Abstract
Heart valves are constantly exposed to high dynamic loading and are prone to degeneration. Therefore, it is a challenge to develop a durable heart valve substitute. A promising approach in heart valve engineering is the development of hybrid scaffolds which are composed of a mechanically strong inorganic mesh enclosed by valvular tissue. In order to engineer an efficient, durable and very thin heart valve for transcatheter implantations, we developed a fabrication process for microstructured heart valve leaflets made from a nickel-titanium (NiTi) thin film shape memory alloy. To examine the capability of microstructured NiTi thin film as a matrix scaffold for tissue engineered hybrid heart valves, leaflets were successfully seeded with smooth muscle cells (SMCs). In vitro pulsatile hydrodynamic testing of the NiTi thin film valve leaflets demonstrated that the SMC layer significantly improved the diastolic sufficiency of the microstructured leaflets, without affecting the systolic efficiency. Compared to an established porcine reference valve model, magnetron sputtered NiTi thin film material demonstrated its suitability for hybrid tissue engineered heart valves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several different types of biological and artificial heart valve replacements are available today, but yet the ideal valve substitute to replace the natural heart valve has not yet been found. Since heart valves are permanently exposed to high pressures and shear stress due to blood flow it is a challenge to develop a long lasting valve that also features all desirable characteristics of a heart valve substitute.4,9 Biological heart valve bioprostheses show the best hemodynamic properties, but their durability is limited. Mechanical valve replacements exhibit very good durability, but patients need a lifelong anticoagulation therapy.16
Several attempts have been made to develop a hybrid valve with a strong scaffold backbone, which withstands strong mechanical forces and additionally serves as extra cellular matrix (ECM) for tissue engineered heart valve leaflets.17
One approach states the use of organic decellularized xenografts, using the ECM to culture autologous cells in vitro prior to the implantation.15 These pulmonary heart valved stents demonstrated good valve functions in animal testing. However, calcification which might be induced by an immunological reaction against the leftover antigens of the xenograft was observed. Further studies also report the issue of the complete decellularization of the biological ECM and possible increase of the risk of inflammation or immunogenic response due to cell remnants.24 A major factor determining the durability of biological valves is their ECM composition, which serves as a strong backbone for withstanding the stresses.19 In natural valves, valvular interstitial cells (VIC) are able to translate macroscopic forces into specific biomechanical response, therefore a higher stress on the leaflets results in a higher stiffness induced by the VICs.14 This mechanism does not exist in bioprosthetic heart valves, resulting in an inferior durability of tissue valves when exposed to cardiac cycling.20
Regarding the limitations of bioprosthetic valves, several studies investigate inorganic materials as leaflet backbone material for tissue engineered hybrid valves. To mimic the performance of a native heart valve leaflet, Hinderer et al. fabricated an inorganic electrospun poly (l-lactide) (PLA) based scaffold matrix adapted to the structure and mechanical properties of native valve leaflet. This matrix was seeded with valvular interstitial cells and valvular endothelial cells to engineer a bio-functionalized hybrid heart valve.10
To overcome the disadvantages of polymeric, decellularized and biological scaffolds, Alavi et al. developed a strong woven stainless steel mesh backbone, enclosed with a biological membrane of smooth muscle cells (SMCs), fibroblasts and endothelial cells (ECs).1 One issue of this scaffold might include the lack of adequate recoil of stainless steel required for dynamic heart valve leaflets. In a further study, Alavi et al. performed a similar tissue seeding procedure with a 76 µm thick NiTi core mesh backbone. The investigation of the inflammatory response demonstrated a superior biocompatibility for NiTi compared to stainless steel.3 Hybrid valves with leaflets produced by the same method and with a NiTi scaffold thickness of 25 µm were evaluated in a recent study by Alavi et al. Tests in a heart flow simulator confirmed good opening properties of the trileaflet valve and a good attachment of the enclosing tissue of smooth muscle and fibroblast/myofibroblast and a final layer of endothelial cells under physiological flow conditions.2
NiTi thin film is ideally suited for the development of minimal invasive transcatheter based implants, due to its superelastic properties and its excellent biocompatibility.8,11,13,18,21 Furthermore the fabrication of thin films by means of sputter deposition of NiTi allows complex designing and miniaturization.5, 11 In this regard, sputter deposited NiTi thin film demonstrated high potential as a material for heart valve leaflets.12,23 Due to its high cyclic mechanical stability of NiTi-based thin films, an enhanced valve durability with the right design is expected.6,22
In this study we fabricated microstructured NiTi thin film heart valve leaflets with a thickness of 10 µm by means of sputter deposition, lithography, wet etching and a 3D-shape setting annealing procedure. The structured valve leaflets were seeded with SMCs from a sheep. The performance of the valves in hydrodynamic pulsatile testing with and without cells were evaluated and compared an established porcine valve.
Materials and Methods
Fabrication of NiTi Thin Film Heart Valve Leaflets
Free standing NiTi thin films for heart valve leaflets were fabricated by means of magnetron sputter deposition.12 Planar leaflet scaffolds were structured by UV-lithography process and wet etching technique. The detailed process flow is described by Lima de Miranda et al. 11 The leaflets were fabricated with a meshed scaffold structure (Fig. 1a) with a strut width of 9.5 ± 0.2 µm, a rhombic short axis of 24.6 ± 0.2 µm, a rhombic long axis of 84.9 ± 0.2 µm, resulting in a rhombic hole size of 1245 ± 55 µm2. Further NiTi thin film valve leaflets were fabricated without a microstructure (full material NiTi thin film leaflets). All thin film heart valve leaflets have a thickness of 10 µm. Planar and structured NiTi thin films were shaped into a 3D-heart valve form, using a specifically designed steel mold during an annealing procedure (Fig. 1b). The 3D thin film valve design has a diameter of 20 mm and the leaflet height of 14 mm (Fig. 1c).
(a) Schematic planar 2D design of free standing NiTi thin film heart valve leaflets. Microscope image shows the rhombic mesh structure with a strut width of 9.5 µm and rhombic hole size of 1245 µm2. Full material NiTi thin film leaflets have the same form, but without the rhombic microstructure of the leaflets. (b) Schematic of a steel mold to shape the 3D heart valve leaflet form. Films are wrapped around the bottom part and a form-negative was used to press the films into valve form during an annealing procedure. (c) Fabricated microstructured NiTi thin film heart valve leaflets.
Cell Harvest
For cell harvest a 5 cm long segment of an ovine carotid artery was removed under sterile conditions. Therefore, first the ECs were isolated using 0.2% Collagenase A (Roche Diagnostics GmbH) and cultured in a 24-well plate (Nunc GmbH) in cell culture medium [M199-Medium (Biochrom)], containing 1% Penicillin–Streptomycin, 10% fetal calf serum and recombinant human fibroblast growth factor [Fibroblast growth factor (FGF); Peprotech]. For gaining the smooth muscle cells (SMCs) the carotid artery segments were cut into 1–2 mm pieces, divided into two wells of a 6-well plate and cultured in the cell culture medium (M199-Medium) as described above. After 5 days the SMCs grew out of the carotid artery segments. Both cell populations were incubated at 37 °C and 5% CO2 and serially passaged with Accutase™ (PAA Laboratories GmbH). For this experiment only the SMCs were used.
Cell Adhesion Assay
100.000 cells/cm2 were seeded onto the autoclaved NiTi thin film valve leaflets by dropping cell suspension onto the leaflets. Seeded NiTi valves were cultured statically in Dulbecco’s Modified Eagle’s Medium (DMEM, Biochrom, Germany), supplemented with 10% fetal bovine serum (FBS, Biochrom, Germany), 1% penicillin and streptomycin (100U/ml, Biochrom, Germany) and 5 ng/ml fibroblast growth factor (FGF, PeproTech, Germany) at 37 °C, with 5% CO2 for seven days. Afterwards the cells on the seeded leaflets were fixed and kept in 4% formalin until testing.
Pulsatile Testing
Fabricated heart valve leaflets with cells and without cells were tested with a custom built pulse duplicator apparatus (Fig. 2).7 For this study, a NiTi full film valve without microstructured leaflets, structured valve leaflets without cells as well as structured NiTi thin film valve leaflets seeded with SMCs for seven days were evaluated and compared to a porcine reference valve. Measurements were carried out at a heart rate from 40 beats/min to 75 beats/min and a stroke volume of 70 ml/stroke. The heart rate and the stroke volume determines the cardiac output to 2.8, 3.5, 4.2, 4.9, and 5.25 l/min for heart rates of 40, 50, 60, 70 and 75 beats/min respectively. Pressure sensors, mounted in subvalvular and supravalvular position were used to obtain the transvalvular pressure difference during systole and diastole. The operating valve was recorded with a camera to gain information on the opening behavior and to determine the performance index (PI), which is defined as the ratio of the maximum opening area during systole and the total cross section area of the valve. All tests were carried out at room temperature with ultrapure water as test fluid.
Pulse duplicator apparatus used for hydrodynamic pulsatile measurements at heart rates from 40 to 75 bpm with a stroke volume of 70 ml/stroke and a cardiac output from 2.8 to 5.25 l/min.7
Results
SEM Micrographs of Cell Seeded Valve Leaflets
For preparation of the SEM investigation, the valve leaflets were washed with PBS for 5 min and dried with alcohol at ascending concentrations up to 99% to remove excess water. SEM micrographs of NiTi thin film valve leaflets are presented in Fig. 3. The images clearly show a confluent growth of the cells/ECM over the NiTi leaflet after 7 days of cell seeding with smooth muscle cells. The struts and the pores in the meshed structure are almost completely covered with cell material. After hydrodynamic pulsatile testing with heart rates ranging from 40 to 75 beats/min and maximum diastolic transvalvular pressure differences up to 150 mmHg and 200 cycles in total, SMCs still adhere to the NiTi surface. The covering is not confluent anymore, but the holes are still covered with cells to a large extent.
SEM overview and image with higher magnification of the NiTi scaffold: (a) SEM images of the valve leaflet scaffold without SMCs, (b) NiTi thin film scaffold seeded with SCMs for seven days before pulsatile testing. A thin cell and fiber layer covers the structured NiTi film and closes the holes; (c) NiTi thin film scaffold seeded with SMCs after pulsatile testing at heart rates from 40 to 75 beats/min. After pulsatile testing some fiber and cell material detached, but the rhombic holes are still closed to a large extent.
Systolic Pressure Difference
The valves efficiency correlates with the transvalvular systolic pressure differences. In order to estimate the efficiency of the tested valves, the mean pressure difference during systole was determined. A low pressure difference refers to better opening behavior and a high opening area. The NiTi thin film heart valve leaflets without cells, as well as the NiTi leaflets seeded with SMCs show very low mean systolic pressure differences (ranging from 1.7 mmHg at 40 beats/min to 4.4 mmHg at 75 beats/min) (Fig. 4). The mean systolic pressures of the full material NiTi thin film leaflets is 3.5 mmHg at 40 beats/min and increasing up to 4.7 mmHg at 75 beats/min. Compared to the NiTi leaflets the mean systolic pressure difference for the porcine reference valve is about five times higher (9.5 mmHg at 40 beats/min and 25.5 mmHg at 75 beats/min).
Opening Area
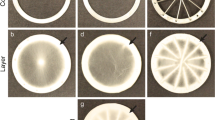
Figure 5 shows the opening and closing phase of the tested valves at a heart rate of 70 bpm. Microstructured NiTi thin film leaflets with and without cells exhibit a high opening area and a simultaneous opening and closing behavior of the leaflets was observed. The porcine valve has a smaller opening area compared to NiTi valves, mainly due to the design and the coalescence of the three valve leaflets. The PI of the microstructured NiTi thin film heart valves with and without SMCs is in the range of 60 to 73% and much higher than the PI of the non-structured full material NiTi thin film leaflets (40 to 60%) and the porcine valve with 32% (Fig. 6). In a previous study, Haaf et al. determined a PI of a human aortic valve of 56%.7
Opening and closing phase of NiTi heart valve leaflets, NiTi leaflets seeded with SMCs, the full material NiTi thin film valve and the porcine heart valve at a heart rate of 70 beats/min. The sequence shows the closed state at a1–a4, beginning of the systolic phase b1–b4, the maximum opening c1–c4, leaflet closing at the beginning of the diastolic phase d1–d4 and the complete closing during diastole. The microstructured leaflets with and without SMCs open simultaneously and exhibit a high effective opening area and a good leaflet compliance. The valvular opening area is larger in all NiTi valves compared to the porcine valve.
Performance index (PI) of microstructured NiTi thin film heart valves with and without SMCs is much higher compared to the full material NiTi thin film leaflets and the porcine valve. PI were determined from the maximum opening areas of the valves (Fig. 5).
Diastolic Pressure Difference
The diastolic pressure difference across the valve is related to the valves sufficiency. High pressure differences across the closed valve during diastole refer to a better closing behavior and a sufficient leak tightness. The non-structured full material NiTi thin film leaflets and the porcine reference valve reached the highest diastolic transvalvular pressure difference (17.5 mmHg at 40 beats/min–58.3 mmHg at 75 beats/min) followed by the NiTi thin film leaflets seeded with SMCs (14.9 mmHg–50.8 mmHg) (Fig. 7a). The mean diastolic transvalvular pressure difference of the SMC cultured NiTi leaflets did not deteriorate after 200 dynamic cycles (Fig. 7b). As expected, the microstructured NiTi thin film leaflets without SMCs showed the lowest diastolic pressures (9.1–27.4 mmHg).
(a) Mean diastolic pressure for heart rates from 40 to 75 beats/min. The full material NiTi thin film leaflets and the porcine valve exhibit the highest transvalvular pressure difference. Seeding of the microstructured leaflets increased the leak tightness and leads to higher diastolic transvalvular pressure differences. (b) Mean diastolic pressure difference at a heart rate of 70 bpm. After more than 200 cycles in total the pressure difference of the SMC cultured leaflets has the same value compared to the first cycles and two times higher pressure difference compared to leaflets without SMCs.
Discussion
This study investigates microstructured NiTi thin film as a matrix scaffold material for tissue engineering of heart valve leaflets for transcatheter valve implants. A fabrication process for 3D shaped NiTi thin film leaflets by means of magnetron sputtering, lithography, wet etching and a subsequent shape setting procedure was introduced. The leaflets of a microstructured NiTi thin film valves were seeded with SMCs in vitro for 7 days. The functional performance of SMC seeded NiTi thin film leaflets, microstructured leaflets without SMCs as well as full material NiTi thin film leaflets was determined under pulsatile conditions in a pulse duplicator apparatus. These in vitro hydrodynamic experiments provide essential information on the hemodynamic function of the valves. For evaluation of the performance of the NiTi thin film valve leaflets an established porcine valve was tested and used as a reference.
All tested NiTi thin film valves show higher effective valvular opening areas compared to the porcine reference model throughout the operating range from 40 to 75 beats/min. Furthermore, a low systolic transvalvular pressure difference relates to a high cardiac output and a low flow resistance of the leaflets. In this regard all NiTi leaflets show a good performance in terms of reduced ventricular workload. The recorded image sequences reveal a good leaflet compliance of all microstructured NiTi leaflets, as a simultaneous opening and closing behavior of the three leaflets was observed. A major issue of the full material NiTi thin film leaflets is the leaflet compliance, since one leaflet shows only a slight opening at the maximum opening area and during the closing phase the leaflets do not close simultaneously. Compared to the microstructured leaflets, the full material leaflets reveal a higher stiffness and therefore a lower opening area, especially at low heart rates. The porcine valve has a significantly lower opening area and is therefore characterized with a much higher transvalvular pressure drop.
During diastole the closing characteristics of the valves are determined. The recorded sequences show a complete closing of all tested NiTi thin film valve leaflets. Nevertheless, all microstructured NiTi leaflets exhibit lower diastolic pressure differences compared to the control valve. The full material NiTi thin film leaflets are able to maintain the same diastolic transvalvular pressure difference as the porcine reference valve.
In order to close the rhombic holes of the meshed NiTi structure and thereby increasing the leak tightness of the valve, the microstructured leaflets were seeded with smooth muscle cells (SMCs) for 7 days. SEM micrographs reveal a confluent grown monolayer of SMCs after 7 days of cell seeding.
In pulsatile hydrodynamic experiments the smooth muscle cell layer on the meshed NiTi thin film structure could increase diastolic characteristics of the valve significantly, without affecting the systolic functional properties. The mean diastolic transvalvular pressure stays constant, comparing the first dynamic cycle with the mean diastolic pressure difference tested after more than 200 cycles in total. Opening area and systolic pressure drop are similar for the valves with and without smooth muscle cell layer.
After the functional test of the seeded valves at heart rates from 40 to 75 beats/min and 200 cycles in total, a follow up SEM investigation was carried out. SEM micrographs after testing of the seeded NiTi thin film leaflets show that cells are still attached to the meshed NiTi surface. The diastolic pressure measurements indicate a much higher degree of leak tightness of the cell seeded films. Since after 200 dynamic testing cycles the mean transvalvular diastolic pressure difference is the same as for the first cycle at 70 bpm, we think that weak adhering cells and fibers detach during an early stage of the dynamic test. Nevertheless, the stronger adhering SMCs and fibers close a large part of the rhombic holes and are still sufficient enough to reduce regurgitation and enable the leaflets to maintain significantly higher transvalvular pressure differences during diastole for more than 200 dynamic cycles.
In a recent study, Alavi et al. reported promising results for the structured NiTi valve leaflets, fabricated by acid etching from 25 µm thick NiTi sheet. The leaflets were equipped with holes of a diameter of 240 µm and a central distance of 320 µm. The hybrid valve proved a good opening behavior and a strong tissue attachment of smooth muscle, fibroblast/myofibroblast and an endothelial layer during dynamic heart flow simulation testing.2
In contrast to the approach by Alavi et al., we use magnetron sputtering and photo lithography to fabricate the thin film leaflets. An annealing procedure is carried out to shape the trileaflet film into a 3D heart valve form. The film thickness of 10 µm and rhombic hole structure with 9.5 µm struts and a hole size of 1245 µm2 is much smaller compared to the structure of Alavi et al. The sputtering process allows a miniaturization and the fabrication of smaller structures compared to wet etching techniques as well as other novel design opportunities.5 In our approach we try to close the holes in the leaflets with a thin tissue layer, presumably a cell monolayer to assure a dynamic function of the leaflets, while keeping the total thickness of the leaflets as thin as possible. A further tissue formation in vivo with a following endothelialization of the SMC cultured leaflets has to be investigated. The culturing of the SMC monolayer leads to very thin valve leaflets which allow a possible transcatheter implantation with smaller catheters. Figure 8 shows the crimping of a NiTi thin film device (20 mm diameter, 5 µm thick NiTi thin film rhombic microstructure and a 50 µm thick NiTi thin film frame) into an 8 Fr catheter. The thin film device unfolds and regains its original shape immediately after it is deployed from the catheter. The device did not show any damage after the crimping process.
Sequence of the crimping procedure of a NiTi thin film device (20 mm diameter, 5 µm thick NiTi thin film rhombic microstructure and a 50 µm thick NiTi thin film frame). (a)–(d) shows the insertion of the device into a 8 Fr catheter. When the device is deployed from the catheter it initially regains its original shape (e–h). No damage was observed after the crimping procedure.
The magnetron sputtered films demonstrated a very good fatigue resistance in tensile tests as well as high corrosion resistance compared to conventional NiTi material.22,25 This high resistance is related to the lack of inclusions and the smooth surface of the film. Furthermore, subsequent sputter deposition and lithography steps enable the fabrication of 2.5 dimensional films with different thicknesses, i.e. the thickness of leaflet parts with higher expected loads can be adjusted as required.
In this study, we used a generic leaflet design to demonstrate the feasibility of magnetron sputtered and structured NiTi thin film as a strong backbone scaffold for tissue engineered heart valve leaflets. The novel leaflets (without seeded cells) underwent 5 × 105 opening cycles in a pulsatile setup under low pressures without any apparent damage. We believe a design optimization based on finite element modeling and tissue strengthening with longer seeding periods with additional fibroblasts and endothelial cells will allow the engineering of durable thin film heart valve leaflets with improved functional performance.
References
Alavi, S., and A. Kheradvar. Metal mesh scaffold for tissue engineering of membranes. Tissue Eng. Part C 18(4):293–301, 2012.
Alavi, S., and A. Kheradvar. A hybrid tissue-engineered heart valve. Ann. Thorac. Surg. 99:2183–2187, 2015.
Alavi, S., W. Liu, and A. Kheradvar. Inflammatory response assessment of a hybrid tissue-engineered heart valve leaflet. Ann. Biomed. Eng. 41:316–326, 2013.
Arjunon, S., S. Rathan, H. Jo, and A. Yoganathan. Aortic valve: mechanical environment and mechanobiology. Ann. Biomed. Eng. 41(7):1331–1346, 2013.
Bechtold, C., R. Lima de Miranda, and E. Quandt. Capability of sputtered micro-patterned NiTi thick films. Shap Mem. Superelast. 1(3):286–293, 2015.
Chluba, C., W. Ge, R. Lima de Miranda, J. Strobel, L. Kienle, E. Quandt, and M. Wuttig. Ultralow-fatigue shape memory alloy films. Science 348(6238):1004–1007, 2015.
Haaf, P., M. Steiner, T. Attmann, G. Pfister, J. Cremer, and G. Lutter. A novel pulse duplicator system: evaluation of different valve prostheses. Thorac. Cardiov. Surg. 57:10–17, 2009.
Habijan, T., R. Lima de Miranda, C. Zamponi, E. Quandt, C. Greulich, T. Schildhauer, and M. Köller. The biocompatibility and mechanical properties of cylindrical NiTi thin films produced by magnetron sputtering. Mater. Sci. Eng. C 32(8):2523–2528, 2012.
Harken, D. E., W. Taylor, A. Lefemine, S. Lunzer, H. Low, M. Cohen, and J. Jacobey. Aortic valve replacement with a caged ball valve. J. Am. Cardiol. 9:292–299, 1962.
Hinderer, S., J. Seifert, M. Votteler, N. Shenb, J. Rheinlaender, T. Schäffer, and K. Schenke-Layland. Engineering of a bio-functionalized hybrid off-the-shelf heart valve. Biomaterials 35(7):2130–2139, 2014.
Lima de Miranda, R., C. Zamponi, and E. Quandt. Micropatterned freestanding superelastic TiNi films. Adv. Eng. Mater. 15(1–2):66–69, 2013.
Loger, K., R. Lima de Miranda, A. Engel, M. Marczynski-Bühlow, G. Lutter, and E. Quandt. Fabrication and evaluation of nitinol thin film heart valves. Cardiovasc. Eng. Technol. 5(4):308–316, 2014.
Meltzer, A., and D. Stoeckel. Function and performance of Nitinol vascular implants. Open Med. Dev. J. 2(2):32–41, 2010.
Merryman, W., I. Youn, H. Lukoff, P. Krueger, F. Guilak, R. Hopkins, and M. Sacks. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am. J. Physiol. Heart Circ. Physiol. 290:H224–H231, 2006.
Metzner, A., U. Stock, K. Iino, G. Fischer, T. Huemme, J. Boldt, J. Braesen, B. Bein, J. Renner, J. Cremer, and G. Lutter. Percutaneous pulmonary valve replacement: autologous tissue-engineered valved stents. Cardiovasc. Res. 88(3):453–461, 2010.
Pibarot, P., and J. Dumesnil. Prosthetic heart valves selection of the optimal prosthesis and long-term management. Circulation 119:1034–1048, 2009.
Plimpton, W. Liu, and A. Kheradvar. Immunological and phenotypic considerations in supplementing cardiac biomaterials with cells. In: Biomaterials for Cardiac Regeneration, edited by M. Ruel, and E. Suuronen. Switzerland: Springer, 2015, pp. 239–273.
Ryhänen, J. Biocompatibility evolution of nickel-titanium shape memory alloy. Finland: Faculty of Medicine, University of Oulu, 1999.
Schoen, F. Future directions in tissue heart valves: impact of recent insights from biology and pathology. J. Heart Valve Dis. 8(4):350–358, 1999.
Schoen, F., and R. Levy. Pathology of substitute heart valves. J. Card. Surg. 9:222–227, 1994.
Shabalovskaya, S. Surface, corrosion and biocompatibility aspects of nitinol as an implant material. Bio-Med. Mater. Eng. 12(1):69–109, 2002.
Siekmeyer, G., A. Schüßler, R. Lima de Miranda, and E. Quandt. Comparison of the fatigue performance of comercially produced samples versus sputter-deposited NiTi. J. Mater. Eng. Perform. 23(7):2437–2445, 2014.
Stepan, L., D. Levi, and G. Carman. A thin film nitinol heart valve. J. Biomech. Eng. 127(6):915–918, 2005.
Stock, U., and K. Schenke-Layland. Performance of decellularized xenogeneic tissue in heart valve replacement. Biomaterials 27(1):1–2, 2006.
Wohlschlögel, M., R. Lima de Miranda, A. Schüßler, and E. Quandt. Nitinol: tubing versus sputtered film–microcleanliness and corrosion behavior. J. Biomed. Mater. Res. B 2015. doi:10.1002/jbm.b.33449.
Acknowledgements
Project funding by the Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged.
Conflict of interest
Authors Klaas Loger, Alexander Engel and Jessica Haupt declare that they have no conflict of interest. Author Rodrigo Lima de Miranda is partner of the Acquandas GmbH (Kiel) and holds several patents on shape memory thin films techniques. Author Eckhard Quandt reports funding of the project by the German Research Foundation (DFG). He is partner of the Acquandas GmbH (Kiel) and holds several patents on shape memory thin films techniques. Author Georg Lutter reports funding of the project by the German Research Foundation (DFG) and the German Center for Heart and Circulation (DZHK).
Human and Animal Rights
No human or animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Loger, K., Engel, A., Haupt, J. et al. Microstructured Nickel-Titanium Thin Film Leaflets for Hybrid Tissue Engineered Heart Valves Fabricated by Magnetron Sputter Deposition. Cardiovasc Eng Tech 7, 69–77 (2016). https://doi.org/10.1007/s13239-015-0254-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-015-0254-6