Abstract
MicroRNA-421 (miR-421) plays crucial roles during carcinogenesis and is a potential tumor marker in the diagnosis of several types of cancers. However, whether miR-421 in gastric juice, which is specific for gastric tissue, can be used as a biomarker for gastric cancer screening is unclear. In the present study, real-time quantitative reverse transcription-polymerase chain reaction was used to analyze miR-421 levels in gastric juice from patients with gastric cancer or benign gastric disease, or normal. A receiver operating characteristic (ROC) curve was constructed to evaluate the diagnostic values. The results showed that gastric juice levels of miR-421 in patients with gastric cancer were significantly different from those in benign gastric diseases (P < 0.001). The area under the ROC curve of miR-421 was up to 0.767 (95 % CI = 0.684–0.850). The levels of miR-421 in gastric juice from gastric patients were not significantly associated with the main clinicopathological factors such as tumor size, Lauren’s classification, and Borrmann’s classification. For the detection of early gastric cancer, the use of gastric juice miR-421showed a remarkable improvement compared with the use of serum carcinoembryonic antigen alone. These results indicated that gastric juice miRNAs such as miR-421 are useful biomarkers for screening gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer remains a major clinical challenge worldwide due to its high prevalence, poor prognosis, and limited treatment options [1]. Although the incidence of gastric cancer has declined over the years, it continues to be the second leading cause of cancer death and the fourth most common malignancy worldwide [1]. Less than 25 % of gastric cancer cases are diagnosed at an early stage, and the 5-year survival rate is only 24 % in the USA and Europe [2]. Despite the rapid development of several anticancer drugs, advanced gastric cancer is still strongly associated with a poor outcome, with a median survival of 7–10 months in patients with metastatic or unresectable disease [3]. If gastric cancer patients are diagnosed at an early stage, the survival rate will be improved to over 60 % [2]. However, common blood-based tumor markers, such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and serum pepsinogen, do not have satisfactory sensitivity [1, 2].
MicroRNAs (miRNAs) are members of a large class of small noncoding RNAs which regulate many genes in human tumorigenesis, including gastric cancer [4]. Altered levels of miRNAs in peripheral blood provide novel tumor markers for gastric cancer. The levels of several peripheral blood miRNAs, including miR-106a, miR-17, miR-10b, miR-223, and miR-21, have been found to be different between gastric cancer patients and healthy subjects [5, 6]. In our previous study, we demonstrated that miR-421 was highly expressed in gastric cancer tissues and gastric cancer cell lines [7]. Among gastric cancer patients, the positive detection rate of miR-421 was higher than that of serum CEA [7].
Cancer-associated miRNAs are readily detectable in the peripheral circulation of patients and are emerging as candidate biomarkers for the detection of cancer [8, 9]. A study showed that body fluid miRNAs were associated with the state of the surrounding tissues [10]. However, most cancer-associated miRNAs are shared by several types of cancer [8]. In this study, considering the high degree of tissue specificity, we used gastric juice as a sample to search for gastric cancer-associated miRNAs. We demonstrated that gastric juice miR-421 levels in gastric cancer patients were significantly different from those in benign gastric diseases. The results indicated that miR-421 may be a potential biomarker of gastric cancer.
Materials and methods
Patients and sample collection
Gastric juices were collected from 141 subjects, including 42 patients with gastric cancer (mean age, 64.2 ± 13.2 years), 34 patients with gastric ulcer (51.8 ± 13.5 years), 18 patients with atrophic gastritis (57.5 ± 13.3 years), and 47 patients with normal mucosa or minimal gastritis (50.2 ± 13.4 years), between 2010 and 2011 from the Endoscopy Center of the Affiliated Hospital of Ningbo University School of Medicine (Table 1). All diagnoses were confirmed by endoscopic examination followed by pathological diagnosis of biopsies. Cases with normal mucosa or minimal gastritis, which had no family history of gastric cancer, were treated as the controls. The exclusion criteria for all 141 subjects were the following: oral ulcer, Barrett’s esophagus, esophagitis, oral and esophageal cancer, duodenal ulcer, stomach polyps, upper gastroduodenal bleeding, the use of proton pump inhibitors or nonsteroidal anti-inflammatory drugs within 2 weeks of the study, and the coexistence of other malignant diseases. Biopsy specimens were routinely processed and analyzed by experienced pathologists who were blind to the designs of this study. According to the criteria proposed by the Japanese Research Society for Gastric Cancer [11], we divided gastric cancer patients into early (n = 7) or advanced cases (n = 35). Among the early gastric cancer patients, three had severe dysplasia and others were of type 0-IIa+IIc or 0-IIc+III. In accordance with Borrmann’s advanced gastric cancer classification [12], we further classified gastric cancer patients as type I (n = 6), type II (n = 9), type III (n = 13), or type IV (n = 7). Finally, in accordance with Lauren’s classification [13], gastric cancer patients were classified as of intestinal type (n = 29), diffuse type (n = 5), or mixed type (n = 8).
Endoscopy was performed using an Olympus GIF H260 (Olympus Corp., Tokyo, Japan) after a 12-h overnight fast. Immediately after insertion of the endoscope into the stomach, 3 ml gastric juice without bile, blood, and gross food residues was aspirated through the matching washing pipe PW-2L-1 (Olympus Corp.) and collected in a sterile centrifuge tube. Next, routine inspection was performed and a biopsy taken.
Gastric juice specimens were centrifuged at 2,000×g for 30 min at 4 °C to remove cell fragments and mucus. Then, the pH was measured using a glass electrode pH meter. Finally, the supernatants were stored at −80 °C until use.
Ten pairs of randomly selected mucosa biopsies were collected from advanced gastric cancer patients. Cancer and adjacent normal mucosa (at least 5 cm away from the tumor) were biopsied. Mucosa specimens were soaked in RNAfixer Reagent (Bioteke, Beijing, China) and stored at −80 °C until use.
Informed consent was obtained from all participants. This study was approved by the Human Research Ethics Committee of Ningbo University.
RNA extraction
Before RNA extraction, mucosa biopsies were placed at room temperature for 1 h. Approximately 10 mg mucosa biopsy was added to 1 ml Trizol reagent (Invitrogen, Karlsruhe, Germany) and then homogenized by grinding in an RNase-free mortar. Finally, RNA was extracted following the manufacturer’s instructions.
Gastric juice was also placed at room temperature for 1 h. For gastric juice RNA extraction, 750 μl Trizol LS reagent (Invitrogen) was mixed with 250 μl gastric juice. After vortex mixing for 30 s and then standing for 5 min, 200 μl chloroform was added. The Trizol–chloroform mixture was vortex-mixed for 15 s and then centrifuged at 12,000×g for 15 min at 4 °C. The upper aqueous phase was transferred to a fresh tube. Finally, RNA was extracted following the manufacturer’s instructions. Total RNA was quantified using a SmartSpec Plus spectrophotometer (Bio-Rad, Hercules, CA, USA). The A 260/A 280 ratio was used to evaluate RNA purity.
Real-time quantitative reverse transcription-polymerase chain reaction
Reverse transcription (RT) was performed using the miScript RT kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s instructions. The RT product was diluted fourfold by adding RNase-free water. Quantitative polymerase chain reaction (qPCR) was performed using the miScript SYBR Green PCR Kit (Qiagen) on an Mx3005P QPCR System (Stratagene, La Jolla, CA, USA). The 20-μl PCR mixture included 6 μl RT product, 10 μl 2× QuantiTect SYBR Green PCR Master Mix, 1 μl 10× miScript Universal Primer (downstream PCR primer for small RNA; Qiagen), 1 μl 10× miRNA upstream PCR primer, and 2 μl RNase-free water. The upstream primers for amplifying miR-16 and miR-421 were 5′-TAGCAGCACGTAAATATTGGCG-3′ and 5′-AACAGACATTAATTGGGCGC-3′, respectively. They were synthesized by Shanghai GenePharma Co. (Shanghai, China). The reaction mixtures were incubated at 95 °C for 15 min, followed by 45 amplification cycles of 94 °C for 15 s, 60 °C for 30 s, and 70 °C for 30 s. The quantification cycle (C q), previously known as the threshold cycle (C t) or crossing point (C p) [6, 7, 14], was defined as the number of PCR cycles required for the fluorescence to pass the fixed threshold. We also quantified transcripts of U6 small RNA using the Hs_RNU6B miScript Primer Assay (Qiagen) for normalizing the levels of miR-421 in endoscopic mucosa biopsies, and we used miR-16 to normalize the level of miR-421 in gastric juice. The ΔC q method was used for analysis, as described previously [14]. Higher ΔC q values mean lower levels of target miRNA (miR-421). The specificity of the RT-qPCR technique was confirmed by a dissociation curve analysis. The data were analyzed using MxPro Software, version 3.2 (Stratagene). The experiment was repeated at least twice. The investigators were blind to the clinical and pathological diagnoses.
Cloning and sequencing of RT-qPCR products
Gastric juice miRNA RT-qPCR products were first purified using a UNIQ-10 PCR Product Purification Kit (Sangon Biotech, Shanghai, China) and then cloned into the pUCm-T vector (Sangon Biotech) following the manufacturer’s instructions. Finally, DNA sequencing was performed by Sangon Biotech Co., Ltd.
Determining the stability and reproducibility of gastric juice miRNAs
To test the stability of gastric juice miRNAs, four aliquots of gastric juice were randomly selected and stored at room temperature, 4 °C, or −20 °C for 0, 2, 6, and 12 h. These time points were chosen to represent typical short-term transport and storage conditions encountered in clinical laboratory practice [15]. To verify the reproducibility of RT-qPCR for the detection of gastric juice miRNAs, we used a method similar to that previously reported in stool miRNA detection [16]. The miR-421 levels were detected in a set of gastric juice specimens (n = 10) in two independent experiments with an interval of 1 day. The correlation of their C q values was compared.
Detection of gastric juice CEA levels
As the antigen–antibody binding kinetics are affected by the pH status, and at low pH the measured CEA values are lower than the actual values, gastric juice supernatant was adjusted to pH 7.0 with 0.1 M NaOH before CEA analysis [17]. Gastric juice CEA was measured using enzyme-linked immunosorbent assay CEA kits (KBH Diagnosis, Shanghai, China) with a SpectraMax M5 Microplate Reader (Molecular Device, Inc., Sunnyvale, CA, USA). The cutoff value was 5 ng/ml.
Serological tumor marker analysis
Serum CEA and CA19-9 were measured using an Elecsys 2010 machine (Roche Diagnostics, Basel, Switzerland). The cutoff values were 5 ng/ml and 35 U/ml for CEA and CA19-9, respectively.
Statistical analysis
Data were analyzed using Statistical Program for Social Sciences (SPSS) software 17.0 (SPSS Inc., Chicago, IL, USA). Differences in the miRNA levels between paired biopsy mucosa specimens were determined by the paired t test. The stability of gastric juice miRNAs was analyzed using one-way analysis of variance. Experimental reproducibility was determined by the Pearson correlation test. Correlation statistics were analyzed by the Spearman correlation test. The levels of gastric juice miRNAs between different groups were compared using the Mann–Whitney rank sum test. Positive rate comparisons were made using the χ 2 criterion and Fisher’s exact test. A receiver operating characteristic (ROC) curve was established to evaluate the diagnostic value for differentiating between gastric cancer and benign diseases. The level of significance was taken as P < 0.05. All the graphs were plotted using SigmaPlot 12.0 (Systat Software, Inc., Chicago, IL, USA).
Results
Levels of miR-421 in endoscopic mucosa biopsies
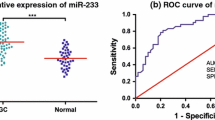
miR-421 is an established oncomiR [7]; therefore, we focused on this miRNA. Since miR-421 has been found upexpressed in surgical removal of gastric cancer tissues and several gastric cell lines [7], here, the levels of miR-421 in gastric mucosa biopsies from gastric cancer specimens (n = 10) were compared with those from adjacent normal specimens. We confirmed that all gastric cancer specimens were upexpressed miR-421 compared with those from adjacent normal specimens (P = 0.001, Fig. 1).
Relative expression levels of miR-421 between mucosa biopsies from gastric cancer specimens and adjacent normal specimens (n = 10). The ΔC q was equal to the difference between the quantification cycle (C q) for miR-421 (target) and that of U6 RNA (reference). The ΔC q value was calculated according to the formula: ΔC q = C q (miR-421) − C q (U6). A lower ΔC q value meant a higher level of miRNA. P values indicated significant differences between paired specimens determined by the paired t test
Determination of miRNA stability in gastric juice
By sequencing the RT-qPCR products of gastric juice miRNAs, we found that the sequences of miR-16 and miR-421 (Fig. 2) were consistent with those from the miRNA database (www.miRBase.org). To test the stability of gastric juice miRNAs, we stored gastric juice at room temperature, 4 °C, and −20 °C for 0, 2, 6, and 12 h. The RT-qPCR results showed that no significant difference of the miR-421 level was found (Fig. 3). This study also demonstrated that gastric juice-based miRNAs were stable with a highly reproducible detection.
Determination of the levels of gastric juice miR-421 from patients with gastric cancer
To search for a new marker for the screening of gastric cancer, the levels of gastric juice miR-421 from gastric cancer patients were compared to those from patients with benign diseases. Interestingly, we found that the gastric juice miR-421 levels in gastric cancer patients were lower than those in patients with benign diseases (Fig. 4a). To evaluate the diagnostic value of miR-421, a ROC curve was constructed. The area under the ROC curve (AUC) of miR-421 was up to 0.767 (95 % CI = 0.684–0.850, P < 0.001; Fig. 4b). The appropriate cutoff value of gastric juice miR-421 for identifying gastric cancer patients was 5.21. The sensitivity and specificity were 71.4 and 71.7 %, respectively. The Youden index of miR-421 was 0.431. In addition, compared with the positive detection rate of gastric juice CEA (69.04 %), miR-421 (71.4 %) was more sensitive (P < 0.001).
Gastric juice miR-421 level among different gastric diseases and ROC analysis. a Gastric juice miR-421 level discriminated patients with gastric cancer (GC) from other benign groups (NMMG normal mucosa or minimal gastritis, AG atrophic gastritis, GU gastric ulcer). The dot line denotes the optimal cutoff values (ΔC q). b ROC curve plotted to discriminate gastric cancer and gastric benign patients
Relationship between gastric juice miR-421 level and clinicopathological factors of patients with gastric cancer
We examined whether the gastric juice miR-421 level correlated with the clinicopathological factors of gastric cancer patients. According to the Borrmann’s classification, advanced gastric cancer patients were classified as four types: from type I to type IV. There was no difference in the gastric juice miR-421 level among them (P = 0.807). Additionally, according to Lauren’s classification, gastric cancer patients were classified into three types: intestinal type, diffuse type, and mixed type. There was also no difference in the gastric juice miR-421 level among them (P = 0.090). Besides, we have not found any difference between the gastric juice miR-421 level with Helicobacter pylori status (P = 0.065), tumor location (P = 0.693), tissue differentiation (P = 0.555), and tumor size (P = 0.098).
Observation of the early diagnostic value of using gastric juice miR-421 as a marker
Finally, we observed whether gastric juice miRNAs could be used as an early maker of gastric cancer. Based on the cutoff value from the ROC curves (Fig. 4b), we compared the positive rates of early gastric cancer and advanced gastric cancer. When gastric juice miR-421 was used as a biomarker, the positive detection rates of early and advanced gastric cancer patients were 71.4 % (5/7) and 71.4 % (25/35), respectively (Table 2). However, when serum CEA was used, the positive detection rates of early and advanced gastric cancer patients were only 14.3 % (1/7) and 40.0 % (14/35), respectively. When gastric juice CEA was used, the positive detection rates of early and advanced gastric cancer patients were only 42.8 % (3/7) and 74.3 % (26/35), respectively. For the detection of early gastric cancer, the combined use of gastric juice miR-421 and juice CEA showed a remarkable improvement compared with the use of serum CEA alone (P = 0.029).
Discussion
The detection of molecular markers in gastric juice samples is a potential strategy for gastric cancer screening. Gastric juice can be easily obtained during endoscopic examination. It has the advantage of being a potential surrogate material for the molecular genetic diagnosis of gastric cancer. Gastric juice only consists in the upper digestive system. Therefore, the use of gastric juice as a gastric cancer diagnosis material has higher specificity compared with serum/plasma. Some studies demonstrated that gastric juice peptides and the intrinsic fluorescence spectrum of gastric juice may be used in the diagnosis of gastric cancer [17, 18]. However, the stability of peptides in gastric juice is unknown and the fluorescent substances in gastric juice are easily quenched.
Previous studies from our group showed that altered expression levels of noncoding RNAs can be used as sensitive biomarkers for detecting gastric cancer [6, 14, 19, 20]. We also found that miR-421 was upexpressed in gastric cancer tissues [7]. Especially, we found that miR-421 might be involved in the early stage of stomach carcinogenesis and was a functional marker of circulating tumor cells in gastric cancer patients [7, 20]. In this study, we confirmed that biopsy mucosa miR-421 level in gastric cancer was higher than that of the adjacent tissues (Fig. 1) and that miR-421 might be an early diagnostic marker of gastric cancer (Table 2).
Although miRNAs have been proposed as tissue-based markers for predicting the outcomes of gastric cancer, the analysis of gastric juice miRNA poses several potential challenges. First, RNase will degenerate RNA. Second, in clinical practice, there might be a time gap between collecting gastric juice from patients and processing the sample for analysis [21]. Thus, it is imperative to investigate whether miRNA is stable in gastric juice and the feasibility of detection of miRNA in gastric juice for the diagnosis of gastric cancer. Using RT-qPCR and sequencing (Fig. 2), we found that gastric juice miR-421 can be detected in a remarkably stable form. There was no significant change within 12 h storage in ambient, 4 °C, or −20 °C (Fig. 3). This can be fully able to meet the clinical test requirements. The results presented here will lay the basis and rationale of using miRNAs as gastric juice-based biomarkers for gastric cancer screening.
Evidences suggest that miRNAs are often aberrantly expressed in human malignancies and can function as either oncogenes or tumor suppressors [22, 23]. Recent reports have shown that miR-421 may be an oncomiR in several human cancers including pancreatic cancer and gastric cancer [7, 24]. Our previous study demonstrated that miR-421 was upexpressed in gastric cancer tissues compared with noncancerous tissues [25]. New evidences indicated that miR-421 was involved in the regulation of tumor-associated nuclear receptors such as estrogen receptor, progesterone receptor, glucocorticoid receptor, liver X receptor, and mineralocorticoid receptor [26]. Zhang et al. [26] showed that farnesoid X receptor (FXR) was a novel target of miR-421 in hepatocellular carcinoma cells, and the downregulation of FXR may be a new oncogenic mechanism of miR-421. Another study demonstrated that miR-421 functions as an oncomiR in biliary tract cancer (BTC) by targeting FXR [27]. FXR has been reported to be a tumor suppressor in hepatocellular carcinoma and breast cancer. The ectopic expression of miR-421 significantly decreased FXR protein concentration in BTC cells and promoted cell proliferation, colony formation, and migration in vitro [27]. Our study showed that the transfection of an miR-421 inhibitor significantly suppressed tumor growth in vivo [20]. miR-421 might play a crucial role in carcinogenesis, and it may be used as a biomarker for monitoring circulating tumor cells in patients with gastric cancer [20].
Our previous study had shown that peripheral blood miR-106a and miR-17 had clinical value in distinguishing gastric cancer patients from healthy controls, with AUC values of 0.684 and 0.743, respectively [6]. Compared with these blood-based miRNAs, gastric juice-based miR-421 had more reliable diagnosis values, with AUC value of 0.767 (Fig. 4b). Its sensitivity and specificity were 71.4 and 71.7 %, respectively (Fig. 4). The Youden index is widely used to evaluate the effectiveness of a biomarker. We found that the Youden index of miR-421 in gastric juice was 0.431. In summary, the detection of gastric juice miR-421may be a novel method for screening gastric cancer.
References
Nardone G, Compare D. Epigenetic alterations due to diet and Helicobacter pylori infection in gastric carcinogenesis. Expert Rev Gastroenterol Hepatol. 2008;2:243–8.
Sapari NS, Loh M, Vaithilingam A, Soong R. Clinical potential of DNA methylation in gastric cancer: a meta-analysis. PLoS One. 2012;7:e36275.
Halon A, Donizy P, Biecek P, Rudno-Rudzinska J, Kielan W, Matkowski R. HER-2 expression in immunohistochemistry has no prognostic significance in gastric cancer patients. ScientificWorldJournal. 2012;2012:941259.
Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–40.
Tsujiura M, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174–9.
Zhou H, et al. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using microRNA as a marker. J Mol Med. 2010;88:709–17.
Jiang Z, et al. Increased expression of miR-421 in human gastric carcinoma and its clinical association. J Gastroenterol. 2010;45:17–23.
Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717:85–90.
Tzimagiorgis G, Michailidou EZ, Kritis A, Markopoulos AK, Kouidou S. Recovering circulating extracellular or cell-free RNA from bodily fluids. Cancer Epidemiol. 2011;35:580–9.
Weber JA, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer. 1998;1:10–24.
Li C, et al. Macroscopic Borrmann type as a simple prognostic indicator in patients with advanced gastric cancer. Oncology. 2009;77:197–204.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Cui L, et al. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clin Biochem. 2011;44:1050–7.
McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011;57:833–40.
Wu CW, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739–45.
Hsu PI, et al. Diagnosis of gastric malignancy using gastric juice alpha1-antitrypsin. Cancer Epidemiol Biomarkers Prev. 2010;19:405–11.
Zhou LY, et al. The intrinsic fluorescence spectrum of dilute gastric juice as a novel diagnostic tool for gastric cancer. J Dig Dis. 2011;12:279–85.
Xiao B, et al. Detection of miR-106a in gastric carcinoma and its clinical significance. Clin Chim Acta. 2009;400:97–102.
Zhou H, et al. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers. 2012;17:104–10.
Xie Y, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67:170–6.
O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–43.
Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467–77.
Hao J, Zhang S, Zhou Y, Liu C, Hu X, Shao C. MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer. Biochem Biophys Res Commun. 2011;406:552–7.
Guo J, et al. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652–7.
Zhang Y, et al. Downregulation of human farnesoid X receptor by miR-421 promotes proliferation and migration of hepatocellular carcinoma cells. Mol Cancer Res. 2012;10:516–22.
Zhong XY, et al. MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene. 2012;493:44–51.
Acknowledgments
This work was supported by the Ningbo Medical Research Project (no. 2010B06), the Zhejiang Provincial Research Project (nos. 2010C33112 and 2012C23127), the National Natural Science Foundation of China (no. 81171660), the Natural Science Foundation of Ningbo (no. 2010A610044 and 2012A610207), the Scientific Innovation Team Project of Ningbo (no. 2011B82014), the Project of Key Disciplines in Ningbo (no. XKL11D2128), the Excellent Dissertation Fund of Ningbo University (no. PY20110020), and the K. C. Wong Magna Fund in Ningbo University.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Cui, L., Ye, G. et al. Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumor Biol. 33, 2349–2355 (2012). https://doi.org/10.1007/s13277-012-0497-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0497-x