Abstract
Purpose
We examined the association between post-diagnosis statin use (3-hydroxy-3-methylglutaryl-coenzyme A [HMG-CoA] inhibitors) and risk of breast cancer recurrence.
Materials and methods
The study included 1945 early stage breast cancer survivors participating in the Life After Cancer Epidemiology (LACE) Study. Women who were diagnosed from 1997 to 2000 and identified from the Kaiser Permanente Northern California (KPNC) Cancer Registry entered the cohort on average 2 years post-diagnosis. Information on statin use was obtained from the KPNC pharmacy database. A total of 210 breast cancer recurrences were reported and verified by medical record review. Cox proportional hazard models were used to estimate rate ratios (RR) and 95% confidence intervals (CI).
Results
The mean duration of statin use in the cohort among those who initiated use post-diagnosis was 1.96 years, and lipophilic statins were mainly used (97.8%). Starting statins after diagnosis was suggestive of a decreased risk of breast cancer recurrence (RR = 0.67; 95% CI: 0.39–1.13). Risk of recurrence decreased with increasing duration of statin use after diagnosis (p linear trend = 0.02).
Conclusion
Our findings provide initial support for an inverse association between post-diagnosis, lipophilic statin use and risk of breast cancer recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Statins (3-hydroxy-3-methylglutaryl-coenzyme A [HMG-CoA] inhibitors) are lipid-lowering drugs that have been proven in clinical trials to prevent cardiovascular disease [1]. They have not only been associated with beneficial effects on heart-related conditions but also with possible anti-carcinogenic activity. For example, laboratory studies have shown that lipophilic statins such as simvastatin and fluvastatin inhibit mammary tumor growth by ∼50% at doses equivalent to those used in humans for reducing cholesterol [2]. Thus far, observational studies of the association between statins and risk of developing breast cancer have yielded mixed results, with the majority finding no association [3–10]. To our knowledge, no studies have examined statin use and breast cancer prognosis.
We investigated the potential association between use of statins after breast cancer diagnosis and breast cancer recurrence among participants in the Life After Cancer Epidemiology (LACE) Study, a prospective cohort study of 2,292 early stage breast cancer survivors. Using data from the KPNC pharmacy prescription database, statins were analyzed according to initiation and duration of use after cancer diagnosis.
Materials and methods
Study cohort
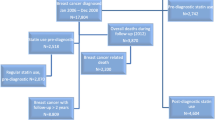
The LACE cohort has been described previously [11]. Briefly, the cohort consists of women diagnosed with invasive breast cancer from 1997 to 2000 recruited primarily from the Kaiser Permanente Northern California (KPNC) Cancer Registry (82%) and the Utah Cancer Registry (12%). Additionally, a subset of women who declined participation in the Women’s Healthy Eating and Living (WHEL) Trial, a dietary intervention trial to prevent recurrence of breast cancer, was used as a third source of participants (6%). Women were eligible if they were diagnosed from age 18–79 years with a first primary breast cancer (Stage I ≥1 cm, II, or IIIA), had completed breast cancer treatment (except for adjuvant hormonal therapy), were within 11–39 months post-diagnosis (to capture behavioral factors in the early post-diagnosis period and to ensure that women were free of symptoms due to treatment), had no history of other cancer within 5 years before enrollment, and were recurrence-free at enrollment. Subsequently, after medical record review, any woman who had a breast cancer recurrence, new breast primary, or death within 3 months after enrollment was deemed ineligible since these events may have been present at enrollment but missed. Therefore, the start of time at risk in the cohort was 3 months post-enrollment.
Between January 2000 and April 2002, 5,656 women who were presumed to meet the LACE eligibility criteria were sent a recruitment package. Of these, 2,614 (46%) agreed to participate and completed the questionnaires. Subsequent medical record review to confirm eligibility resulted in 322 exclusions. Reasons for exclusions were breast cancer recurrence, new primary breast cancer, or death within 3 months after enrollment (37%), incorrect stage (34%), other cancer within the 5 years before enrollment (10%), prior breast cancer (6%), more than 39 months since diagnosis (6%), incomplete demographic and medical data (3%), still receiving treatment (2%), and language difficulty (2%). The remaining 2,292 women constitute the LACE cohort. This analysis was restricted to the 1,945 (85%) who were recruited from KPNC because of the availability of automated pharmacy data on this group. The KPNC participants did not differ significantly from other women in the cohort on age, stage of disease, and body mass index (BMI) (not shown). Subjects entered the cohort on average 1.90 years after diagnosis (range 0.92–3.24 years) and were subsequently followed on average for an additional 5.00 years (range 0.25–6.86) post-enrollment. The study was approved by the institutional review boards of KPNC and University of Utah. Written informed consent was obtained from all participating subjects.
Medication use and other covariates
Information on lipid-lowering agents such as label name, generic name, date dispensed, and days supply was obtained from the KPNC pharmacy database for all Kaiser LACE cohort members (n = 1,945) as of August 24, 2006. To avoid potential confounding by use of lipid-lowering medications before breast cancer diagnosis, individuals who had statin prescriptions (n = 134) prior to diagnosis were excluded, thus leaving 1,811 subjects for analysis.
Initiation and duration of post-diagnosis use was categorized as (1) unexposed (≤100 days supply) and exposed (>100 days supply) and (2) unexposed (≤100 days supply), 101 days–≤2 years supply, and >2 years supply, respectively. A record of at least 100 days supply was considered the minimum exposure since Kaiser health insurance plans commonly allow 100 days supply per dispensing for chronic medications. Therefore, >100 days supply was equivalent to at least two or more prescriptions and deemed as regular use. Results were similar when we excluded the small number of concurrent statin and nonstatin antilipemic users from our analysis (n = 16), so these individuals were retained in the exposure group in our final analyses. Age, race/ethnicity, education, height and weight, smoking status, family history of breast cancer, and breast cancer treatment were collected on the baseline questionnaire at cohort entry. Data on stage, nodal status, and tumor hormone receptor status were obtained from the KPNC Cancer Registry.
Outcomes
Subsequent health outcomes were ascertained semi-annually, and after 5 years of follow-up annually, by mailed questionnaire and verified by medical record. The average response rate to the mailed health status updates was 84%. All nonrespondents were called to complete a report by phone, increasing the response rate to 99%. KPNC computerized mortality files were regularly searched or participant families were contacted for any cohort members not reached (1%). For study subjects who were known to have died, copies of death certificates were obtained and cause of death recorded. Recurrences were defined as subsequent local, regional, or distant disease, and new breast primary cancers in the contralateral breast. Excluding new breast primary cancers from our analysis (n = 30) did not produce substantially different results, so the outcome was included as a recurrence. For this analysis, 210 breast cancer recurrences (of which 73% were distant metastases) were ascertained through December 12, 2006. Mean follow-up time from cohort entry until the endpoint of recurrence was 2.60 years (range 0.25–6.57).
Data analysis
To compare relevant characteristics of users, Pearson chi-square tests were applied. Follow-up began at date of cohort entry and ended at date of first confirmed cancer recurrence, date of death, study drop-out date, or December 12, 2006, whichever occurred first. Rate ratios (RR) and 95% confidence intervals (CI) for breast cancer outcomes were estimated by delayed entry Cox proportional hazard models, with months since diagnosis as the time scale [12, 13]. Statin use was modeled as a time-dependent covariate for which cumulative use based on days supply of every prescription was updated at each failure time. The delayed entry model ensures that a woman who enrolled in the study t years after her initial breast cancer diagnosis was not considered at risk for a possible recurrence prior to t years by removing each woman from the risk set between the time of diagnosis and the time of cohort entry. A linear test for trend was estimated by modeling categorical variables of exposure on an ordinal scale. Confidence intervals not overlapping with 1.00 or p < 0.05 were considered consistent with statistical significance.
Age at diagnosis (<45, 45–54, 55–64, and ≥65 years), race (White, Black, Hispanic, Asian, and Other), BMI (<25, 25–29, and ≥30 kg/m2), cancer stage (Stages I, IIA, IIB, IIIA), and tamoxifen treatment (never, past, and current) were included in the final model as confounders because they had a statistically significant effect on the relative risk associated with statin use when added individually to the Cox model. We also looked at whether the associations between statin use and recurrence varied by menopausal status at diagnosis, BMI, tumor hormone receptor status, and stage of initial breast cancer by first generating strata-specific estimates and then including an interaction term in the model to test for statistical significance. Little evidence was found that the association between exposure and study endpoint varied by the above factors.
Results
Most of the cohort members were white nonsmokers who had no family history of breast cancer (Table 1). Women who used statins post-diagnosis were more likely to be older, obese, or post-menopausal at diagnosis compared to nonusers. Statin users and nonusers were similar with respect to family history of breast cancer, stage of breast cancer, and nodal status (Table 1).
Among the total KP subcohort of 1,811 subjects used in this analysis, 367 (18.9%) used statins and 36 (1.8%) used nonstatin antilipemic drugs post-diagnosis (Table 2). The mean duration of post-diagnosis use of statins and nonstatin antilipemics was 1.96 and 1.31 years, respectively. Lovastatin was the primary statin prescribed (84.4%), followed by simvastatin (10.9%), atorvastatin (2.5%), and pravastatin (2.2%). Among the nonstatin antilipemics, gemfibrozil was mainly prescribed (61.1%), followed by niacin (22.2%), cholestyramine (8.3%), colestipol (5.6%), and ezetimibe (2.8%).
Use of statins for more than 100 days after diagnosis compared to use ≤100 days was associated with a nonstatistically significant reduction in risk of breast cancer recurrence (RR = 0.67; 95% CI: 0.39–1.13), adjusting for age at diagnosis, race, BMI, stage of breast cancer, and tamoxifen treatment (Table 3). Risk of breast cancer recurrence decreased with increasing duration of post-diagnosis statin use (p for linear trend = 0.02) (Table 3). The association between post-diagnosis use of nonstatin antilipemics and recurrence could not be examined adequately since the number of users was small (n = 36), thereby producing unstable risk estimates (not shown).
Discussion
To our knowledge, our study is the first to examine the potential relationship between use of statins and breast cancer prognosis. We found that use of primarily lipophilic statins after diagnosis appeared to be associated with a reduced risk of recurrence, and that this risk decreased with increasing duration of use. Given the largely nonstatistically significant results, the impact of chance cannot be ruled out.
Biological evidence exists which supports a possible protective role of statins, particularly lipophilic statins, on breast cancer outcomes [14]. It appears that only lipophilic statins influence cell proliferation, survival, and motility by permeating the cell membrane [15], specifically extrahepatic cell membranes [16]. In vitro laboratory studies have found that lipophilic statins can inhibit the proliferation of breast cancer cells [2, 17–19], with critical proteins regulating cell survival and proliferation mechanisms being most influenced by statin treatment [2]. Interestingly, this reduction in cell proliferation was stronger in estrogen receptor (ER) -negative cells, suggesting that ER-negative cell lines might be more sensitive to growth inhibition by statins than ER-positive cell lines [2, 17, 20]. However, in our study, we did not observe a difference in the association of statin use and breast cancer recurrence by hormone receptor status, which could be due to small subgroup sizes and thus limited statistical power. In addition, in vivo mouse mammary tumor models demonstrated that lovastatin, fluvastatin, and simvastatin decreased tumor formation and/or inhibited metastasis [2, 21, 22]. Furthermore, three clinical studies, including one randomized trial, suggest that statins may enhance standard chemotherapy or radiotherapy for treatment of cancers such as prostate, rectal, and hepatoma at doses used for cardiovascular disease [15].
Observational studies thus far have yielded mixed results regarding the effect of statins on breast cancer incidence. A large case–control study from the General Practice Research Database, an automated database containing drug prescription and medical information on more than three million people in the UK, found no association between current statin use and breast cancer risk [10]. Interestingly, another large case–control study with cases from a population-based tumor registry reported no overall association of statins with breast cancer incidence but did find that women who had used statins for more than 5 years had an ∼30% lower breast cancer incidence than never users [4]. Two other case–control studies observed no association between statin use and breast cancer risk [6, 9]. Similarly, four large cohort studies found no association between statin use and risk of breast cancer [3, 5, 7, 8], although one study observed an 18% lower breast cancer incidence with use of lipophilic statins [5]. A meta-analysis [23] conducted in late 2005 of nine observational studies, including those mentioned above, and seven clinical trials found that statin use did not significantly affect the risk of breast cancer using either a fixed-effects model (RR = 1.03; 95% CI: 0.93–1.14) or random effects model (RR = 1.02; 95% CI = 0.89–1.18). Stratification by study design did not appreciably change the effect estimates. Notably, this meta-analysis did not include two large retrospective studies, both of which suggested a protective benefit of statin use on breast cancer risk [24, 25].
Strengths of this study include being one of the few existing cohorts of early stage breast cancer survivors, one of the first studies to examine the association of post-diagnosis statin use and breast cancer outcomes, and use of pharmacy prescription data such that recall bias is not a methodological issue. In addition, almost all Kaiser members have pharmacy benefits and thus fill their prescriptions at a Kaiser pharmacy, thereby assuring nearly complete capture of medication dispensings [26]. A limitation of this study is the inability to examine the impact of lipophilic compared to lipophobic statins on breast cancer endpoints since only eight participants had a record of lipophobic statin use, yet our data were homogeneous in terms of statin lipophilicity. In addition, we could not adequately examine confounding by indication with comparison to nonstatin antilipemics due to limited numbers, and the pharmacy database does not contain information on indication. Thus, one cannot exclude the possibility that statin use could be a surrogate for the effect of an associated condition like hyperlipidemia. When interpreting our results, one should also consider that long-term statin users (5 years or more) tend to be healthier and more adherent to therapy and screening than nonusers such that our risk estimates could be biased toward a protective effect [27]. Considering that the average duration of post-diagnosis statin use in our cohort was more short-term (1.96 years), the impact of this bias would most likely be minimal. Finally, our results cannot be generalized to recurrences that may have occurred in the early post-diagnosis period because women did not enter the cohort until they had completed chemotherapy and/or radiation treatment (on average 2 years post-diagnosis).
In conclusion, our study provides initial support for an inverse association between primarily lipophilic statins and risk of breast cancer recurrence. Given our results and the fact that statins are currently among the most widely prescribed drugs in the US [28], more studies are warranted to fully assess the anti-oncogenic potential of statins on breast cancer prognosis.
References
Baigent C, Keech A, Kearney PM et al (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366(9493):1267–1278
Campbell MJ, Esserman LJ, Zhou Y et al (2006) Breast cancer growth prevention by statins. Cancer Res 66(17):8707–8714
Beck P, Wysowski DK, Downey W, Butler-Jones D (2003) Statin use and the risk of breast cancer. J Clin Epidemiol 56(3):280–285
Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR (2004) The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer 100(11):2308–2316
Cauley JA, McTiernan A, Rodabough RJ et al (2006) Statin use and breast cancer: prospective results from the Womens Health Initiative. J Natl Cancer Inst 98(10):700–707
Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Shapiro S (2002) Statin use and the risk of breast and prostate cancer. Epidemiology 13(3):262–267
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE (2005) Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch Intern Med 165(19):2264–2271
Friis S, Poulsen AH, Johnsen SP et al (2005) Cancer risk among statin users: a population-based cohort study. Int J Cancer 114(4):643–647
Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ (2004) The risk of cancer in users of statins. J Clin Oncol 22(12):2388–2394
Kaye JA, Jick H (2004) Statin use and cancer risk in the general practice research database. Br J Cancer 90(3):635–637
Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML (2005) Life after cancer epidemiology (LACE) study: a cohort of early stage breast cancer survivors (United States). Cancer Causes Control 16(5):545–556
Allison PD (1995) Survival analysis using SAS: a practical guide. SAS Press, Cary, NC
Hosmer DW, Lemeshow S (1999) Applied survival analysis: regression modeling of time to event data. Wiley, New York
Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM (2005) Statins and cancer prevention. Nat Rev Cancer 5(12):930–942
Katz MS (2005) Therapy insight: potential of statins for cancer chemoprevention and therapy. Nat Clin Pract Oncol 2(2):82–89
Duncan RE, El-Sohemy A, Archer MC (2005) Statins and cancer development. Cancer Epidemiol Biomarkers Prev 14(8):1897–1898
Esserman L, Campbell M, Shoemaker M, Lobo M, Marx C, Benz C (2004) Breast cancer inhibition by statins. J Clin Oncol 22(97s):(Suppl; abstr 1003)
Mueck AO, Seeger H, Wallwiener D (2003) Effect of statins combined with estradiol on the proliferation of human receptor-positive and receptor-negative breast cancer cells. Menopause 10(4):332–336
Seeger H, Wallwiener D, Mueck AO (2003) Statins can inhibit proliferation of human breast cancer cells in vitro. Exp Clin Endocrinol Diabetes 111(1):47–48
Kumar AS, Campbell M, Benz CC, Esserman LJ (2006) A call for clinical trials: lipophilic statins may prove effective in treatment and prevention of particular breast cancer subtypes. J Clin Oncol 24(13):2127; author reply 2127–2128
Alonso DF, Farina HG, Skilton G, Gabri MR, De Lorenzo MS, Gomez DE (1998) Reduction of mouse mammary tumor formation and metastasis by lovastatin, an inhibitor of the mevalonate pathway of cholesterol synthesis. Breast Cancer Res Treat 50(1):83–93
Farina HG, Bublik DR, Alonso DF, Gomez DE (2002) Lovastatin alters cytoskeleton organization and inhibits experimental metastasis of mammary carcinoma cells. Clin Exp Metastasis 19(6):551–559
Bonovas S, Filioussi K, Tsavaris N, Sitaras NM (2005) Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 23(34):8606–8612
Kochhar R, Khurana V, Bejjanki H, Caldito G, Fort C (2005) Statins to reduce breast cancer risk: A case control study in US female veterans. J Clin Oncol 23(7s):(Suppl; abstr 514)
Mortimer J, Axelrod R, Zimbro K (2004) Effect of statins on breast cancer incidence: findings from the Sentara Health Plan. Proc Am Soc Clin Oncol 22(93):(abstr 373)
Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH (2005) Kaiser permanente medical care program. In: BL S (ed) Pharmacoepidemiology. Wiley, West Sussex, pp 241–259
Setoguchi S, Avorn J, Schneeweiss S (2005) Statins and the risk of colorectal cancer. N Engl J Med 353(9):952–954; author reply 952–954
The Internet Drug Index http://www.rxlist.com [Last accessed: December 12, 2006]
Acknowledgments
This study was funded by the National Cancer Institute (R01 CA80027) and by the Utah Cancer Registry (N01 PC67000), with additional support from the State of Utah Department of Health. We thank Natalia Udaltsova and Erin Weltzien for statistical programming support. We thank all LACE Study staff and participants. Financial Support: National Cancer Institute (R01 CA80027 and N01-PC-67000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwan, M.L., Habel, L.A., Flick, E.D. et al. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat 109, 573–579 (2008). https://doi.org/10.1007/s10549-007-9683-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9683-8