Abstract
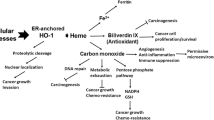
Heme oxygenase-1 (HO-1) catabolizes heme into carbon monoxide, biliverdin, and free iron which mediate its protective effect against oxidative stress. The aim of the present study was to determine the expression level and activity of HO-1 in Korean colon cancer tissues and cell lines. HO-1 protein expression was higher (>1.5-fold) in tumor tissues than in adjacent normal tissues in 14 of 20 colon cancer patients, and HO-1 protein expression was closely correlated with HO-1 enzyme activity in cancer tissues. Immunohistochemical data confirmed that HO-1 protein was expressed at a higher level in colon cancer tissues than in normal mucosa. Furthermore, HO-1 mRNA and protein expression and enzyme activity were higher in the colon cancer cell lines Caco-2, SNU-407, SNU-1033, HT-29, and SW-403 than in the normal fetal human colon cell line FHC. Treatment with the HO-1 inhibitor zinc protoporphyrin decreased the viability of colon cancer cell lines. These data indicate that HO-1 may serve as a clinically useful biomarker of colon cancer and as a target for anticolon cancer drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly one million cases of colon cancer are diagnosed worldwide each year, and an increasing trend in the incidence of colon cancer in Asian countries, including Korea, has been reported in recent years [1, 2]. Recent epidemiologic studies have indicated that a western style diet is closely associated with a high incidence of colon cancer [3, 4]. The presence of iron ions in the colon increases the production of reactive oxygen species (ROS) from peroxides via the Fenton reaction, and the prooxidant environment of the colon may contribute to greater cancer susceptibility [5, 6]. However, the mechanism by which colon cancers acquire resistance to ROS-induced cell death is not well understood. Therefore, a precise understanding of the relationship between colon cancer and oxidative stress-induced molecules is very important. Heme oxygenase-1 (HO-1) represents a prime cellular defense mechanism against oxidative stress via the antioxidant function of its catalytic products including bilirubin, carbon monoxide, and concomitant induction of iron-sequestering ferritin [7, 8]. HO-1 expression is induced in response to oxidative stresses caused by various chemical or physiological factors in cells and tissues, reflecting the main role of this enzyme in the protection against oxidative injury [9–11]. In addition, HO-1 regulates cell proliferation, modulates the inflammatory response, and facilitates angiogenesis [12, 13]. HO-1 is expressed at higher levels in oral squamous carcinoma, pancreatic cancer, hepatoma, and colon cancer than in surrounding normal tissue [14–17], suggesting that highly HO-expressed cancer cells offer a growth advantage and provide cellular resistance against ROS-mediated anticancer therapy [18, 19]. A recent research has identified that HO-1 could be considered as a biomarker and potential therapeutic target for advanced prostate cancer [20]. The present study investigates the involvement of HO-1 in Korean human colon cancers and colon cancer cell lines.
Materials and methods
Reagents
Primary rabbit polyclonal HO-1 antibody was purchased from Calbiochem (San Diego, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and zinc protoporphyrin (ZnPP) were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
Patient tissues
Twenty tissue samples from human colon cancer patients were obtained from Jeju National University Hospital (Jeju, Republic of Korea). This study was approved by the institutional review board for ethics of Jeju National University Hospital (IRB:2011-38) and by informed written consent from patients.
Cell culture
The human colon cancer cell lines Caco-2, SNU-407, SNU-1033, HT-29, and SW-403 were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea), and the normal fetal human colon cell line FHC was purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were maintained at 37°C in an incubator with a humidified atmosphere of 5% CO2. SNU-407, SNU-1033, HT-29, and SW-403 cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), streptomycin (100 μg/ml), and penicillin (100 units/ml). Caco-2 cells were cultured in MEM medium containing 10% heat-inactivated FCS, streptomycin (100 μg/ml), and penicillin (100 units/ml). Human colon FHC cells were cultured in a 1:1 mixture of Ham’s F12 and DMEM containing HEPES (25 mM), cholera toxin (10 ng/ml; Calbiochem-Novabiochem Corp., La Jolla, CA, USA), insulin (5 μg/ml), transferrin (5 μg/ml), hydrocortisone (100 ng/ml), and 10% FCS.
Western blot analysis
Cells were lysed on ice for 30 min in 100 μl of lysis buffer (120 mM NaCl, 40 mM Tris (pH 8), 0.1% NP-40) and centrifuged at 13,000×g for 15 min. Supernatants were collected, and the protein concentration was determined. Aliquots containing 40 μg of protein were boiled for 5 min and electrophoresed on a 10% SDS-polyacrylamide gel. Proteins were transferred onto nitrocellulose membranes, which were subsequently incubated with HO-1 antibody overnight at 4°C. The membranes were further incubated with secondary anti-immunoglobulin-G-horseradish peroxidase conjugates (Pierce, Rockford, IL, USA). Protein bands were detected using an enhanced chemiluminescence western blotting detection kit (Amersham, Little Chalfont, Buckinghamshire, UK).
HO-1 activity
HO-1 enzyme activity in colon tissues was measured as described previously [20]. Briefly, colon tissues or cells were homogenized in 0.5 ml ice-cold 0.25 M sucrose solution containing 50 mM potassium phosphate buffer (pH 7.4). Homogenates were centrifuged at 200×g for 10 min, and the supernatants were further centrifuged at 15,000×g for 60 min. The pellet was then resuspended in 50 mM potassium phosphate buffer (pH 7.4), and the amount of protein was determined. The reaction mixture (200 μl) containing 0.2 mM of the substrate hemin, 500 μg/ml of cell lysate, 0.5 mg/ml rat liver cytosol as a source of biliverdin reductase, 0.2 mM MgCl2, 2 mM glucose-6-phosphate, 1 U/ml glucose-6-phosphate dehydrogenase, 1 mM NADPH, and 50 mM potassium phosphate buffer (pH 7.4) was incubated at 37°C for 2 h. The reaction was stopped with 0.6 ml of chloroform and, after extraction, the chloroform layer was measured spectrophotometrically at 464 nm. HO-1 activity is expressed as nanomole of bilirubin per milligram of protein.
Immunohistochemistry for HO-1
Tissue specimens were fixed in 10% buffered formalin and embedded in paraffin. The same paraffin-embedded tissues as those used for the original hematoxylin-and-eosin-stained sections were used for immunohistochemistry. Tissue blocks were cut into 3-μm-thick slices and mounted on Superfrost Plus-coated slides. Sections were then deparaffinized in xylene and rehydrated through a graded ethanol series. A standard immunohistochemical technique was performed using a Ventana BenchMark XT immunostainer with HO-1 antibody at a dilution of 1:100. Antigen retrieval on the immunostainer was done for 30 min. The HO-1 antibody was incubated at 37°C for 60 min, and 3,3′-diaminobenzidine was used as a chromogen; slides were counterstained with hematoxylin prior to mounting. All staining procedures were performed according to the manufacturer’s recommendations. Intramucosal mononuclear cells in the tissue samples served as internal positive controls based on their strong staining intensity. Negative controls for nonspecific binding were obtained by omitting the primary antibody. The immunohistochemical slides were evaluated and interpreted by a pathologist.
Reverse transcription polymerase chain reaction
Total RNA was isolated using Trizol (GibcoBRL, Grand Island, NY, USA). Reverse transcription polymerase chain reaction (RT-PCR) was performed as described previously [21]. PCR conditions for HO-1 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 35 cycles of 94°C for 45 s, 53°C for 45 s, and 72°C for 60 s. The primer pairs (Bionics, Seoul, Republic of Korea) were as follows (forward and reverse, respectively): HO-1, 5′-GAGAATGCTGAGTTCATG-3′ and 5′-ATGTTGAGCAGGAAGGC-3′; and GAPDH, 5′-AAGGTCGGAGTCAACGGATTT-3′ and 5′-GCAGTGAGGGTCTCTCTCCT-3′. Amplified products were resolved by 1% agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light.
Quantitative real-time PCR
A total of 2 μg RNA was used in the first strand cDNA synthesis using the SuperScript kit (Invitrogen, Carlsbad, CA, USA). The cDNA was then diluted to a volume of 50 μl, of which 1 μl was used for amplification. Quantitative real-time PCR was performed with 2× SYBR Green Master Mix (Invitrogen, Carlsbad, CA, USA) and 900 nM primers for human HO-1 and 18 s RNA. The real-time PCR machine iQ5 (Bio-Rad Laboratories, Hercules, CA, USA) was used to amplify and detect the transcripts of interest. The real-time PCR parameters used were as follows: 50°C for 2 min, Taq activation at 95°C for 10 min, and 40 cycles of 95°C for 15 s and 55°C for 1 min. Reactions were performed in duplicate, and specificity was monitored using melting curve analysis after cycling. Primers were designed using Bioneer (Seoul, Republic of Korea). The primers used were as follows (3′–5′): 18 s RNA, 5′-CAGCCACCCCAGATTGAGCA-3′ and 5'-TAGTAGCGACGGGCGGTGTG-3′; and HO-1, 5′-TGAGGAACTTTCAGAAGGGCC-3′ and 5′-TGTTGCGCTCAATCCCTCC-3′. Data were analyzed using the iQ5 software package. The relative standard curve method was used to calculate relative mRNA abundance between samples, which was then presented as mean ± standard error of the mean of gene expression. All data were first tested for normality, and data with nonnormal distribution were subjected to square root transformation prior to statistical analyses. The comparative cycle threshold (Ct) method was used to calculate the relative changes in gene expression in the iQ5 real-time PCR system. The 2-delta-delta Ct value was calculated after 18 s RNA normalization [22].
Quantification of HO-1 concentration
Cultured colon cancer cells were homogenized in RIPA buffer and 10 mM PMSF and centrifuged at 15,000×g for 15 min at 4°C to remove tissue debris. HO-1 production was quantified using a human HO-1 ELISA kit (Assay Designs Inc., Ann Arbor, MI, USA) following the manufacturer’s protocol. Briefly, cell lysates were added to the coated plates and incubated for 1 h at room temperature with antihuman HO-1 antibody. After several washes, the plates were incubated with antirabbit IgG-horseradish peroxidase conjugate for 30 min and then treated with tetramethylbenzidine, a substrate for peroxidase. The reaction was stopped after 15 min, and the optical density at 450 nm was read using a microplate reader. HO-1 concentration is expressed as nanogram per milliliter.
Cell viability
The effect of the HO-1 inhibitor ZnPP on the viability of human colon cancer cells was determined using the MTT assay, which is based on the reduction of a tetrazolium salt by mitochondrial dehydrogenase in viable cells [23]. Cells were treated with ZnPP at a concentration of 10 μM, and 48 h later, 50 μl of the MTT stock solution (2 mg/ml) was added to each well to obtain a total reaction volume of 200 μl. After incubation for 4 h, the plate was centrifuged at 800×g for 5 min, followed by aspiration of the supernatants. Formazan crystals present in each well were dissolved in 150 μl of dimethyl sulfoxide, and the absorbance at 540 nm was measured on a scanning multi-well spectrophotometer.
Statistical analysis
The results were subjected to analysis of variance using Tukey’s test to analyze differences. The p < 0.05 was considered statistically significant.
Results
HO-1 protein expression and enzyme activity in normal and colon carcinoma tissues
HO-1 protein expression level and enzyme activity were assessed in colon cancer tissues from 20 patients using western blotting and enzyme activity assay. HO-1 protein expression was detected in 70% (14/20) of tumor tissues from colon cancer patients, and the expression level was more than 1.5-fold higher than that in the corresponding normal tissues (Fig. 1a). HO-1 enzyme activity was higher in tumor tissues than in normal tissues from colon cancer patients, almost in agreement with the western blot data (Fig. 1b). Immunohistochemical analysis detected the faint expression of HO-1 protein in normal colonic epithelial cells (Fig. 1c(b)). The carcinoma cells showed diffusely increased expression in the cell cytoplasm (Fig. 1c(c–e)), while some of which reveal strong positivity (Fig. 1c(f)). Intramucosal mononuclear cells showed strong immunoreactivity in all specimens. These results show that HO-1 is highly expressed in tumor tissues from Korean colon cancer patients, suggesting that this protein could be used as a biomarker for cancer detection.
Protein level and activity of HO-1 in colon cancer patients. a The expression of the HO-1 protein was detected using an HO-1-specific antibody. b HO-1 enzyme activity in colon tissues was measured as described in the “Materials and methods”. HO-1 activity is expressed as nanomole of bilirubin per milligram of protein. c Immunohistochemistry for HO-1 in colon carcinoma tissues showed (a) hematoxylin–eosin staining (×100), (b) positive-stained mucosal histocytes and faint expression in normal epithelial cells (×200), (c) transition area from normal to carcinoma (×100), (d) and (e) diffuse moderate expression in carcinoma cells (×200), and (f) strongly positive carcinoma cells (×400)
HO-1 mRNA and protein expression and activity in human colon cancer cell lines
The assessment of HO-1 expression in colon cancer cell lines by RT-PCR and western blotting showed that HO-1 mRNA (Fig. 2a, b) and protein expression (Fig. 2c) in the colon cancer cell lines Caco-2, SNU-407, SNU-1033, HT-29, and SW-403 were higher than those in the normal colon cell line FHC. HO-1 enzyme concentration and activity were also higher in cancer cell lines and were consistent with the pattern of HO-1 protein expression (Fig. 2d, e), although both HO-1 expression and activity level differed among the cell lines. Despite these differences in the expression pattern of different cell lines, the data indicate that HO-1 is a useful marker for the detection of colon cancer.
HO-1 mRNA and protein expression and protein concentration and activity in normal colon and cancer cell lines. The HO-1 mRNA level was detected using a RT-PCR and b real-time PCR analyses. *p < 0.05, significantly different from FHC cells. c HO-1 protein level was detected using western blot analysis. d HO-1 enzyme concentration in colon cell lines was quantified using a human HO-1 ELISA kit. HO-1 concentration is expressed as nanogram per milliliter. *p < 0.05, significantly different from FHC cells. e HO-1 enzyme activity in normal colon and cancer cell lines was measured as described in the “Materials and methods”. HO-1 activity is expressed as nanomole of bilirubin per milligram of protein. *p < 0.05, significantly different from FHC cells
Downregulation of HO-1 levels decreased cell viability
The effect of the HO-1 inhibitor ZnPP [18] on cell viability was assessed in colon cell lines. ZnPP suppressed HO-1 concentration in the normal colon and cancer cell lines (Fig. 3a) and reduced the cell viability of colon cancer cell lines without affecting the viability of normal colon cells (Fig. 3b).
The effect of the HO-1 inhibitor ZnPP on the cell viability of colon cell lines. a After ZnPP treatment for 48 h in colon cell lines, HO-1 enzyme concentration was measured and is expressed as nanogram per milliliter. *p < 0.05, significantly different from ZnPP-untreated cells in each cell line. b Cell viability was measured using the MTT assay. *p < 0.05, significantly different from ZnPP-untreated cells in each cell line
Discussion
HO-1 is a cytoprotective enzyme that is induced in response to a variety of stimuli including oxidative stress. HO-1 induction plays a role in resistance to apoptosis in various human cancer cells [24–26]. In the present study, HO-1 protein expression and activity were examined in tumor and normal specimens obtained from Korean colon cancer patients. HO-1 protein expression and enzyme activity were higher in colon cancer tissues than in the surrounding normal tissues. This was also similar to the case for colon cancer cell lines. The expression of HO-1 in colon cancer tissues was confirmed by histological analysis. Inhibition of HO-1 reduces tumor growth and increases sensitivity to chemotherapy [27, 28]. Overexpression of HO-1, which may exert beneficial effects in a number of pathological conditions [29], has been suggested to play a protective role in cancer cells. Malignant cancers express HO-1 and provide cellular resistance against ROS-mediated anticancer therapy [18, 19], and elevated levels of HO-1 protein are associated with neoplastic growth [29, 30]. This suggests that HO-1 acts as a survival factor for colon carcinoma cells. ROS are mutagenic and thereby induce carcinogenesis. Normally, ROS levels are controlled by an inducible antioxidant system that responds to cellular stress and are predominantly regulated by the transcription factor NF-E2-regulated factor 2 (Nrf2) and its repressor protein Kelch-like ECH-associated protein 1 (Keap1) [31]. In contrast to the acute physiological regulation of Nrf2, in neoplasia, there is evidence for increased basal activation of Nrf2. Recent research has identified that somatic mutations disrupt the Nrf2–Keap1 interaction to stabilize Nrf2 and increase the transcription of Nrf2 target genes, indicating that enhanced ROS detoxification and additional Nrf2 functions may be protumorigenic [32, 33]. HO-1, an inducible antioxidant enzyme, has been reported to be upregulated via activation of Nrf2 [34]. Thus, the upregulation of HO-1 in tumor tissue and colorectal cancer lines may be due to the activation of Nrf2. Future studies will be necessary to determine the role of Nrf2 on HO-1 expression in colorectal cancer and its mechanism involved. HO-1 inhibition by ZnPP derivative enhances the cytotoxicity of hydroperoxides and anticancer drugs in SW-480 cells and mice bearing sarcoma S-180 cells [28]. In the present study, ZnPP enhanced cytotoxicity in cancer cell lines. In the human cancer cell line COLO-201, on the other hand, flavonoid-induced cytotoxicity was found to be mediated by the induction of HO-1 [35]. The relationship between HO-1 and cytotoxicity remains to be elucidated.
In conclusion, the present results indicate that HO-1 may serve as a clinically useful biomarker of colon cancer and a target for anticancer drugs.
References
Ministry of Health and Welfare of Korea. Reports of cancer incidence 07-27. Available from: http://www.mohw.go.kr/in-dex.jsp.
Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastoenterol. 2005;11:4685–8.
Matos E, Brandani A. Review on meat consumption and cancer in South America. Mutat Res. 2002;506–507:243–9.
Nkondjock A, Ghadirian P. Associated nutritional risk of breast and colon cancers: a population-based case control study in Montreal, Canada. Cancer Lett. 2005;223:85–91.
Glei M, Latunde-Dada GO, Klinder A, Becker TW, Hermann U, Voigt K, et al. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res. 2002;519:151–61.
Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Carroll RJ, et al. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett. 2004;208:155–61.
Ryter SW, Choi AM. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal. 2002;4:625–32.
Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–8.
Rizzardini M, Carelli M, Cabello Porras MR, Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: synergism by glutathione depletion and protection by N-acetylcysteine. Biochem J. 1994;304:477–83.
Rossi A, Santoro MG. Induction by prostaglandin A1 of haem oxygenase in myoblastic cells: an effect independent of expression of the 70 kDa heat shock protein. Biochem J. 1995;308:455–63.
Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19.
Cisowski J, Loboda A, Józkowicz A, Chen S, Agarwal A, Dulak J. Role of heme oxygenase-1 in hydrogen peroxide-induced VEGF synthesis: effect of HO-1 knockout. Biochem Biophys Res Commun. 2005;326:670–6.
Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets Rev. 2010;11:1551–70.
Lee J, Lee SK, Lee BU, Lee HJ, Cho NP, Yoon JH, et al. Upregulation of heme oxygenase-1 in oral epithelial dysplasias. Int J Oral Maxillofac Surg. 2008;37:287–92.
Nuhn P, Künzli BM, Hennig R, Mitkus T, Ramanauskas T, Nobiling R, et al. Heme oxygenase-1 and its metabolites affect pancreatic tumor growth in vivo. Mol Cancer. 2009;8:37.
Sass G, Leukel P, Schmitz V, Raskopf E, Ocker M, Neureiter D, et al. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int J Cancer. 2008;123:1269–77.
Becker JC, Fukui H, Imai Y, Sekikawa A, Kimura T, Yamagishi H, et al. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol. 2007;42:852–8.
Fang J, Akaike T, Maeda H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004;9:27–35.
Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003;88:902–9.
Alaoui-Jamali MA, Bismar TA, Gupta A, Szarek WA, Su J, Song W, et al. A novel experimental heme oxygenase-1-targeted therapy for hormone-refractory prostate cancer. Cancer Res. 2009;69:8017–24.
Clark JE, Green CJ, Motterlini R. Involvement of the human oxygenase-carbon monoxide pathway in keratinocyte proliferation. Biochem Biophys Res Commun. 1997;241:215–20.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8.
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–41.
Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503–13.
Sasaki T, Yoshida K, Kondo H, Ohmori H, Kuniyasu H. Heme oxygenase-1 accelerates protumoral effects of nitric oxide in cancer cells. Virchows Arch. 2005;446:525–31.
Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumor: is it a false friend? Antioxid Redox Signal. 2007;9:2099–118.
Fang J, Sawa T, Akaike T, Akuta T, Sahoo SK, Khaled G, et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003;63:3567–74.
Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109:1–8.
Alcaraz MJ, Fernandez P, Guillen MI. Antiinflammatory actions of the heme oxygenase-1 pathway. Curr Pharm Des. 2003;9:2541–51.
Mayerhofer M, Florian S, Krauth MT, Aichberger KJ, Bilban M, Marculescu R, et al. Identification of heme oxygenase-1 as a novel BCR/ABL-dependent survival factor in chronic myeloid leukemia. Cancer Res. 2004;64:3148–54.
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86.
Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–88.
DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9.
Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY, Kang MY, et al. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol. 2010;42:297–305.
Imai M, Kikuchi H, Denda T, Ohyama K, Hirobe C, Toyoda H. Cytotoxic effects of flavonoids against a human colon cancer derived cell line, COLO 201: a potential natural anti-cancer substance. Cancer Lett. 2009;276:74–80.
Acknowledgment
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1120340).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, K.A., Maeng, Y.H., Zhang, R. et al. Involvement of heme oxygenase-1 in Korean colon cancer. Tumor Biol. 33, 1031–1038 (2012). https://doi.org/10.1007/s13277-012-0336-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0336-0