Abstract
It is known that leukemia patients with extramedullary infiltration (EMI) have a worse prognosis than patients without it. Recent data indicate that the amyloid precursor protein (APP) is involved in cell adhesion, motility, and proliferation. The expression of APP and its prognostic significance in acute myeloid leukemia (AML) have not been studied. Our study shows that AML/ETO+ leukemia patients that overexpress APP easily get EMI and that their long-term survival rate is lower than patients without overexpression of APP. In an in vitro study, we knocked down APP in Kasumi-1 cells using small interfering RNA (siRNA). Transwell data show that siRNA/APP substantially impairs cell migration, but it does not inhibit cell proliferation. Furthermore, by quantitative real-time polymerase chain reaction and Western blot, we found that siRNA/APP decreases MMP-2 expression in vitro. Our study provides a novel clue that APP is involved in the extramedullary infiltration of leukemia by MMP-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hematological malignancies account for approximately 7 % of all new cancer cases annually. Acute myeloid leukemia (AML) is the most common acute leukemia in adults. In 2010, there were an estimated 12,330 new cases and 8,950 deaths from AML [1]. Extramedullary leukemia (EML) is common in AML patients, and the frequency of extramedullary infiltration (EMI) in AML patients is reported to be up to 40 %. Extramedullary tissue infiltration of leukemia cells is a major obstacle in the chemotherapy treatment of leukemia, leading to a poor response and relapse.
Translocation (8;21)(q22;q22) is the most common chromosomal aberration with a single structural abnormality found in AML. The translocation fuses the AML1 gene on chromosome 21 with the ETO gene on chromosome 8. The t(8;21) abnormality can be detected by fluorescence in situ hybridization (FISH). Patients with the t(8;21) translocation have a unique cell morphology characterized by maturation (French–American–British classification, FAB M2) and clinical features. It has been reported that the t(8;21) abnormality is identified in 15 % of all adult AML patients and that these patients carry a particularly good prognosis with a complete remission rate of 90 % and a 5-year disease-free survival rate of more than 50 %[2–4]. However, our data are not entirely consistent with these outcomes (as seen in Fig. 1).
Amyloid precursor protein (APP) is a type I integral membrane protein and is located in chromosome 21q21 [5, 6]. It is well known that APP is implicated in synapse formation and synaptic plasticity, and cleavage of APP is directly linked to the pathogenesis of neurodegenerative disorders such as Alzheimer's disease [7, 8]. In recent surveys, the role of APP has been suggested in cell adhesion, calcium metabolism, and signal transduction [9, 10]. Also, APP has been linked to the malignant progression or growth in several different tumor types [11–16]. Baldus et al. [17] reported that the APP gene was overexpressed in AML patients with complex karyotypes and abnormal chromosome 21. However, they did not report about the function of APP in leukemia.
The mechanisms responsible for the metastasis of leukemic cells are far from being fully elucidated. Hence, the identification of factors associated with tumor metastasis and their underlying mechanisms are especially important. The matrix metalloproteinase (MMP) family like MMP-2 and MMP-9 is known to be associated with tumor metastasis because of its capacity to degrade collagen IV, the major extracellular matrix (ECM) component [18]. Earlier reports show an association of MMP-2 expression with the invasive behavior of leukemic cells in AML and acute lymphoblastic leukemia [19, 20].
The aim of this study was to describe the function of APP in relation to leukemia cell metastasis and its mechanism. Here, we report for the first time that the overexpression of APP in AML1/ETO+ patients makes these patients much more susceptible to EML, and we provide evidence that APP plays an important role in AML1/ETO+ AML invasiveness by increasing MMP-2 expression. These findings may provide new insights into therapeutic strategies to obtain a favorable outcome in the treatment of AML patients.
Material and methods
Patient samples
Thirty-one bone marrow samples were obtained from AML/ETO+ leukemia patients of the Nanfang Hospital and used for the analysis of mRNA expression. All samples were obtained as approved by the ethics review board (for characteristics of the patients, see Table 1).
Cell culture
The leukemic cell line Kasumi-1, which carried the t(8;21) giving rise to the fusion gene AML1/ETO+, was used. The cells were grown in RPMI-1640 media (Gibco, USA), routinely supplemented with 20 % fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The medium was changed every 48 h.
Fluorescence in situ hybridization and karyotype analysis
The LSI AML1/ETO dual color, dual fusion translocation probe was used to detect AML1/ETO fusion genes according to the manufacturer's instructions. LSI ETO is a 480-kb probe labeled directly with the Spectrum Orange fluorophore, and it hybridizes to 8q22, which contains the ETO gene. The approximately 1.3-Mb LSI AML1 probe is labeled directly with Spectrum Green and hybridizes to 21q22, which contains the AML1 gene. In normal cells, two orange signals (normal ETO) and two green signals (normal AML1) are observed. In cells with t(8;21)(q22;q22), one orange (normal ETO), one green (normal AML1), and two yellow fusion signals are observed because the DNA probes encompass both sides of both chromosomal breakpoints. The two yellow fusion signals are derived from the AML1/ETO and ETO/AML1 fusion genes on der(8)t(8;21) and der(21)t(8;21), respectively.
Chromosome analysis was performed using short-term cultures following standard protocols [21]. The chromosomes were interpreted according to the International System for Human Cytogenetic Nomenclature [22].
Quantitative real-time PCR
Total RNA was extracted with TRIZOL reagents according to the manufacturer's protocol (Invitrogen). The cDNA was synthesized from 1,000 ng of total RNA using PrimerScript RT Reagent Kit (TaKaRa). The real-time PCR was carried out using a SYBR Green PCR mix in Applied Biosystems StepOne and StepOne Plus Real-Time PCR Systems. Expressions of APP and MMP-2 mRNA were detected using specific primers (APP, MMP-2 primer sense and antisense; Table 2).
Lentivirus conduction and infection
The complementary DNA sequence of APP was designed from the full-length APP sequence by Shanghai GeneChem Company (Shanghai, China; siRNA target sequence of APP: CATCTTTGACCGAAACGAA). After testing knockdown efficiencies, stem–loop oligonucleotides were synthesized and cloned into the lentivirus-based vector GV118. A nontargeting scrambled RNA GV112 vector was generated as a negative control (NC: TTCTCCGAACGTGTCACGT). Recombinant lentiviruses were produced by co-transfecting 293 T cells with a lentivirus expression plasmid and a packaging plasmid using Lipofectamine 2000. Infectious lentiviruses were harvested 48 h post-transfection, centrifuged to remove cell debris, and then filtered through 0.45-μm cellulose acetate filters [23–25]. The virus titer was determined by fluorescence-activated cell sorting analysis of green fluorescent protein (GFP)-positive 293 T cells and was approximately 8 × 108 transducing units/ml medium. Approximately 1 × 105 Kasumi-1 cells were seeded in six-well plates before the siRNA transfection. The lentivirus was added and co-incubated with the cells for an additional 18 h in 10 % fetal bovine serum 1640 (Gibco) at 37 °C. The infection efficiency of GFP in Kasumi-1 cells was about 60 % at a multiplicity of infection of 100. The Kasumi-1 cells were transfected with the APP siRNA and with the scramble siRNA that was used as a negative control.
Western blot
The cells were washed three times with cold PBS and lysed in RIPA buffer with 1 mM PMSF and protease inhibitor cocktail for 15 min on ice. The resolved proteins underwent centrifugation at 15,000 × g for 30 min at 4 °C. The protein quantification of supernatants was determined using BCA protein assay, and all samples were separated using 10 % sodium by electrophoresis in ProGel Tris–Glycin with a 10 % gradient and then transferred onto PVDF membranes (Millipore). The analysis used the following primary antibodies as described by the manufacturers of the antibodies: monoclonal anti-APP (Abcam), MMP-2(Santa Cruz Biotechnology Inc.), and β-actin (Abcam). After the final rinsing with TBST, the membrane was incubated with secondary HRP anti-rabbit IgG or anti-mouse IgG for 2 h. After washing, the immunoblots were visualized using chemiluminescence HRP substrate and analyzed with an image analyzer.
CCK-8 assay
Cells were divided into the three following groups: wild Kasumi-1 cells (wild), siRNA/APP-treated Kasumi-1 cells (siAPP), and scramble siRNA-treated Kasumi-1 cells (NC). Cells were seeded at 5,000 cells per well in 96-well plates. The cells were cultured for 24, 48, 72, and 96 h. At the end of the incubation time, 10 μl of 5 mg/ml CCK-8 (Sigma) was added to each well. After that, cells were incubated at 37 °C with gentle shaking for another 30 min. After 4 h, the optical density was determined with a microplate reader at 450 nm. Each experiment was repeated three times.
Cell migration assay
Co-culture Transwell© plates with Millipore membranes (8-μm pores) were obtained from Costar, and the membranes were pretreated under moist conditions. Cells were divided into three groups as stated above, suspended in 100 μl of serum-free 1640 medium, and seeded at a final density of 3 × 105 cells/well in triplicate in the upper chamber. The lower compartment of the invasion chamber was filled with 500 μl of 1640 supplemented with 10 % fetal bovine serum. The migration was allowed to proceed at 37 °C in a 5 % CO2 atmosphere for 18 h. Cells that had migrated into the lower compartment were counted, and the ratio of migrated cells was calculated by dividing the number of cells that migrated to the lower compartment by the total number of cells loaded in the upper compartment. Photographs were captured using a camera (Canon). Each invasion experiment was performed in triplicate.
Statistical analysis
SPSS 13.0 software was used for the statistical analysis. Data were obtained from three independent experiments and expressed as means ± standard deviation. Statistical analysis was performed by one-way ANOVA followed by the Fisher's post-hoc test procedure for significance (p < 0.05).
Results
APP expressions in leukemia patients with AML1/ETO+
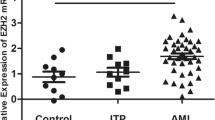
When the clinical data of the AML1/ETO+ leukemia patients with low expression of APP were compared with the clinical data of AML/ETO+ leukemia patients with high expression of APP, it was shown that the patients with high expression were much more prone to EML (p < 0.05; Table 1) and had a lower relapse-free survival (RFS) rate and overall survival (OS) rate (p < 0.05; Fig. 1a, b). These data indicate that APP levels were directly related to the RFS and OS rates.
Characteristics of Kasumi-1 cells
FISH images showed that Kasumi-1 cells were AML/ETO+ (Fig. 2a) positive, and karyotype analysis showed that Kasumi-1 cells contain t(8;21) (Fig. 2b). This cell line was matched with AML1/ETO+ leukemia patients.
Targeted APP knockdown by lentivirus siAPP
Kasumi-1 cells were transfected with siRNA/APP (siAPP) and scramble siRNA (NC). Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot for APP confirmed suppression in the siAPP group, but not in the NC group. qRT-PCR showed that the mRNA of APP was significantly reduced in the siAPP group (p < 0.01), and WB showed that the protein was markedly inhibited in the siAPP group (Fig. 3). This experiment confirmed that the siRNA/APP effectively reduces APP expression.
Lentivirus vectors for the APP siRNA were constructed and shown to be specific and potent for silencing APP expression in the Kasumi-1 leukemia cell line. a APP mRNA levels of Kasumi-1 cells infected with the siRNA/APP by real-time PCR. The NC and wild group were used as controls (asterisk denotes p < 0.05). b Western blot analysis of APP protein expression in wild Kasumi-1 cells infected with siRNA/APP and NC
SiRNA/APP inhibits cell migration, but not proliferation
As seen in Fig. 4a–f, siRNA/APP does not affect OD values at different observation points when compared to controls (p > 0.05), indicating that siRNA/APP does not inhibit cell proliferation. However, siRNA/APP does affect cell migration. This is shown in Fig. 5a, which shows pictures taken from the lower compartments of the invasion chambers; the number of Kasumi-1 cells with siRNA/APP is much less than the number of cells in the control groups. When the ratio of migrated cells is compared, the cells with siRNA/APP have a significantly lower ratio than those in the control groups (p < 0.05; Fig. 5b).
Cell proliferation at different times. a OD value of cells at the start time; b OD value at the incubation time of 24 h; c OD value at the incubation time of 48 h; d OD value at the incubation time of 72 h; e OD value at the incubation time of 96 h; f cell proliferation curves among different groups. There is no statistic difference among the groups in 24, 48, 72, and 96 h (p > 0.05)
The effects of siRNA/APP on cell migration as determined by Transwell assay. a Cells in the lower chamber of Transwell plates. b When comparing the ratio of migrated cells, it is shown that the ratio of cells with siRNA/APP is significantly less than the ratio of cells in the control groups (asterisk denotes p < 0.05)
In order to explore the underlying molecular mechanism of how APP affects cell migration, we analyzed the expression levels of MMP-2 using Western blot and qRT-PCR. The results indicated that knockdown of APP downregulates MMP-2 in siRNA/APP-transfected cells, suggesting that APP mediates Kasumi-1 cell migration by MMP-2 (Fig. 6).
APP regulates MMP-2 expression. a Real-time PCR of MMP-2 mRNA level from wild Kasumi-1 cells, cells infected with siRNA/APP and scr-siRNA (NC) (asterisk denotes p < 0.05). There is no difference between the wild group and NC group (p > 0.05). b Western blot analysis of MMP-2 protein expression in Kasumi-1 cells infected with siRNA/APP, NC, and wild cells
Discussion
APP is an integral and widely expressed membrane protein, and it is initially expressed as a precursor protein which undergoes proteolytic peptide cleavage. Overexpression of APP has been recently reported in several types of human malignancies. In this study, we determined that overexpression of APP significantly increase EML in AML1/ETO+ patients, suggesting that APP may serve an important role in leukemia cell migration. In an in vitro study, we further demonstrated that there is a relationship between APP and EML.
In this study, we constructed the siRNA/APP lentivirus vector, which efficiently knocked down the expression levels of the APP mRNA and protein. We found that siRNA/APP inhibited the migration of Kasumi-1 cells when compared with controls. Overexpression of APP has been linked to the growth of pancreatic cancer, keratinocyte, and thyroid cells [13–15]. However, in our study, leukemia cell proliferation was not affected. As such, we argue that the knockdown of APP by lentivirus-delivered siRNA may serve as a potential future precaution for leukemia in which APP is overexpressed.
AML is a hematopoietic malignancy, and it is characterized by the excessive egress of leukemic blasts from the bone marrow and their infiltration of organs. These processes require leukemic cells to cross matrix barriers and penetrate blood vessel walls, which depend on the catalytic modification of ECM and basement membranes [26]. As one of the key ECM-degrading enzymes, MMP-2 has been documented to participate in tumor invasion and metastasis [27–29]. Feng et al. [30] discovered that MMP-2 and MMP-9 secreted by leukemic cells increase the permeability of the blood–brain barrier by disrupting tight junction proteins. In our study, we interfered the APP gene and found that MMP-2 was significantly reduced when APP was silenced. Based on these data, we argue that APP is an important regulator of MMP-2 expression and leukemic cell invasion, and possible mechanisms will be further investigated.
In conclusion, our results established a correlation between MMP-2 and APP overexpression in leukemic cells. This information provides an important clue in the mechanism of leukemic cell extramedullary infiltration, which may provide additional insight into a potential target for therapeutic intervention. Further studies will have to clarify the precise mechanism between APP and MMP-2.
References
Cornell RF, Palmer J. Adult acute leukemia. Disease-a-month: DM. 2012;58:219–38.
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from cancer and leukemia group B. Blood. 2002;100:4325–36.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–33.
Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–5.
Robakis NK, Wisniewski HM, Jenkins EC, Devine-Gage EA, Houck GE, Yao XL, et al. Chromosome 21q21 sublocalisation of gene encoding beta-amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet. 1987;1:384–5.
Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19:7793–803.
Yang G, Gong YD, Gong K, Jiang WL, Kwon E, Wang P, et al. Reduced synaptic vesicle density and active zone size in mice lacking amyloid precursor protein (APP) and APP-like protein 2. Neurosci Lett. 2005;384:66–71.
Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5.
Muller T, Meyer HE, Egensperger R, Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer's disease. Prog Neurobiol. 2008;85:393–406.
Nakagawa T, Kabuto M, Kubota T, Kodera T, Sato K. Production of amyloid beta protein precursor as a proteinase inhibitor by human astrocytic tumors. Anticancer Res. 1999;19:2963–8.
Meng JY, Kataoka H, Itoh H, Koono M. Amyloid beta protein precursor is involved in the growth of human colon carcinoma cell in vitro and in vivo. Int J Cancer. 2001;92:31–9.
Hansel DE, Rahman A, Wehner S, Herzog V, Yeo CJ, Maitra A. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res. 2003;63:7032–7.
Ko SY, Lin SC, Chang KW, Wong YK, Liu CJ, Chi CW, et al. Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int J Cancer. 2004;111:727–32.
Krause K, Karger S, Sheu SY, Aigner T, Kursawe R, Gimm O, et al. Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J Endocrinol. 2008;198:291–9.
Takayama K, Tsutsumi S, Suzuki T, Horie-Inoue K, Ikeda K, Kaneshiro K, et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009;69:137–42.
Baldus CD, Liyanarachchi S, Mrozek K, Auer H, Tanner SM, Guimond M, et al. Acute myeloid leukemia with complex karyotypes and abnormal chromosome 21: amplification discloses overexpression of APP, ETS2, and ERG genes. Proc Natl Acad Sci USA. 2004;101:3915–20.
Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100.
Lin LI, Lin DT, Chang CJ, Lee CY, Tang JL, et al. Marrow matrix metalloproteinases (MMPs) and tissue inhibitors of MMP in acute leukaemia: potential role of MMP-9 as a surrogate marker to monitor leukaemic status in patients with acute myelogenous leukaemia. Br J Haematol. 2002;117:835–41.
Suminoe A, Matsuzaki A, Hattori H, Koga Y, Ishii E. Expression of matrix metalloproteinase (MMP) and tissue inhibitor of MMP (TIMP) genes in blasts of infant acute lymphoblastic leukemia with organ involvement. Leuk Res. 2007;31:1437–40.
Schoch C, Schnittger S, Bursch S, Gerstner D, Hochhaus A, Berger U, et al. Comparison of chromosome banding analysis, interphase- and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and for follow-up in chronic myeloid leukemia: a study on 350 cases. Leukemia. 2002;16:53–9.
An International System for Human Cytogenetic Nomenclature (1985) ISCN 1985. Report of the Standing Committee on Human Cytogenetic Nomenclature. Birth Defects Orig Artic Ser. 1985;21:1–117.
Federico M. From lentiviruses to lentivirus vectors. Methods Mol Biol. 2003;229:3–15.
Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–5.
Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–9.
Janowska-Wieczorek A, Marquez LA, Matsuzaki A, Hashmi HR, Larratt LM, Boshkov LM, et al. Expression of matrix metalloproteinases (MMP-2 and −9) and tissue inhibitors of metalloproteinases (TIMP-1 and −2) in acute myelogenous leukaemia blasts: comparison with normal bone marrow cells. Br J Haematol. 1999;105:402–11.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74.
Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20:4782–93.
Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–92.
Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, et al. Matrix metalloproteinase-2 and −9 secreted by leukemic cells increase the permeability of blood–brain barrier by disrupting tight junction proteins. PLoS One. 2011;6:e20599.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jiang, L., Yu, G., Meng, W. et al. Overexpression of amyloid precursor protein in acute myeloid leukemia enhances extramedullary infiltration by MMP-2. Tumor Biol. 34, 629–636 (2013). https://doi.org/10.1007/s13277-012-0589-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0589-7