Abstract
The purpose of this study was to investigate the expression of autophagy-related proteins Beclin 1 and LC3 in cervical normal epithelial cells and squamous cancer cells, and to evaluate the prognostic significance of Beclin 1 and LC3 expression in FIGO stage I–II cervical squamous cell carcinoma. The immunohistochemical expression of Beclin 1 and LC3 were evaluated in 26 formalin-fixed paraffin-embedded cervical normal tissue samples and 50 tumor samples of FIGO stage I–II cervical squamous cell carcinoma, respectively. Correlations with clinicopathologic characteristics were determined by Chi-square test. The prognostic impact of Beclin 1 and LC3 expression in regard to overall survival was determined by Kaplan–Meier method. Cervical normal squamous epithelial cells and cancer cells expressed high Beclin 1 immunoreactivity in 96.2 % (25/26) and 28.0 % (14/50) of patients (p = 0.000), and expressed high LC3 immunoreactivity in 76.9 % (20/26) and 26.0 % (13/50) of patients, respectively (p = 0.000). The expression of both Beclin 1 and LC3 were not associated with age, FIGO stage, pathologic differentiation, and lymph node metastasis. The 3-year overall survival (OS) rates with low and high expressions of Beclin 1 were 72.2 and 100.0 %, respectively (p = 0.034). The 3-year OS rates with low and high expressions of LC3 were 75.7 and 92.3 %, respectively (p = 0.224). These results show that expression level of both Beclin-1 and LC3 were significantly lower in cervical squamous cancer cells than normal squamous epithelial cells. The expression of Beclin 1 and LC3 may have prognostic significance in early stage cervical squamous cell carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is second only to breast cancer in women as the most common gynecologic malignancies, and it remains one of the most important causes of mortality in women worldwide [1]. More than 90 % of cervical cancer is squamous cell carcinoma in pathologic classification. The radical surgery or radiotherapy can cure the majority patients of FIGO stage I–II cervical cancer. Komaki at al. [2] reported that the 5-year overall survival (OS) rates were 89.4 and 79.3 % in patients with FIGO stage I and IIcervical cancer, respectively. The traditional pathological factors, such as lymph node metastasis, parametrial invasion, positive tumor margins, and deep stromal and lymphovascular invasion, are currently considered important prognostic factors for survival [3]. However, the accuracy of these prognostic factors is still controversial, except for the presence of lymph node metastasis [4, 5]. Therefore, it seems reasonable to develop novel markers to accurately predict the prognosis of patients with cervical cancer.
Autophagy has recently emerged as an important area in cancer research, with the main interest focusing on tumor growth and progression, prognosis, and potential therapy [6, 7]. Autophagy is an intracellular pathway for the degradation of long-lived proteins and damaged organelles [8], and may be considered a form of non-apoptotic programmed cell death, the so-called “typeII” or “autophagic” cell death [9]. Up to now, approximately thirty specific genes regulating autophagy has been discovered in yeasts so far, with 16 homologues in man [10]. Beclin 1 and LC3 (microtubule-associated protein 1A/1B-light chain 3) genes play a pivotal role in mammalian autophagy [11]. Beclin 1 is involved in both the signaling pathway activating autophagy and in the initial step of autophagosome formation [11, 12]. LC3 comprises both a soluble LC3 I and a lapidated form, called LC3II. LC3II correlates with autophagy, being recruited into autophagosomes. Various types of stressors upregulate LC3 and promote the conjugation of its cytosolic form, LC3 I to phosphatidylethanolamine, to constitute the autophagosome-specific LC3II, which is so far considered the most reliable marker of autophagy [12, 13].
To our knowledge, Beclin 1 and LC3 are widely expressed in solid tumors. Moreover, biologic significance and clinical impact of Beclin 1 and LC3 expression in these cancers seem to be associated with tumor type [8, 9, 12, 14–17]. However, few studies have reported the expression level of Beclin 1 and LC3 and its prognostic significance in cervical cancer, especially in FIGO stage I–II cervical squamous cell carcinoma. Therefore, we set out to investigate the expression of Beclin 1 and LC3 in cervical normal squamous epithelial cells and cancer cells, and to evaluate the prognostic significance of Beclin 1 and LC3 expression in FIGO stage I–II cervical squamous cell carcinoma.
Materials and methods
Patients and specimen selection
The paraffin-embedded postoperative tissue samples were obtained from the archives of the Department of Pathology, the Second Affiliated Hospital of Soochow University, between January 2007 and December 2008. We retrieved retrospectively 50 tumor samples from patients with FIGO stage I–II cervical squamous cell carcinoma and 26 samples with cervical normal tissue, respectively. Approval for current project was obtained from the local ethics committee together with written informed consent from each patient.
The main characteristics of 50 patients with FIGO stage I–II were summarized in Table 1. Ages of all patients in this study range from 20 to 68 years (median, 42 years). According to FIGO stage, there enrolled 36 patients with stage I and 14 patients with stage II. All patients underwent radical surgery. Seven patients with pelvic lymph node metastasis received radiotherapy. Systemic adjuvant treatment was administered to seven patients.
Immunohistochemistry
Three serial slides, each 3-um thick, were cut from paraffin-embedded tissue. One slide was used to give HE staining again. Immunohistochemical staining was performed on the other two slides using the two-step procedure. The anti-human Beclin 1 rabbit monoclonal antibody (ab51031) (Abcam, Cambridge, MA; diluted 1: 100) and anti-human LC3B rabbit monoclonal antibody (ab63817) (Abcam, Cambridge, MA; diluted 1:100) were used. After deparaffinization and hydration, the slides were subjected to antigen retrieval by pressure-cooking for 30 min. Endogenous peroxidase activity was neutralized using peroxide block placement on the slides for 15 min at room temperature. The slides were then incubated with anti-Beclin 1 and anti-LC3B monoclonal antibody for 30 min at 4°C, respectively. This was followed by incubation with peroxidase-conjugated polymer (ChemMate EnVision/HRP; Gene Tech, Shanghai, China) for 30 min at room temperature. The chromogen reaction was developed in 3,3′-diaminobenzidine (Gene Tech, Shanghai, China) tetrahydrochloride for 10 min. Finally, hematoxylin was used as a light nuclear counterstain. The negative control used was an IgG2b isotype antibody (Dako), ensuring the same concentration of immunoglobins as for the anti-Beclin 1 and anti-LC3B.
Assessment of Beclin 1 and LC3 expression
All slides were evaluated independently by two experienced pathologists (Li F and Zhang Y). The intensity of staining was recorded as strong, weak, and negative. According to Giatromanolaki’s description, only strong staining cell was regarded as positive cell [16]. The extent of staining was expressed as a percentage of positive cells to total and was recorded after examining all optical fields at ×200 magnification. The mean value was used to score for all samples. The percentages of staining-positive cells were scored into four categories: 1 (0–24 %), 2 (25–49 %), 3 (50–74 %), and 4 (75–100 %). The scoring pattern was defined as follows: 1–2, low expression (<50 % positive cells); 3–4, high expression (≥50 % positive cells).
Statistical analysis
The relationship between Bcelin 1, LC3 expression, and clincopathologic characteristics was examined with Chi-square test. OS rates were performed by the Kaplan–Meier method and log-rank test. Overall survival time was defined from the day of surgery to the day of death or last follow-up. For all tests, a two-sided p < 0.05 was considered significant.
Results
The expression of Beclin 1 and LC3 in cervical normal squamous epithelial cells and cancer cells
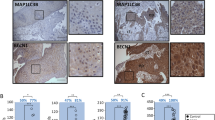
The expression of Beclin 1 and LC3 was observed mainly in the cytoplasm within cervical normal squamous epithelial cells and cancer cells. In addition, the nucleus in some of normal epithelial or cancer cells also have LC3 expression. Stone-like pattern of LC3 expression, previously described in breast carcinoma, lung cancer. and endometrial adenocarcinoma [8, 9], was not observed in both cervical normal squamous epithelial cells and cancer cells. The mean percentages of Beclin 1 expression were 77.4 % (range 5–95 %) and 33.7 % (1–95 %) in cervical normal squamous epithelial cells and cancer cells, respectively (Fig. 1). Cervical normal squamous epithelial cells and cancer cells expressed high Beclin 1 immunoreactivity in 96.2 % (25/26) and 28.0 % (14/50) of patients, respectively (p = 0.000, Table 2).
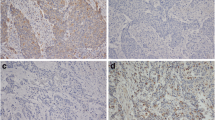
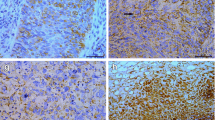
Expression of Beclin 1 and LC3 by immunohistochemistry in cervical normal squamous epithelial cells and cancer cells (magnification ×1,000). a HE staining of cervical normal squamous epithelium; b HE staining of cervical squamous cell cancer; c High expression of Beclin 1 in cervical normal squamous epithelial cells; d High expression of Beclin 1 in cervical squamous cancer cells; e Low expression of Beclin 1 in cervical squamous cancer cells; f High expression of LC3 in cervical normal squamous epithelial cells; g High expression of LC3 in cervical squamous cancer cells; h Low expression of LC3 in cervical squamous cancer cells
The mean percentages of LC3 expression were 68.4 % (range 8–97 %) and 32.7 % (range 1–94 %) in cervical normal squamous epithelial cells and cancer cells, respectively (Fig. 1). Cervical normal squamous epithelial cells and cancer cells expressed high LC3 immunoreactivity in 76.9 % (20/26) and 26.0 % (13/50) patients, respectively (p = 0.000, Table 2).
The association of Beclin 1 and LC3 expression with clincopathologic characteristics
The expression level of Beclin 1 and LC3 in 50 patients with FIGO stage I–II cervical squamous cell carcinoma with respect to several standard clinicopathologic characteristics were detailed in Table 3. The statistical results demonstrated that there was no significant association of Beclin 1 and LC3 expression with any of the following parameters: age, FIGO stage, pathologic differentiation, and lymph node metastasis (Table 3). There was also no significant correlation between Beclin 1 and LC3 expression (χ2 = 0.954, p = 0.329, Chi-squared test).
The correlation between Beclin 1 and LC3 expression and overall survival
The average duration of follow-up was 41 months (range, 2–57 months). The Kaplan–Meier plots showed that the 3-year OS rate of all patients was 80.0 %. The 3-year OS rates with low and high expression of Beclin 1 were 72.2 and 100.0 %, respectively (χ2 = 4.49, p = 0.034; Fig. 2a). The 3-year OS rates with low and high expression of LC3 were 75.7 and 92.3 %, respectively (χ2 = 1.48, p = 0.224; Fig. 2b).
Discussion
The expression level of the autophagy-related protein Beclin 1 is frequently lower in malignant cells than their normal counterparts, such as malignant melanoma, lung cancer, hepatocellular carcinoma, and gastric carcinoma [8, 12, 14, 15, 19]. In contrast, LC3 has different expression level in different tumor types. The studies reported that LC3 expression was either decreased in brain and ovarian cancer [20, 21] or increased in esophageal and gastrointestinal cancers [22]. In the present study, our results revealed that the expression level of both Beclin-1 and LC3 were significantly downregulated in cervical squamous cancer cells compared to normal squamous epithelial cells. Moreover, expression of Beclin 1 and LC3 were mainly located in the cytoplasm of both cervical normal squamous epithelial cells and cancer cells by using immunohistochemistry method. In addition, the nucleus in some of normal or cancer cells also expressed LC3.
Emerging evidence has demonstrated that autophagy was involved in the pathogenesis of most of common cancers [18, 19]. However, biologic significance and clinical impact of the variations in Beclin 1 and LC3 in cancer seem to be associated with tumor type. Beclin 1 has been identified as a haplo-insufficient tumor-suppressor gene, which plays important role in autophagy, apoptosis, and differentiation of cancer [15, 23]. Several research have shown that decreased expression of Beclin 1 was correlated with tumor progression, and with a lower survival rate in gastric, esophageal, colorectal, hepatocellular, and breast cancer [12, 15]. Chen et al. [15] found that decreased expression of Beclin 1 in gastric cancer cells was significantly associated with poor differentiation, nodal and distant metastasis, advanced TNM stage, and tumor relapse, and with shorter survival. Similarly, Beclin 1 expression was related significantly to depth of invasion, lymph node metastasis, and clinical stage in esophageal squamous cell carcinoma. Furthermore, the survival rate of Beclin 1-positive group was better than that of the Beclin 1-negative group [18]. However, Giatromanolaki et al. [16] found that increased expression of Beclin 1 was associated with high tumor grade, high myometrial invasion, and a poor 5-year survival. The previous research results suggested that Beclin 1 might be a biphasic protein in cancer, and both low and high Beclin 1 expression might be related to tumor progression and prognosis [8, 24]. In the present study, our results demonstrated that high Beclin 1 expression group had a higher 3-year overall survival rate than that of the low Beclin 1 expression group in FIGO stage I–IIcervical squamous cell carcinoma, evidenced by univariate analysis (χ2 = 4.49, p = 0.034; Fig. 2a). However, there was no significant association of Beclin 1 expression with clincopathologic characteristics, including age, FIGO stage, pathologic differentiation, and lymph node metastasis.
With respect to clinical significance of LC3 expression in cancer, its has different impact in different tumors. Fujii et al. [25] found that high expression of LC3 at the peripheral areas of pancreatic tumors was associated with poor overall survival and shorter disease-free periods. There was also a relationship with tumor size and tumor necrosis. Similarly, Karpathiou et al. [9] reported that high LC3 expression was strongly correlated with a reduction of the overall median survival in non-small cell lung carcinoma. In cutaneous malignant melanomas, Sivridis et al. [8] revealed that LC3 expression was not related to either histopathological parameters or prognosis. However, Cheng et al. [26] demonstrated that low LC3 expression was a low risk factor for progressive-free survival in cervical squamous cell carcinoma. In the present study, we also found that the patients whose tumor had low LC3 expression had an unfavorable trend of 3-year overall survival rate in FIGO stage I–IIcervical squamous cell carcinoma (Fig. 2b).
In conclusion, we found that expression level of both Beclin-1 and LC3 were significantly lower in cervical squamous cancer cells than normal squamous epithelial cells. The expression of Beclin 1 and LC3 may have prognostic significance in early stage cervical squamous cell carcinoma. However, this retrospective study is potentially limited by the relatively small numbers of patients, and the results may not be the most representative for the whole cases. Therefore, more and larger studies are required.
References
Sun Y, Liu JH, Jin L, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010;294:204–10.
Komaki R, Brickner TJ, Hanlon AL, et al. Long-term results of treatment of cervical carcinoma in the United States in 1973, 1978 and 1983: patterns of care study (PCS). Int J Radiat Oncol Biol Phys. 1995;31:973–82.
Jermal A, Siegel R, Ward E. Cancer statistics. CA Cancer J Clin. 2009;59:225–49.
Lee YY, Choi CH, Kim CJ, et al. The prognostic significance of the SUVmax (maximum standardized uptake value for F-18 fluorodeoxyglucose) of the cervical tumor in PET imaging for early cervical cancer: preliminary results. Gynecol Oncol. 2009;115:65–8.
Kim BS, Kim IJ, Kim SJ, et al. The prognostic value of the metabolic tumor volume in FIGO stage IA to IIB cervical cancer for tumor recurrence: measured by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011;45:36–42.
Eisenberg-Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis. 2009;14:376–91.
Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Autophagy. 2009;5:442–50.
Sivridis E, Koukourakis MI, Mendrinos SE, et al. Beclin-1 and LC3A expression in cutaneous malignant melanomas: a biphasic survival pattern for beclin-1. Melanoma Res. 2011;21:188–95.
Karpathiou G, Sivridis E, Koukourakis MI, et al. Light-chain 3A autophagic activity and prognostic significance in non-small cell lung carcinomas. Chest. 2011;140:127–34.
Cheong H, Klionsky DJ. Biochemical methods to monitor autophagy-related processes in yeast. Methods Enzymol. 2008;451:1–26.
Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–73.
Miracco C, Meng GC, Franchi A, et al. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Human Pathol. 2010;41:503–12.
Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in high eukaryotes. Autophagy. 2008;4:151–75.
Ding ZB, Shi YH, Zhou J, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–75.
Chen YB, Hou JH, Feng XY, et al. Decreased expression of Beclin 1 correlates with a metastatic phenotypic feature and adverse prognosis of gastric carcinomas. J Surg Oncol. 2012;105:542–7.
Giatromanolaki A, Koukourakis MI, Koutsopoulos A, et al. High Beclin 1 expression defines a poor prognosis in endometrial adenocarcinomas. Gynecol Oncol. 2011;123:147–51.
Sivridis E, Koukourakis MI, Zois CE, et al. LC3A-positive light microscopy detected patterns of autophagy and prognosis in operable breast carcinomas. Am J Pathol. 2010;176:2477–89.
Chen Y, Lu Y, Lu C, et al. Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1α expression. Pathol Oncol Res. 2009;15:487–93.
Jiang ZF, Shao LJ, Wang WM, et al. Decreased expression of Beclin-1 and LC3 in the human lung cancer. Mol Biol Rep. 2012;39:259–67.
Aoki H, Kondo Y, Aldape K, et al. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy. 2008;4:467–75.
Shen Y, Li DD, Wang LL, et al. Decreased expression of autophagy-related proteins in malignant epithelial ovarian cancer. Autophagy. 2008;16:1067–8.
Yoshioka A, Miyata H, Doki Y, et al. LC3, an autophagosome marker, is highly expressed in gastrointestinal cancer. Int J Oncol. 2008;33:461–8.
Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–96.
Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Beclin 1 over- and underexpression in colorectal cancer: distinct patterns relate to prognosis and tumor hypoxia. Br J Cancer. 2010;103:1209–14.
Fujii S, Mitsunaga S, Yamazaki M, et al. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 2008;99:1813–9.
Cheng HY, Zhang YN, Wu QL, et al. Expression and clinical significance of LC3 in cervical cancer tissues. Chin J Cancer Prev Treat. 2011;18:1247–51.
Acknowledgments
This study was supported by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions and Jiangsu Province’s Key Medical Department in 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, W., Pan, X., Li, F. et al. Expression of Beclin 1 and LC3 in FIGO stage I–II cervical squamous cell carcinoma and relationship to survival. Tumor Biol. 33, 1653–1659 (2012). https://doi.org/10.1007/s13277-012-0421-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0421-4