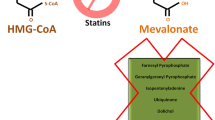
Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most common lethal tumors in the world. Thus, it is very urgent to develop new therapeutic targets against this disease. The mevalonate (MVA) pathway, paced by its rate-limiting enzyme, hydroxymethylglutaryl coenzyme A reductase, is required for the generation of several fundamental end products including cholesterol and isoprenoids. The function of the MVA pathway in ESCC has not been investigated. In this study, it was found that the MVA pathway was upregulated in ESCC clinical samples. Statin, the inhibitor of the MVA pathway, exerted potent cytotoxicity against human ESCC cells by inhibiting cell growth and proliferation, while it exerted lesser effects on non-tumorigenic SHEE cells. Further study revealed that statin could potently induce cell apoptosis and cell cycle arrest and also dose-dependently inhibit the growth of xenograft tumors in nude mice. With regard to the molecular mechanism, statin treatment was related to decreased extracellular signal-regulated kinase activation and proliferating cell nuclear antigen, cyclin D1 expression, and increased cleavage of poly(ADP-ribose) polymerase. Taken together, our findings suggest that the MVA pathway plays an important role in the progression of ESCC by modulating cell growth and statin might be a potential therapeutic agent in ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is the ninth most frequently occurring malignancy in the world. Recent evidence shows that the incidence of this malignancy is increasing [1]. Esophageal adenocarcinoma and esophageal squamous carcinoma (ESCC) are the two major types of esophageal cancer, and the latter is more prevalent in China and other Asian countries [2, 3]. Over the past two decades, although the 5-year survival rate for ESCC patients has been improved owing to the application of successful surgery and the development of therapeutic drugs, survival still remains low [4]. Thus, it is urgent to develop novel therapeutic agents to treat this disease.
The mevalonate (MVA) pathway is a complex biochemical pathway, which is required for the generation of several fundamental end products including cholesterol, isoprenoids, dolichol, ubiquinone, and isopentenyladenine [5]. The heart of this pathway is the rate-limiting enzyme hydroxymethylglutaryl coenzyme A reductase (HMGCR). Both HMGCR and the MVA pathway received considerable interest decades ago primarily because of the cholesterol-lowering drugs known as statins [6]. Inhibition of HMGCR by statins in normal cells triggers a robust homeostatic feedback response, which ensures that the cells upregulate and restore the MVA pathway [7]. Recently, it has been reported that either deficient feedback control of HMGCR or increased HMGCR expression is present in a number of tumors [8, 9]. Administration of exogenous MVA to xenograft-bearing mice was also shown to promote tumor growth [10]. Finally, recent epidemiological studies have shown that patients taking certain statins for cholesterol control displayed a decreased risk of developing some cancers [11–17]. Taken together, these reports suggest that HMGCR may play an important role in human malignancies. However, the expression of HMGCR and other enzymes involved in the MVA pathway in ESCC samples and the therapeutic effects of statins for ESCC remain unknown.
In this study, we investigated the expression of HMGCR and other enzymes involved in the MVA pathway in ESCC clinical samples. It was found that the expression of HMGCR, mevalonate diphosphate decarboxylase (MVD), and acetoacetyl-CoA thiolase 2 (ACAT2) was elevated in the clinical ESCC samples. Statin, the inhibitor of the MVA pathway, inhibited cell growth in vitro and attenuated the tumorigenicity of ESCC cells in vivo. Mechanically, it was shown that statin activated the apoptosis pathway by upregulation of cleaved poly(ADP-ribose) polymerase (PARP) and downregulation of cyclin D1, proliferating cell nuclear antigen (PCNA), and phosphorylated extracellular signal-regulated kinase (ERK). Taken together, our study revealed that the MVA pathway is a therapeutic target in ESCC.
Materials and methods
Compounds and reagents
All chemicals were of analytical grade. Lovastatin was bought from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The ECL Plus system was purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). All cell culture supplies were obtained from Invitrogen-Gibco Co. ERK, p-ERK, PARP, and cyclin D1 antibodies together with all secondary antibodies (anti-mouse and anti-rabbit immunoglobulin G) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Primary ESCC samples
Primary tissues were collected from patients who received surgery for ESCC at Shanghai Chest Hospital of Shanghai Jiao Tong University and Changhai Hospital of Second Military Medical University. All patients had given informed consent. Dissected samples were frozen immediately after surgery and stored at −80 °C until needed.
Cell culture
ESCC cell lines KYSE180 and Caes17 and immortalized esophageal epithelial cell line SHEE were originally obtained from ATCC and cultured in RPMI 1640 medium (Invitrogen) with 10 % fetal bovine serum, 10 units/ml penicillin G, and 10 mg/ml streptomycin. The cells were incubated at 37 °C in 5 % CO2 humidified air.
Cell growth assay
Cell growth and viability were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer’s protocol (Roche Applied Science). In brief, 2 × 103 cells (per well) were seeded in 96-well plates and were treated either for 48 h with lovastatin at serial concentrations (0, 5, and 10 μM) or for various times (0, 24, 48, and 72 h) with lovastatin at concentrations of 0, 5, and 10 μM. After treatment, 20 μl of MTT solution was added to each well. The plates were incubated for an additional 4 h, after which the absorbance at 540 nm was recorded using a SpectraMax190 microplate reader (Molecular Devices, USA) to calculate cell survival percentages.
Cell cycle and apoptosis analysis
Briefly, 5 × 105 cells were seeded in six-well plates and allowed to adhere. Twenty-four hours later, the cells were harvested by centrifugation at 1,000 rpm for 5 min. The cell pellets were washed twice with phosphate-buffered saline (PBS), followed by fixation with ice-cold 70 % ethanol, and stored at −20 °C overnight. Then, the pellets were washed with cold PBS; suspended in 500 ml PBS containing 50 μg/ml propidium iodide, 0.1 mg/ml RNase A, and 0.05 % Triton X-100; and incubated at 37 °C for 40 min in the dark. The cell cycle distribution was determined on the Becton Dickinson FACSCalibur. The experiment was repeated thrice under the same conditions.
Western blotting analysis
Cells were harvested and lysed in cold lysis buffer (50 mM Tris/HCl, pH 7.6; 150 mM NaCl; 0.1 % sodium dodecyl sulfate (SDS); 1 % Nonidet P-40; 1 mg/ml PMSF) at 4 °C for 20 min. Cellular lysates were cleared by centrifugation, and protein concentrations were determined by the Bradford method. Equal amounts of total cellular proteins were resolved by 8 % SDS–polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore). After blocking, the membranes were incubated with primary and secondary antibodies. Protein bands were visualized by the ECL system (Amersham Biosciences).
Tumorigenesis assay
Briefly, KYSE180 cells were harvested and re-suspended in serum-free RPMI 1640 medium containing 20 % (v/v) Matrigel (BD Biosciences, Bedford, MA, USA). Aliquots of cells (2.5 × 104 cells/0.1 ml) were injected subcutaneously into the left inguinal area of the mice. Tumor mass (weight in grams) was determined by the formula 1/2a × b 2, where a is the long diameter and b is the short diameter. Mice bearing tumors (about 1 week after tumor cell inoculation) were randomly divided into control and treatment groups (three mice/group). Lovastatin was administered at doses of 20 mg/kg (5 days/week for 4 weeks). The control group received saline only. The mice were killed, and tumors were carefully excised.
Result
MVA pathway was upregulated in clinical ESCC samples
In order to investigate the expression of enzymes involved in the MVA pathway in ESCC samples, the expression of HMGCR, MVD, and ACAT2 was examined by real-time PCR analysis in a series of randomly selected tumors and the paired normal tissues. Upregulation of HMGCR, MVD, and ACAT2 was observed in ESCC samples (Fig. 1a–c). Moreover, statistical analysis showed that the expression of HMGCR, MVD, and ACAT2 was significantly higher in the ESCC tumor tissues compared with the normal tissues (Fig. 1d). These results indicated that the MVA pathway might play an oncogenic role in the progression of ESCC.
Upregulation of MVA pathway in ESCC samples. a–c Relative expression of HMGCR, MVD, and ACAT2 mRNA in human ESCC samples and paired normal esophageal tissues. Semi-quantitative RT-PCR was performed on 20 paired ESCC RNA samples. The expression of HMGCR, MVD, and ACAT2 was normalized to that of beta-actin. Data were calculated in triplicate. Each bar was the log2 value of the ratio of MGCR, MVD, and ACAT2 expression levels between ESCC (C) and matched normal tissues (N) from the same patient. Because log22 = 1, a bar value >1 represents more than twofold increase (C > N), whereas a bar value < −1 represents more than twofold decrease (C < N). d The expression of HMGCR, MVD, and ACAT2 was shown as box plots, with the horizontal lines representing the median; the bottom and top of the boxes representing the 25th and 75th percentiles, respectively; and the vertical bars representing the range of data. We compared the expression of HMGCR, MVD, and ACAT2 in cancer tissues and normal tissues using the t test. n = 20. Single asterisk indicates p < 0.05; double asterisk indicates p < 0.01
Statin, the inhibitor of MVA pathway, inhibited the growth of ESCC cells
To determine whether endogenous MVA pathway played an important role in the progression of ESCC, we used lovastatin to modulate the activity of the MVA pathway in KYSE180 and Caes17 cells. The in vitro cytotoxicity of lovastatin to KYSE180 and Caes17 cells were first assessed using the MTT assay. Caes17 and KYSE180 cells were exposed to lovastatin at various concentrations for 72 h (Fig. 2a). The apoptotic morphology of Caes17 and KYSE180 cells exposed to lovastatin is shown in Fig. 2a. In addition, lovastatin inhibited the proliferation of Caes17 and KYSE180 cells in a dose-dependent manner (Fig. 2b, c), while its anti-proliferative effects were much less evident in the SHEE cells (immortalized non-tumorigenic esophageal epithelial cells) treated with the same concentrations of lovastatin (Fig. 2d). In fact, lovastatin significantly inhibited the proliferation of Caes17 and KYSE180 cells beginning at the 5-μM concentration (Fig. 2b, c; p < 0.05), while there was no significant inhibition in the SHEE cells at any of the concentrations (up to 10 μM; Fig. 2d). In order to examine whether statin inhibited the MVA pathway specifically in the ESCC cell lines, we checked the activity of Ras which is tightly regulated by the MVA pathway. In the glutathione S-transferase (GST) pull-down assay, it was found that statin blocked the activity of Ras dramatically, suggesting the inhibition of the MVA pathway by statin (Fig. 2e). These observations suggested that lovastatin selectively inhibited the growth of KYSE180 and Caes17 ESCC cells by inhibiting the MVA pathway, while it exerted less potent effects on non-tumorigenic SHEE cells.
Lovastatin preferentially decreased the viability of Caes17 and KYSE180 cells. a The morphology of Caes17 and KYSE180 cells treated with lovastatin. b–d Cell growth inhibition after 0, 24, 48, or 72 h of exposure of Caes17 (b), KYSE180 (c), and SHEE (d) cells to lovastatin (at concentrations of 0, 5, and 10 μM). All values were representative of at least three independent experiments with similar results and were presented as the percentage of cell growth inhibition, where vehicle-treated cells were regarded as 100 % viable (0 % growth inhibition). e GST pull-down assay in Caes17 cells. The GST or GST-Raf binding domain (RBD) fusion protein was added to the lysate of the cells treated with statin. The expression of Ras pulled down by the GST-RBD (active Ras) was detected by Western blot analysis
Statin induced cell cycle arrest and promoted apoptosis of ESCC cells
Since lovastatin dramatically reduced the proliferation of Caes17 and KYSE180 cells, we next investigated the underlying mechanism. First, we evaluated whether lovastatin could induce the apoptosis of KYSE180 cells. As shown in Fig. 3a, b, lovastatin strongly induced apoptosis in a dose-dependent manner during 24-h exposure. At 5-μM concentration, lovastatin increased the apoptosis of KYSE180 cells by nearly threefold (Fig. 3b; p < 0.05). In conclusion, our data demonstrated that lovastatin could induce significant apoptosis in the KYSE180 cells in a dose-dependent manner. We also investigated whether lovastatin could affect the cell cycle progression in KYSE180 cells. For this assay, we decreased the lovastatin incubation time to 12 h to avoid high apoptosis induction. As illustrated in Fig. 3c, d, after a 12-h treatment, lovastatin induced significant increases in the number of cells in the G0/G1 phase. In addition, lovastatin led to a significant decrease in the number of cells in the S phase. Taken together, our data suggested that the inhibitory effects of lovastatin might be associated with its induction of cell cycle arrest and apoptosis.
Lovastatin induced apoptosis and G0/G1 cell cycle arrest in KYSE180 cells in a dose-dependent manner. a Apoptosis of KYSE180 cells treated with serial concentrations of lovastatin for 24 h. b Data summary and analysis of the apoptotic index. c Cell cycle evaluation of KYSE180 cells treated with serial concentrations of lovastatin for 12 h. d Data analysis of cells presented as the percent distribution of a specific phase. Data are representative of values from at least three independent experiments with similar results
Lovastatin regulated the expression of cell cycle and apoptosis-related genes
In the previous study, we have found that lovastatin induced cell cycle arrest and apoptosis. To further define the effects of lovastatin on cell apoptosis and proliferation, we investigated the expression of a panel of proteins involved in these pathways. After treating Caes17 and KYSE180 cells with lovastatin, we observed an increased cleavage of PARP (Fig. 4). We further investigated the possible mechanism responsible for the anti-proliferative effects of lovastatin. As mentioned above, we evaluated the expression of various proteins associated with proliferation and the cell cycle progression. It was shown that treating the Caes17 and KYSE180 cells with lovastatin downregulated the expression of cyclin D1, PCNA, and the activated ERK (indicated by phosphorylated ERK). Taken together, lovastatin might exert in vitro effects through modulating cell growth and apoptosis pathway.
Lovastatin exerted potent anti-tumor activity in vivo
Our in vitro results suggested that lovastatin suppressed cell growth. Therefore, we evaluated whether lovastatin could influence the tumorigenicity of ESCC cells in vivo by utilizing the mouse xenograft tumor model. The compound was administered 5 days a week at a dose of 20 mg/kg. Therapeutic effects were evaluated by examining tumor growth. As shown in Fig. 5a, lovastatin exhibited significant tumor inhibition. After 18 days of treatment, lovastatin resulted in about 60 % tumor growth inhibition (Fig. 5b; p < 0.05). Moreover, although the mice receiving the 20 mg/kg dose experienced a slight loss in body weight, there were no significant differences in body weight compared with the control group (data not shown), indicating that lovastatin might have an acceptable safety profile.
Discussion
In previous studies, it was shown that statin induced DNA damage, cell apoptosis, cell cycle arrest, and proliferation inhibition in esophageal adenocarcinoma and pancreatic cancer [18, 19]. However, the anti-tumor activities of statin in ESCC have not been elucidated. Here, we are the first to study the expression of the MVA pathway in ESCC clinical samples and systematically investigate the anti-tumor effects of lovastatin in vitro and in vivo. We demonstrated that the MVA pathway was activated in ESCC and lovastatin exerted its anti-cancer activities on Caes17 and KYSE180 cells by inhibition of cell growth and proliferation. Further study revealed that it could induce cell apoptosis and cell cycle arrest. Such functional outcomes were achieved through a cascade of reactions including downregulation of phosphorylated ERK, cyclin D1, and PCNA. The focus of the current study was to determine whether lovastatin could represent a promising candidate for future anti-cancer drug development and to further elucidate its mechanism(s) of action.
It has been reported that HMGCR, the rate-limiting enzyme in the MVA pathway, directly promoted transformation of MCF-10A cells [20]. Here, we provide evidence demonstrating upregulation of the MVA pathway in ESCC samples. However, the mechanisms for dysregulation of the MVA pathway and HMGCR expression are not yet well defined [5]. The MVA pathway is highly regulated in non-transformed cells, and loss of any dysregulation of this critical pathway would drive tumorigenesis. Recently, for example, it was determined that HMGCR can be regulated through the transcriptional activity of hypoxia-inducible factor 1 alpha [21], a transcription factor that plays a fundamental role in the response to hypoxia and in tumor cell metabolism. These reports suggested that activation of upstream signaling cascades might lead to the dysregulation of HMGCR expression and the MVA pathway activation.
Statins have shown cancer chemopreventive effects [22]. They can trigger some tumor cells to undergo apoptosis in vitro and suppress tumor growth in vivo. They also have an anti-metastatic property, which is evident in their suppression of tumor cell invasiveness in Matrigel as well as in animal experiments [23]. In addition, statins, especially at high concentrations, can inhibit capillary tube formation by endothelial cells in vitro and in vivo [24]. The effects of statins were thought to be mediated through inhibition of Ras and RhoA activity [22].
In conclusion, our study demonstrated that lovastatin could exert potent cytotoxicity and inhibit cell proliferation. Besides the induction of apoptosis, it can also induce cell cycle arrest and inhibit the growth of ESCC xenograft tumors. In the future, the present study, together with the previous reported findings, will surely improve our understanding about the mechanisms of action of statins and will also provide a basis for their future clinical development as a novel anti-cancer agent.
References
Siegel R. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41.
Koshiol J, et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127:93–100.
Zhao X, et al. Loss of heterozygosity at 6p21 and HLA class I expression in esophageal squamous cell carcinomas in China. Asian Pac J Cancer Prev. 2011;12:2741–5.
Chen YH, et al. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One. 2012;7:e42766.
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30.
Endo A, et al. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–82.
Davidson MH, et al. Safety profiles for the HMG CoA reductase inhibitors: treatment and trust. Drugs. 2001;61:197–206.
Larsson O, et al. HMG-CoA reductase inhibitors: role in normal and malignant cells. Crit Rev Oncol Hematol. 1996;22:197–212.
Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med. 2004;229:567–85.
Duncan RE, El-Sohemy A, Archer MC. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J Biol Chem. 2004;279:33079–84.
Cauley JA, et al. Statin use and breast cancer: prospective results from the Women’s Health Initiative. J Natl Cancer Inst. 2006;98:700–7.
Kumar AS, et al. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev. 2008;17:1028–33.
Kochhar R, Khurana V, Bejjanki H, Caldito G, Fort C. Statins reduce breast cancer risk: a case control study in US female veterans. J Clin Oncol. 2005;23:514.
Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109:573–9.
Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk? Trends Endocrinol Metab. 2008;19:113–21.
Karp I, Behlouli H, Lelorier J, Pilote L. Statins and cancer risk. Am J Med. 2008;121:302–9.
Lipkin SM, et al. Genetic variation in 3-hydroxy-3-methylglutaryl CoA reductase modifies the chemopreventive activity of statins for colorectal cancer. Cancer Prev Res. 2010;3:597–603.
Kantor ED, et al. Use of statin medications and risk of esophageal adenocarcinoma in persons with Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2012;21:456–61.
Mohammed A, et al. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48(Cre/+) LSL-Kras(G12D/+) mice. Int J Cancer. 2012;131:1951–62.
James W, et al. Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci U S A. 2010;107:15051–6.
Pallottini V, et al. Regulation of HMG-CoA reductase expression by hypoxia. J Cell Biochem. 2008;104:701–9.
Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42.
Graaf MR, Richel DJ, van Noorden CJ, et al. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–41.
Vincent L, Chen W, Hong L, et al. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: contribution to its anti-angiogenic effect. FEBS Lett. 2001;495:159–66.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jianxin Shi and Ji Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shi, J., Zhu, J., Zhao, H. et al. Mevalonate pathway is a therapeutic target in esophageal squamous cell carcinoma. Tumor Biol. 34, 429–435 (2013). https://doi.org/10.1007/s13277-012-0567-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0567-0