Abstract
In this study, the effects of the plant growth-promoting rhizobacterium (PGPR), Bacillus sp. JS on the growth of tobacco (Nicotiana tabacum ‘Xanthi’) and lettuce (Lactuca sativa ‘Crispa’), were evaluated by comparing various growth parameters between plants treated with the bacterium and those exposed to water or nutrient broth as control. In both tobacco and lettuce, fresh weight and length of shoots were increased upon exposure to Bacillus sp. JS. To explain the overall de novo expression of plant proteins by bacterial volatiles, two-dimensional gel electrophoresis was performed on samples from PGPR-treated tobacco plants. Our results showed that chlorophyll a/b binding proteins were significantly up-regulated, and total chlorophyll content was also increased. Our findings indicate the potential benefits of using Bacillus sp. JS as a growth-promoting factor in agricultural practice, and highlight the need for further research to explore these benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth may be influenced by a number of abiotic and biotic factors, including some soil microorganisms. The soil and root-colonizing bacteria that promote plant growth are called plant growth-promoting rhizobacteria (PGPR). Since the term PGPR was first used by Kloepper and coworkers (Kloepper and Schroth 1978), numerous studies have addressed PGPR-driven improvements in plant growth. PGPR are thought to enhance plant growth by (1) increasing the nutrient availability to plants, (2) producing plant growth regulators, and (3) enabling plants to withstand biotic and abiotic stresses.
Leguminous plants obtain their nitrogen (N) requirements through N-fixation by rhizobia, such as Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, Rhizobium, and Sinorhizobium, which reside in their root nodules (Vessey 2003). However, non-leguminous crops depend on atmospheric nitrogen that is fixed and made available by PGPR (Kennedy and Tchan 1992). Soil fertility, including the available soil nitrogen, is one of the most important factors affecting plant growth, development, and crop yield. Azospirillum in maize (Garcia de Salamone et al. 1996), rice (Malik et al. 1997), and wheat (Boddey et al. 1986), Burkholderia sp. in rice (Baldani et al. 2000), Gluconacetobacter diazotrophicus in sugarcane (Boddey et al. 2001; Sevilla et al. 2001), and Herbaspirillum sp. in sorghum and rice (James et al. 1997, 2002) are documented PGPR species that improve the growth of crop plants through nitrogen fixation. PGPR may, therefore, be considered as biofertilizers in that they are microorganisms, which colonize the rhizosphere and promote plant growth (Vessey 2003).
By playing the role of an elicitor and by triggering the induced systemic resistance (Ryu et al. 2004), PGPR may enable plants to endure biotic stresses (Raj et al. 2003; Guo et al. 2004). Drought/salt stress is a common challenge in crop production (Hu and Schmidhalter 2005). Some studies have shown that PGPR confer tolerance to stress, particularly to abiotic stresses, such as drought, salt stress, and extreme temperatures (Yang et al. 2009). For example, Mayak et al. (2004) reported that Achromobacter piechaudii ARV8 increased the fresh and dry weights of both tomato (Lycopersicum esculentum Mill ‘F144’) and pepper (Capsicum annuum L. ‘Maor’) seedlings under transient water stress. The mechanism suggested for this positive effect was that the production of ACC deaminase by PGPR causes the degradation of the ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC), which in turn, prevents the formation of ethylene (Khan et al. 2009); high ethylene concentrations are known to inhibit plant growth and development (Davies 2004). In addition, wheat (Triticum aestivum) inoculated with Azospirillum brasilense Sp245 showed enhanced lateral root growth, root hair development, higher water content, and greater grain yield than did the non-inoculated plants under water stress; this effect could be the result of nitric oxide production by Azospirillum brasilense Sp245 (Creus et al. 2004, 2005).
The repeated physical and chemical treatment of soil is known to reduce its fertility and is environmentally unsustainable. In contrast, the use of PGPR that confer growth benefits to plants may be a more sustainable approach over the long term. Among the PGPR studied in recent years, Bacillus subtilis GB03 and B. amyloliquefaciens IN937a were reported to promote Arabidopsis growth via the production of bacterial volatile 2,3-butanediol (Ryu et al. 2003). Moreover, Bacillus megaterium XTBG34 promoted the growth in Arabidopsis by emitting 2-pentylfuran (Zou et al. 2010).
A noble bacterial strain was characterized and designated as Bacillus sp. JS in previous research (Song et al. 2012). The aim of this study was to determine the growth-promoting effects of the volatiles of Bacillus sp. JS on plants and to elucidate the mechanistic underpinnings of these effects.
Materials and methods
Bacterial culture
Bacillus sp. JS, Escherichia coli DH5α, Pseudomonas syringae pv. tomato, Xanthomonas campestris pv. vesicatoria, B. megaterium, P. putida, B. cereus BS101, and B. cereus BS107 were streaked on nutrient agar medium (NA, Difro, Detroit, USA) and cultured at 28 °C.
A single bacterial colony was transferred from NA to 30 mL of nutrient broth (NB, Difro, USA) and grown in a reciprocating shaker (110 rpm) at 28 °C for 14 h. The bacterial concentration was adjusted to 1 × 107 colony-forming units (CFU)/mL for the in vitro test, and to 1 × 108 CFU/mL for the field test.
In vitro plant growth promotion
Seeds of Nicotiana tabacum ‘Xanthi’ were sterilized by treating with 70% ethanol for 30 s, 1% sodium hypochlorite for 2 min, and were finally rinsed four times with sterile distilled water. A divided Petri dish test was performed in which ten tobacco seeds were placed on one side of the divided Petri dish (100 × 15 mm) containing half strength Murashige–Skoog’s (MS) medium with 1.5% sucrose. After 7 days, 50 μL of Bacillus sp. JS suspension was added onto the side of the divided Petri dish with no tobacco seeds. In the vertical plate test, seeds were placed on the upper side of a square plate (120 × 120 mm) containing half strength MS medium with 1.5% sucrose. After 7 days, 50 μL each of Bacillus sp. JS, E. coli DH5α, P. syringae pv. tomato, X. campestris pv. vesicatoria, B. megaterium, P. putida, B. cereus BS101, and B. cereus BS107 suspensions were spread on the lower side of the plate. The square plate was kept vertically and incubated under 16-h light/8-h dark conditions, at 26 °C. For growth evaluation, 30 seedlings were randomly collected from three or four plates at 5 and 10 DAT (days after treatment) for the vertical test and after 8 days for the divided plate test. The data were calculated by analysis of variance (ANOVA), with means separated by Tukey’s HSD test at p < 0.01.
Growth response of lettuce
Lettuce seeds were sown in 50-plug trays and grown for 10 days. The 10-day-old lettuce seedlings were then placed in a top-open container (with a 5-cm gap from the bottom) containing 250 mL of the bacterial culture diluted with 750 mL dH2O. The bacterial suspension was changed every 5 days. The containers with one-half strength NB, instead of bacterial suspension, were used as controls. Fifty lettuce plants were randomly selected from three plug trays. The fresh weight of shoots was measured at 25 and 35 DAT (i.e., 35- and 45-days-old shoots). These shoot-weight data were compared using the independent-samples t test at p < 0.01.
Determination of total chlorophyll content
Seven tobacco seedlings were grown on half-strength MS media containing 1.5% sucrose on one-half of the divided plates. These plates were exposed to 16-h light/8-h dark cycles at 26 °C for 7 days, after which, 50 μL of Bacillus sp. JS suspension (at a concentration of 1 × 107 CFU/mL) was dropped onto the other side of the divided Petri dish. De-ionized water was used, instead of the bacterial suspension, as a control. Seven days after bacterial inoculation, 0.5 g tissue from 20 tobacco seedlings was ground in liquid nitrogen using a mortar and pestle. Thereafter, 50 mL of acetone was added to the sample, and the mixture was centrifuged at 1500× g for 5 min. The absorbance of the supernatant was measured at 645 and 663 nm using a DU 730 UV–Vis spectrophotometer (Beckman, U.S.A.). The total chlorophyll content of the sample was calculated based on the method of Arnon et al. (1949) using the formula:
where A645 and A663 are the absorbance of the sample at 645 and 663 nm, respectively.
Protein extraction and 2-DE analyses
To determine the change in the expression of proteins in the seedlings caused by the volatiles of Bacillus sp. JS, two-dimensional gel electrophoresis (2-DE) was performed with three biological replicates. Tobacco seeds were placed on one side of the divided Petri dishes (100 × 15 mm) containing half strength MS medium with 1.5% sucrose. After 7 days, 50 μL of Bacillus sp. JS suspension was added to the side of the divided Petri dish with no tobacco seeds. The inoculated plates were incubated under 16-h light/8-h dark conditions at 26 °C. Nutrient broth was used as a control. The seedling samples were collected 96 h after Bacillus sp. JS treatment, and were stored at − 80 °C. The protein was extracted using Mg/NP-40 buffer, consisting of 0.5 M Tris–HCl (pH 8.3), 2% v/v Nonidet P-40 (NP-40), 20 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1% w/v polyvinyl polypyrrolidone, following the method described by Kim et al. (2004), and was subsequently fractionated with PEG 4000. The isoelectric focusing (IEF) gel mixture consisted of 4.5% w/v acrylamide solution, 9.5 M urea, 2% v/v NP-40, and 2.5% v/v pharmalytes (pH 3–10:5–8:4–6.5 = 1:3.5:2.5; Amersham Biosciences, CA, USA). Each 150-μg protein sample was loaded onto an IEF gel (18-cm tube gel). In the second dimension, SDS–PAGE was conducted following the method of Laemmli (1970), using 12% polyacrylamide gels. The 2-DE gels were CBB-stained in 0.2% w/v Coomassie R-250, 50% v/v methanol, and 10% v/v glacial acetic acid followed by destaining in 10% v/v glycerol and 50% v/v methanol. PDQuest software (Version 6.2.1; Bio-Rad, CA, USA), recommended by the supplier, was used for the analysis of gel image. The intensities of the induced protein spots from the samples were recorded as digital images, using a high-resolution scanner (GS-710 Calibrated Imaging Densitometer; Bio-Rad).
The spots were picked from the gel, washed with 50% v/v acetonitrile in 0.1 M NH4HCO3, and vacuum-dried. The gel fragments were reduced for 45 min at 55 °C in a solution of 10 mM DTT in 0.1M NH4HCO3. After cooling, the DTT solution was immediately replaced with a solution of 55 mM iodoacetamide in 0.1 M NH4HCO3. After washing in a solution of 50% acetonitrile in 0.1M NH4HCO3, the dried gel pieces were made to swell in a minimum volume of 10 mL digestion buffer containing 25 mM NH4HCO3 and 12.5 ng/μL trypsin (sequencing grade; Promega, Madison, USA), and incubated at 37 °C overnight. The trypsin-digested peptides were extracted following the method of Kim et al. (2003).
All the samples were finally analyzed using a Voyager-DE STR MALDI-TOF mass spectrometer (PerSeptive Biosystems, Framingham, MA, USA). The parent ion masses were measured in the reflectron/delayed extraction mode with an accelerating voltage of 20 kV, a grid voltage of 76.000%, a guide wire voltage of 0.010%, and a delay time of 150 ns. A two-point internal standard for calibration was used with des-Arg1-Bradykinin (m/z 904.4681) and angiotensin 1 (m/z 1296.6853). The peptides were selected in the mass range of 500–3000 Da. For data processing, the software package PerSeptive-Grams was used. The database searches were performed using Protein Prospector (http://prospector.ucsf.edu).
Results
In vitro plant growth promotion
The results of the divided plate experiment, in which the effect of Bacillus sp. JS on tobacco was investigated, showed approximately 90% increase in plant growth when tobacco seeds were treated with the PGPR for 8 days over that in the seeds treated with the control (Fig. 1). The divided plate has a central partition through which only volatile materials can move. Therefore, any effect of the bacteria on the plant seedlings would imply that effects are mediated by the volatiles. This positive effect was likely related to the volatile chemicals emitted by Bacillus sp. JS.
The assessment of growth performance in the vertical square plates showed that when compared to the various microorganisms used by us, Bacillus sp. JS had greater overall positive effects on the plant growth (Fig. 2). In contrast to the B. megaterium and B. cereus BS101 treatments where significantly lower plant growth was observed than in the control, the Bacillus sp. JS-treated plants showed significant growth promotion effects after 10 days of treatment (Fig. 2). The treatment with Pseudomonas putida also induced root growth but it had no effect on the fresh weight of shoots as compared to that in the control treatment.
Growth promotion of tobacco seedlings in response to Bacillus sp. JS treatment. Control: water, JS: Bacillus sp. JS, E. coli: Escherichia coli DH5α, PS: Pseudomonas syringae pv. tomato DC3000, XC: Xanthomonas campestris pv. vesicatoria, PP: Pseudomonas putida, BM: Bacillus megaterium, BS101: Bacillus cereus strains BS101, BS107: Bacillus cereus strains BS107. Different letters of the alphabet denote significant difference between the factors (according to Tukey’s HSD at p < 0.01). Each data point represents the mean ± standard error
Growth enhancement in lettuce
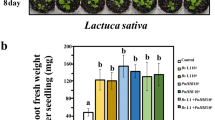
To evaluate the growth promoting effect of the volatiles of Bacillus sp. JS in field, lettuce seedlings in open top plug tray were employed. The results showed that the shoot weight of lettuce plants treated with Bacillus sp. JS was significantly higher (by approximately 78% at 25 DAT by 31% at 35 DAT) than that of plants treated with the control (Fig. 3).
Quantification of growth promotion in lettuce induced by exposure to volatiles of Bacillus sp. JS in the open-plug tray field trial. Bacillus sp. JS suspension was administered at the bottom and lettuce were kept afloat. Each data point represents the mean ± standard error, and asterisks denote significant differences based on the independent samples t test (p < 0.01)
Alteration of protein and chloroplast density in Bacillus sp. JS treated samples
In the 2-DE gel analysis, we detected nine differential spots (Fig. 4), among which spot number 5, corresponding to hairpin binding protein 1, showed decreased intensity (Table 1). The protein group with increased expression consisted of elongation factors TuA and TuB, chloroplast ferredoxin-NADP reductase, chloroplast chlorophyll A-B binding protein 40, chloroplast chlorophyll a/b binding protein cab-BO3-1, mannose-6-phosphate isomerase class I, and small chain of ribulose-bisphosphate carboxylase. The chlorophyll a/b binding protein in particular, which is known to increase during development and with light exposure, was increased by about two-fold (Table 1).
Discussion
The genome sequencing of Bacillus sp. JS was recently completed (Song et al. 2012). The sequence data of Bacillus sp. JS revealed that the Bacillus sp. JS genome consists of a single circular chromosome of 4,120,406 bp with 43.9% GC content, 4240 protein-coding sequences, 10 rRNA operons, and 8669 tRNAs. In addition, the Bacillus sp. JS genome was found to be similar to those of B. subtilis 168 and BSn5 genomes, with a 70 ANIb value of 95.31% (Song et al. 2012). The PGPR bacterium used in the subsequent experiments in this study was designated as Bacillus sp. JS.
The genome sequence has been deposited in GenBank under accession no. CP003492. This study revealed that the volatiles of Bacillus sp. JS enhance the growth in several plants, including tobacco, lettuce, and Arabidopsis. We further aimed to determine the mechanisms of plant growth promotion by Bacillus sp. JS. In the divided plate experiment, JS remarkably increased the fresh weight of the shoots of tobacco seedlings. This observation suggested that the plant growth promoting effect of Bacillus sp. JS is due to the volatiles produced by Bacillus sp. JS. The number of lateral root and root hairs and the primary root length of tobacco seedlings were also increased by the treatment with Bacillus sp. JS (data not shown). Among the mechanisms that might explain these effects is the production of plant hormones by the PGPR, which stimulate cell division and elongation (Davies 2004).
To understand the mechanism of growth promotion by Bacillus sp. JS volatiles, 2-DE analyses were carried out. Eight proteins were up-regulated and one was down-regulated upon treatment with Bacillus sp. JS volatiles. The photosynthesis pathway-related proteins, such as chloroplast chlorophyll A–B binding protein 40 and chloroplast chlorophyll a/b binding protein cab-BO3-1 were up-regulated. The increased chlorophyll A–B binding protein was demonstrated to correlate with the up-regulation of its mRNA in an earlier SSH analysis (Kim et al. 2015). The level of mRNA has been shown to parallel the accumulation of chlorophyll in Pinus palustris seedlings (Peer et al. 1996). The comparison of chlorophyll content in tobacco seedlings treated with Bacillus sp. JS volatiles and those that were not-treated revealed an approximately 60% increase in the chlorophyll content in the PGPR-treated samples (Fig. 5). The positive effect of Bacillus sp. JS volatiles on the chlorophyll content, reported here, supports the notion that one of the pathways by which PGPR exerts growth-promoting effects is the increase of chlorophyll content in plants.
The PGPR-induced growth enhancement seen in lettuce seedlings, as evidenced by the increase in fresh weight of the shoots of seedlings, highlights the potential of Bacillus sp. JS application in increasing the yields of lettuce in commercial cultivation. PGPR have previously been shown to exert beneficial effects on plant development; for example, the application of OSU-142 and M3 stimulated the yield and quality parameters of sugar beet, barley (Cakmakci et al. 2001), raspberry (Orhan et al. 2006), and apple (Aslantas et al. 2007) in the field, via direct or indirect mechanisms. However, ours is the first report of growth promotion by PGPR in lettuce. PGPR-mediated increase in the availability of nutrients in the rhizosphere has been proposed as the mechanism by which PGPR enhance the crop yield and increase the fruit size (Bar-Ness et al. 1992; Richardson 2001). However, because in the present study, plants were adequately supplied with all the nutrients, the notion that the observed positive growth effects may be the consequence of hormone production gets credence (Gutierrez-Manero et al. 2001). The growth-promoting effects of phytohormones, produced or induced by PGPR, are thought to alter the assimilation–partitioning patterns in plants, and therefore, alter the growth and the fructification process (Cakmakci et al. 2001; Orhan et al. 2006; Aslantas et al. 2007). Our results suggest that Bacillus sp. JS has the potential to increase the yield of lettuce. However, additional field experiments are required to verify the consistency of these positive effects in the conventional production systems.
We report a novel finding with regard to PGPR and their role in agriculture, i.e., the potential usefulness of Bacillus sp. JS in increasing the crop yield in species, such as tobacco and lettuce. We assume that the volatiles of Bacillus sp. JS act as elicitors and activate the chlorophyll synthesis in plant growth promotion. The application of Bacillus sp. JS can not only enhance the plant growth in agriculturally important species but can also make agriculture environmentally safe and sustainable, thereby contributing to the redressal of problems related with the use of agrochemicals.
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Aslantas R, Cakmakcı R, Sahin F (2007) Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci Hortic 111:371–377
Baldani VLD, Baldani JI, Döbereiner J (2000) Inoculation of rice plants with the endophytic diazatrophs Herbaspirillum seropedicae and Burkholderia spp. Biol Fertil Soils 30:485–491
Bar-Ness E, Hadar Y, Chen Y, Romheld V, Marschner H (1992) Short-term effects of rhizosphere microorganisms on Fe uptake from microbial siderophores by maize and oat. Plant Physiol 100:451–456
Boddey RM, Baldani VLD, Baldani JI, Döbereiner J (1986) Effect of inoculation of Azospirillum spp. on nitrogen accumulation by field-grown wheat. Plant Soil 95:109–121
Boddey RM, Polidoro JC, Resende AS, Alves BJR, Urquiaga S (2001) Use of the 15N natural abundance technique for the quantification of the contribution of N2 fixation to sugar cane and other grasses. Func Plant Biol 28:889–895
Cakmakci R, Kantar F, Sahin F (2001) Effect of N2-fixing bacterial inoculations on yield of sugar beet and barley. J Plant Nutr Soil Sci 164:527–531
Creus CM, Sueldo RJ, Barassi CA (2004) Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can J Bot 82:273–281
Creus CM, Graziano M, Casanovas EM, Pereyra MA, Simontacchi M, Puntarulo S, Barassi CA, Lamattina L (2005) Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 221:297–303
Davies PJ (2004) Plant hormones:biosynthesis, signal transduction, action. Kluwer Academic Publishers, Dordrecht
Garcia de Salamone IE, Dobereiner J, Urquiaga S, Boddey RM (1996) Biological nitrogen fixation in Azospirillum strain-maize genotype associations as evaluated by the 15N isotope dilution technique. Biol Fertil Soils 23:249–256
Guo JH, Qi HY, Guo YH, Ge HL, Gong LY, Zhang LX (2004) Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biol Control 29:66–72
Gutierrez-Manero FJ, Ramos-Solano B, Probanza A, Mehouachi J, Tadeo FR, Talon M (2001) The plant-growthpromoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol Plant 111:206–211
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
James EK, Olivares FL, Baldani JI, Dobereiner J (1997) Herbaspirillum, an endophytic diazotroph colonizing vascular tissue in leaves of Sorghum bicolor L. Moench. J Exp Bot 48:785–797
James EK, Gyaneshwar P, Mathan N, Barraquio QL, Reddy PM, Iannetta PPM, Olivares FL, Ladha JK (2002) Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant-Microbe Interact 15:894–906
Kennedy IR, Tchan YT (1992) Biological nitrogen fixation in non-leguminous field crops: recent advances. In: Ladha JK, George T, Bohlool BB (eds) Biological nitrogen fixation for sustainable agriculture. Springer, Dordrecht, pp 93–118
Khan MS, Almas Z, Javed M (2009) Microbial strategies for crop improvement. Springer, Berlin
Kim ST, Kim HS, Kim HJ, Kim SG, Kang SY, Lim DB, Kang KY (2003) Prefractionation of protein samples for proteome analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Mole Cell 16:316–322
Kim ST, Kim SG, Hwang DH, Kang SY, Kim HJ, Lee BH, Lee JJ, Kang KY (2004) Proteomic analysis of pathogen-responsive proteins from rice leaves induced by rice blast fungus, Magnaporthe grisea. Proteomics 4:3569–3578
Kim JS, Lee J, Seo SG, Lee C, Woo SY, Kim SH (2015) Gene expression profile affected by volatiles of new plant growth promoting rhizobacteria, Bacillus subtilis strain JS in tobacco. Genes Genom 37:387–397
Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th international conference on plant pathogenic bacteria, Gilbert-Clarey, France pp. 879–882
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Malik KA, Bilal R, Mehnaz S, Rasul G, Mirza MS, Ali S (1997) Association of nitrogen-fixing, plant-growth-promoting rhizobacteria (PGPR) with kallar grass and rice. In: Ladha JK, de Bruijn FJ, Malik KA (eds) Opportunities for biological nitrogen fixation in rice and other non-legumes. Springer, Dordrecht, pp 37–44
Mayak S, Tirosh S, Glick BR (2004) Plant growth promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Physiol 166:525–530
Orhan E, Esitken A, Ercisli S, Turan M, Sahin F (2006) Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci Hortic 111:38–43
Peer W, Silverthorne J, Peters JL (1996) Developmental and light-regulated expression of individual members of the light-harvesting complex b gene family in Pinus palustris. Plant Physiol 111:627–634
Raj SN, Deepak SA, Basavaraju P, Shetty HS, Reddy MS, Kloepper JW (2003) Comparative performance of formulations of plant growth promoting rhizobacteria in growth promotion and suppression of downy mildew in pearl millet. Crop Prot 22:579–588
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci 100:4927–4932
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Sevilla M, Burris RH, Gunapala N, Kennedy C (2001) Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif—mutant strains. Mol Plant-Microbe Interact 14:358–366
Song JY, Kim HA, Kim JS, Kim SY, Jeong H, Kang SG, Kim BK, Kwon SK, Lee CH, Yu DS, Kim BK, Kim SH, Kwon SY, Kim JF (2012) Genome sequence of the plant growth-promoting rhizobacterium Bacillus sp. Strain JS. J Bacteriol 194:3760–3761
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Zou C, Li Z, Yu D (2010) Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J Microbiol 48:460–466
Acknowledgements
This work was supported by a grant (Code # S211214L030210) from Forest Science & Technology Projects, Forest Service and by Advanced Production Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (Code # 1100345), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ji Seong Kim declares that he has no conflict of interest. Jeong Eun Lee declares that he has no conflict of interest. Hualin Nie declares that he has no conflict of interest. Yong Jae Lee declares that he has no conflict of interest. Sun Tae Kim declares that he has no conflict of interest. Sun-Hyung Kim declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kim, J.S., Lee, J.E., Nie, H. et al. Physiological and proteomic analysis of plant growth enhancement by the rhizobacteria Bacillus sp. JS. Genes Genom 40, 129–136 (2018). https://doi.org/10.1007/s13258-017-0615-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-017-0615-7