Both readers were blinded to clinicopathologic data and patient outcomes. The expression of FOXC1 was quantified using a visual grading system based on the extent of staining (percentage of positive tumor cells graded on a scale from 0 to 3: 0 = negative, 1 = 1–30 %, 2 = 31–60 %, 3 > 60 %) and the intensity of staining (graded on a scale of 0–3: 0 = none, 1 = weak staining, 2 = moderate staining, 3 = strong staining). The combination of extent (E) and intensity (I) of staining was obtained by the product of E × I called EI, varying from 0 to 9 for each spot. The mean EI score was calculated for each NSCLC specimen. EI scores of 0–3 were considered low expression and EI scores >3 were considered high expression. In 95 % of the samples, the evaluations of the two observers were identical, the remaining slides were reevaluated, and consensus decisions were made.
Statistical analysis
All statistical analyses were carried out using the SPSS 13.0 statistical software package. The χ2 and Fisher exact tests were used to analyze the relationship between FOXC1 expression and the clinicopathologic characteristics. Survival curves were plotted using the Kaplan–Meier method and compared using the log-rank test between the high and low expression of FOXC1 cases. Survival data were evaluated using the Cox proportional hazards model. Independent prognostic factors were determined by multivariate analysis. P < 0.05 was considered statistically significant.
Results
Analysis of FOXC1 mRNA expression
Firstly, quantitative real-time RT-PCR was conducted to detect the FOXC1 mRNA expression in 30 cases of NSCLC and corresponding adjacent lung tissues. We found that 18 of the 30 patients (60 %) showed a higher expression level of FOXC1 mRNA in NSCLC specimens than in non-cancerous tissue specimens (at least 2.1-fold change). In addition, the relative expression of FOXC1 mRNA in the NSCLC specimens was significantly higher than that in the corresponding normal tissues (0.662 ± 0.118 vs 0.325 ± 0.074; P < 0.001, Fig. 1). Thus, FOXC1 may play important roles in the progression of lung cancer.
Analysis of FOXC1 protein expression
FOXC1 protein levels in resected NSCLC samples were measured by Western blotting. Increased FOXC1 expression was detected in 19 of the 30 tumor tissue samples (63.3 %), compared with matched adjacent non-tumor tissue samples (P < 0.001; Fig. 2). These findings were consistent with the quantitative real-time PCR (qRT-PCR) data.
Western blot analysis of FOXC1 expression in NSCLC patients. Relative FOXC1 expression in NSCLC (T) tissues compared to adjacent non-tumor (N) tissues (n = 30), assessed by Western blotting (P < 0.001). Results are shown as expression relative to GAPDH and are means (±SD) of three separate experiments
The degree of FOXC1 immunohistochemical staining correlates with clinicopathological characteristics
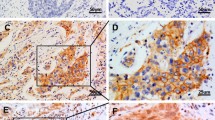
To further determine whether FOXC1 protein upregulation is linked to the clinical parameters of NSCLC patients, we examined the expression of FOXC1 protein in 125 NSCLC tissue samples by immunohistochemistry. According to the FOXC1 immunoreactive intensity, 56 (44.8 %) patients were classified as low-FOXC1 group and 69 (55.2 %) patients were classified as high-FOXC1 group (Fig. 3).
Immunohistochemical analysis of FOXC1 in NSCLC patients. a High expression level of FOXC1 in squamous cell carcinomas. b Low expression level of FOXC1 squamous cell carcinomas. c High expression level of FOXC1 in adenocarcinomas. d Low expression level of FOXC1 in adenocarcinomas.; a, b, c, d Original magnification × 200
Table 1 summarizes the relationships between FOXC1 expression and clinicopathological parameters of NSCLC patients. We found that the expression of FOXC1 protein was significantly correlated with poor tumor differentiation, TNM stage, and lymph node metastasis of NSCLC patients (P < 0.001, 0.007, and 0.004, respectively). However, statistical analysis revealed no significant correlations between FOXC1 expression and age, gender, smoking history, histological type, and tumor classification.
Correlation between FOXC1 expression levels and patient survival
The Kaplan–Meier method was performed to further analyze the association of FOXC1 expression with prognosis of NSCLC patients. We found that the survival of patients with high FOXC1 protein expression was significantly shorter than that of patients with low FOXC1 protein expression (P < 0.001; Fig. 4). Thus, the expression of FOXC1 protein could affect the prognosis of NSCLC patients.
To evaluate the possibility of FOXC1 used as an independent risk factor for poor prognosis, conventional clinicopathological factors and FOXC1 protein levels were assessed by Cox’s univariate and multivariate hazard regression model (Table 2). Univariate analysis indicated that tumor differentiation, TNM stage, lymph node metastasis, and FOXC1 protein expression were significantly associated with overall survival of NSCLC patients. By multivariate analysis, we showed that FOXC1 protein expression and lymph node metastasis were independent prognostic factors for overall survival of NSCLC patients.
Discussion
Genetic alterations are a hallmark of human cancer. In recent years, the field of cancer genomics has made significant advances in the area of cancer-associated genetic lesion identification. Furthermore, the importance of epigenetic changes that occur during NSCLC development was also recognized [16]. Epigenetic changes can take the form of DNA methylation or histone modification [17]. Histone modifications in the form of selective acetylation, phosphorylation, and methylation serve as switches that alter chromatin structure, allowing posttranscriptional activation or repression of downstream proteins [18]. Understanding these epigenetic changes will lead to the identification of novel cancer-related genes, which may represent attractive targets for cancer treatment and provide new insights into the biology of hepatic cancers. Thus, an integrative approach to hepatic cancer research that combines epidemiological, genetic, and epigenetic information has emerged as an important paradigm for cancer therapy [19].
Members of the FOX family of transcription factors regulate a wide array of biological processes including development, differentiation, and invasion [3]. FOXC1 (Mf1, FKHL7, FREAC3) was originally identified as an important transcription factor that controls development of structures derived from the neural crest, and FOXC1 mutations have long been recognized as a primary cause of Axenfeld–Rieger syndrome [4–6]. A number of studies have documented that FOXC1 expression is upregulated in several types of human cancers, such as hepatocellular carcinoma, breast cancer, and prostate cancer, through duplications or rearrangements of the FOXC1 locus, suggesting that over-expression of FOXC1 is involved in the tumorigenesis of many types of cancer [9, 20, 21]. In this study, we investigated FOXC1 expression in NSCLC and its correlation with clinicopathological features, including the survival of patients with NSCLC.
To address these issues, we first investigated FOXC1 mRNA and protein expression in NSCLC specimens by qRT-PCR and Western blotting, respectively. FOXC1 transcript and protein levels were determined in 30 pairs of resected specimens (tumor tissue samples and matched adjacent non-tumor tissue samples) from NSCLC patients. We observed that FOXC1 mRNA and protein levels were significantly increased in tumor tissue samples compared with adjacent non-tumor tissues. In addition, the FOXC1 protein and mRNA showed similar expression patterns in the matched samples. These observations support the hypothesis that FOXC1 may function as an oncogene in NSCLC, and also suggest that FOXC1 may play an important role in the tumorigenesis of NSCLC. We further assessed the FOXC1 protein in 125 NSCLC tumor specimens by immunohistochemistry and analyzed its correlation to clinicopathological features. We found that FOXC1 expression was significantly higher in neoplastic than in nonneoplastic tissues, which was consistent with previous findings in other cancer types. Additionally, FOXC1 positively correlates with poor tumor differentiation, TNM stage, and lymph node metastasis in human NSCLC patients. It agrees with the fact that FOXC1 plays a role in enhancing cell proliferation of human lung cancer cells and may thereby contribute to the early progression of carcinoma tumors.
More importantly, our results also showed that FOXC1 expression is associated with patient survival. Collectively, these findings suggest that FOXC1 is upregulated in NSCLC tissues and positively participates in lung cancer progression. In the present study, univariate and multivariate analyses revealed that FOXC1 expression was recognized as an independent prognostic factor for patient outcome. Our results not only suggest a potentially promising use of FOXC1 as a valuable prognostic indicator, but also imply a possible link between the biological function of FOXC1 and the pathogenesis of NSCLC. This could lead to the development of a novel anti-lung cancer strategy. Nonetheless, further studies are needed to elucidate the molecular mechanisms by which FOXC1 participates in the development and progression of lung cancer and to address whether FOXC1 could be used as a target for novel therapeutic approaches.
In summary, the data from the current study have shown that FOXC1 is over-expressed in NSCLC and associated with poor tumor differentiation, TNM stage, and lymph node metastasis, as well as poor prognosis of NSCLC patients. Furthermore, FOXC1 levels appear to be an independent predictor of survival for patients with NSCLC.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi:10.3322/CA.2007.0010.
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi:10.1200/JCO.2005.05.2308.
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59. doi:10.1038/nrc2223.
Kidson SH, Kume T, Deng K, Winfrey V, Hogan BL. The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev Biol. 1999;211:306–22.
Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell. 1998;93:985–96.
Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, Ritch R, Koop B, Kuo WL, Collins C, Marshall J, Gould DB, Pearce W, Carlsson P, Enerbäck S, Morissette J, Bhattacharya S, Hogan B, Raymond V, Walter MA. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld–Rieger anomaly. Am J Hum Genet. 1998;63:1316–28.
Du J, Li L, Ou Z, Kong C, Zhang Y, Dong Z, Zhu S, Jiang H, Shao Z, Huang B, Lu J. FOXC1, a target of polycomb, inhibits metastasis of breast cancer cells. Breast Cancer Res Treat. 2012;131:65–73.
Chung TK, Lau TS, Cheung TH, Yim SF, Lo KW, Siu NS, Chan LK, Yu MY, Kwong J, Doran G, Barroilhet LM, Ng AS, Wong RR, Wang VW, Mok SC, Smith DI, Berkowitz RS, Wong YF. Dysregulation of microRNA-204 mediates migration and invasion of endometrial cancer by regulating FOXC1. Int J Cancer. 2012;130:1036–45. doi:10.1002/ijc.26060.
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, Walter MA, Martens JW, Richardson AL, Giuliano AE, Cui X. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–6. doi:10.1158/0008-5472.
Bloushtain-Qimron N, Yao J, Snyder EL, Shipitsin M, Campbell LL, Mani SA, Hu M, Chen H, Ustyansky V, Antosiewicz JE, Argani P, Halushka MK, Thomson JA, Pharoah P, Porgador A, Sukumar S, Parsons R, Richardson AL, Stampfer MR, Gelman RS, Nikolskaya T, Nikolsky Y, Polyak K. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci U S A. 2008;105:14076–81. doi:10.1073/pnas.0805206105.
Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP, Lee AV, Lin X, Bagaria SP, Giuliano AE, Cui X. FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-κB signaling. Oncogene. 2012;31:4798–802. doi:10.1038/onc.2011.635.
Ray PS, Bagaria SP, Wang J, Shamonki JM, Ye X, Sim MS, Steen S, Qu Y, Cui X, Giuliano AE. Basal-like breast cancer defined by FOXC1 expression offers superior prognostic value: a retrospective immunohistochemical study. Ann Surg Oncol. 2011;18:3839–47.
Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, Hollier BG, Ram PT, Lander ES, Rosen JM, Weinberg RA, Mani SA. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449–54. doi:10.1073/pnas.1004900107.
Sobin LH, Fleming ID. TNM classification of malignant tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–4.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Piperi C, Vlastos F, Farmaki E, Martinet N, Papavassiliou AG. Epigenetic effects of lung cancer predisposing factors impact on clinical diagnosis and prognosis. J Cell Mol Med. 2008;12:1495–501. doi:10.1111/j.1582-4934.2008.00309.x.
Seligson DB, Horvath S, McBrian MA, Mah V, Yu H, Tze S, Wang Q, Chia D, Goodglick L, Kurdistani SK. Global levels of histone modifications predict prognosis in different cancers. Am J Pathol. 2009;174:1619–28. doi:10.2353/ajpath.2009.080874.
Yang S. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829–33. doi:10.1038/nrg2218.
Risch A, Plass C. Lung cancer epigenetics and genetics. Int J Cancer. 2008;123:1–7. doi:10.1002/ijc.23605.
Xia L, Huang W, Tian D, Zhu H, Qi X, Chen Z, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2012. doi:10.1002/hep.26029.
Peraldo-Neia C, Migliardi G, Mello-Grand M, Montemurro F, Segir R, Pignochino Y, Cavalloni G, Torchio B, Mosso L, Chiorino G, Aglietta M. Epidermal growth factor receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer. BMC Cancer. 2011;11:31. doi:10.1186/1471-2407-11-31.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, LX., Zhou, RS., Xu, HF. et al. High expression of FOXC1 is associated with poor clinical outcome in non-small cell lung cancer patients. Tumor Biol. 34, 941–946 (2013). https://doi.org/10.1007/s13277-012-0629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0629-3