Abstract
Cenchrus ciliaris, commonly known as buffelgrass, is an apomictic perennial range grass usually grown in arid/semi-arid regions. Because of the difficulties faced in conventional breeding of this polymorphic polyploid grass, the development of an efficient protocol for genetic transformation is warranted. Such a protocol would enable functional genomic studies needed to elucidate the mechanism of apomixis in this species. The embryogenic calli for genetic transformation experiments were obtained by in vitro culture of the immature inflorescences of buffelgrass cv. IGFRI-3108. Four developmental stages of immature inflorescences were compared for callus induction, and the most mature stage produced most callus. Embryogenic calli were bombarded at three distances, 6 cm (L1), 9 cm (L2), or 12 cm (L3) with a marker gene uid A present in pCAMBIA1301, under the same vacuum (85 kPa) and at constant pressure (900 psi). The Agrobacterium-mediated genetic transformation was also performed using the same construct. Transient and stable expression as well as PCR amplification of the GUS gene was used for comparative analysis as well as for validation of the transformants. Transient GUS expression was present in a significantly higher percentage of bombarded calli (56.33%) than of Agrobacterium treated calli (11.17%), but the number of GUS positive cells per callus was similar. Among the three different bombardment distances, transient GUS expression was highest at L2, but stable GUS expression was highest at L1. Shoot development from the bombarded calli could be accomplished, which failed from the Agrobacterium-mediated transformed calli. Thus, the results indicate that C. ciliaris cv. IGFRI-3108 can be successfully transformed through Biolistic particle bombardment, while Agrobacterium-mediated transformation requires further optimization of transformation protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cenchrus ciliaris L. (syn. Pennisetum ciliare), commonly known as buffelgrass, belongs to family Poaceae and reproduces predominantly through apomixis [11, 24]. It is mainly used as fodder with high biomass productivity [26, 29] and tolerance to drought and extreme conditions of temperature [1, 25]. Due to its high resilience to extreme environmental conditions and higher productivity, it is cultivated in India [4] as one of the major forage crops. Owing to its predominantly apomictic mode of reproduction and being polypoid with varying chromosome numbers from 2n = 4x = 36 to 2n = 6x = 54 [6, 12, 34], its genetic improvement through conventional breeding methods is extremely difficult and restricted to the selection of elite lines from natural variants [18, 21]. Many candidate genes for apomixis have been isolated from Cenchrus, but the detailed functional analysis could not be carried out due to the lack of an efficient genetic transformation protocol. Tissue culture followed by genetic transformation constitutes one of the powerful techniques for functional genomic studies as well as genetic improvement of plants [19, 28]. Plant regeneration from embryogenic calli induced from immature inflorescence and other explants of Cenchrus has been reported earlier [2, 16, 22, 23, 32, 35]; and the detailed analysis of somatic embryogenesis and shoot regeneration from different explants too was demonstrated [35].
Among apomictic C. ciliaris genotypes, the highest shoot induction frequency was found in buffelgrass cv. IGFRI-3108 [32]. Production of plenty of embryogenic calli, an efficient medium for long term maintenance of embryogenic calli and plantlet regeneration are some of the prerequisites for tissue culture mediated genetic transformation. The response of the plant to the tissue culture medium is also dependent on the genotype as well as the type of explant used [17, 31, 35]. Although many research articles have already been published on embryogenic callus induction, plant regeneration and genetic transformation of Cenchrus spp., it is still difficult to find a universal protocol or a common method for every explant or genotype. Therefore, this study aimed at comparative evaluation of two genetic transformation methods for buffelgrass cv. IGFRI-3108 in terms of transient GUS expression and shoot development efficiency of the transformed calli.
Materials and methods
Induction and maintenance of embryogenic calli
Immature inflorescences of buffelgrass cv. IGFRI-3108 were collected when the flag leaf was not fully emerged out from the first subtending leaf and were classified as stages I, II, III, and IV depending on the developmental stage of the immature inflorescences. Before removing them from the leaf sheaths, immature inflorescence was surface sterilized with 70% ethanol for 3 min, rinsed with sterile distilled water 3 to 4 times and blot-dried using sterile tissue papers. Later, the immature inflorescences were dissected out carefully and cut into approximately 1 cm long pieces. The pieces of immature inflorescences were inoculated on MS medium [27] containing 3 mg/l 2, 4-D, 0.5 mg/l BAP, 3% sucrose, gelled with 0.2% Gelrite (pH adjusted to 5.8 before autoclaving at 121 °C for 21 min) for callus induction [32] by slightly submerging the basal part of the explant into the medium. Callus induction on immature inflorescences of four stages was recorded for comparative analysis. Compact, fragile embryogenic calli were selected and subcultured on callus maintenance media by keeping them in the dark at 25 ± 2 °C. For maintaining the embryogenic nature of calli, the medium was also supplemented with 300 mg/l casein hydrolysate, 400 mg/l l-proline, 400 mg/l l-glutamine [32]. Subculturing of the calli was done at an interval of 21 days. The regenerative response of the calli after every subsequent subculture, up to four subculture cycles was examined discretely, before using them for genetic transformation experiments. For inducing shoot regeneration, the calli were kept in the regeneration medium for 16 h photoperiod under the fluorescent lamp at 25 ± 2 °C. For genetic transformation experiments, the calli growing at either second or third subcultures were only used. The detail of the media used is given in Table 2.
Plasmid isolation
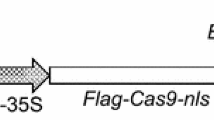
Plasmid pCAMBIA1301 was isolated from E. coli DH5α harboring the plasmid using QIAprep Spin Miniprep Kit (Qiagen). GUS gene is cloned in this plasmid under the transcriptional control of promoter CaMV35S, and the hygromycin resistance (hptII) gene is present as a plant selection marker.
Biolistic genetic transformation
Biolistic optimization kit (Bio-Rad) was used for the gene gun-mediated genetic transformation of calli using a Helium-driven particle delivery system (Bio-Rad, PDS-1000) with plasmid DNA-coated gold particles (1.6 µ) as microcarriers [4, 10] and other physical parameters (Table 1). The embryogenic calli were arranged on MS media containing 1 mg/l 2,4-D, and 1 mg/l BAP and 0.2 M Mannitol as osmoticum on 90 mm Petri plates a day before bombardment. Plasmid DNA was coated on gold particles after sterilizing them with absolute ethanol, followed by three washing with sterile distilled water. For DNA coating, 60 mg sterilized gold particles were re-suspended in 1 ml sterile distilled water, an aliquot of 50 µl was added with 5 µl of plasmid DNA (1 µg/µl) along with 20 µl of 2.5 M CaCl2 and 20 µl of 0.1 M spermidine [4]. The mix was incubated in ice for 15 min, centrifuged to pellet down, and then, re-suspended in 50 µl sterile distilled water. About 15 µl of this mixture was used for each bombardment and each target was bombarded only once and ten replicates were used per treatment (L1, L2 & L3).
Agrobacterium-mediated genetic transformation
Agrobacterium culture and liquid infection were performed at pH 7.2, while co-cultivation of Agrobacterium and embryogenic calli was carried out at pH 5.8 (Table 2). The pCAMBIA1301 plasmid was mobilized into Agrobacterium EHA105 strain by the freeze–thaw method of transformation. The Agrobacterium harboring the pCAMBIA1301 was grown in YEB medium containing 50 µg/ml Kanamycin (Table 2) for primary culture; and 1 ml of primary culture was used as an inoculum to prepare 25 ml of secondary culture in YEB medium containing 50 µg/ml kanamycin grown at 28 °C, 200 rpm until OD600 of the culture reached 0.8. Embryogenic calli were co-cultivated in the liquid infection medium containing 400 µM acetosyringone [32] and 0.05% silwet-77 for 30 min under 50 kPa vacuum at 28 °C. Following the Agroinfection, the excess of bacteria was removed by blotting on dry sterile filter paper. The calli were then transferred to a freshly prepared co-cultivation medium for 3 days under dark at 28 °C. After three days, the co-cultivated calli were submerged while swirling in 500 mg/l cefotaxime solution for 10 min and then washed with sterile distilled water. After blot-drying the calli they were incubated for 3 days for post-transformation culture and were then used to check for the frequency of transient GUS expression and the number of GUS spots per callus by using histochemical GUS assay. The transformation event was repeated ten times for the statistical analysis.
Transient GUS expression analysis
For the bombardment, each treatment was replicated ten times and ~ 30 calli were randomly sampled from each bombarded event and ten such events were considered from each treatment, whereas in the case of Agrobacterium-mediated method, the transformation event was repeated ten times. Transient GUS expression analysis was carried out for the transformed calli from both the methods by randomly selecting ~ 30 calli after 3 days of post-transformation incubation. The standard method of GUS histochemical assay [14] was used to determine the transformation efficiency for the transformation methods [4, 21] in terms of the percentage of transient GUS expression and the GUS spots per callus. The selected calli were submerged in enough GUS assay solution and incubated at 37 °C for 24 h. The number of GUS positive calli per treatment and the number of blue spots per positive callus were counted for both the methods for comparative analysis. For bombarded calli, stable GUS expression analysis was also performed after 30 days of the transformation event.
Selection of transformed calli and shoot development
The putatively transformed calli were cultured on the selection medium and kept in the dark at 25 ± 2 °C for further multiplication of transformed calli. The selected calli were transferred to maintenance medium and multiplied further to use for shoot induction. For shoot induction, the positive calli were cultured on freshly prepared regeneration medium and incubated in 16 h photoperiod under the fluorescent lamps at 25 ± 2 °C. Shoot development from the selected calli was observed after 21–28 days of inoculation. One subculture was made after 21 days, and for shoot elongation the emerged shoots (2–4 cm long) were transferred to the half-MS medium. The leaves from the putative transgenic plants were incubated in GUS assay solution for 48 h at 37 °C, washed properly in 70% ethanol for de-pigmentation, and observed under a stereo zoom microscope.
PCR confirmation of transformants
For PCR analysis of the regenerated putative transgenic plants, genomic DNA was isolated from the leaves using DNeasy Plant Mini Kit (Qiagen). The genomic DNA was used as a template for PCR amplification of the GUS gene using forward 5′ CATGAAGATGCGGACTTACG 3′ and reverse 5′ ATCCACGCCGTATTCGG 3′ primers designed for the gene.
Data analysis
The experiments or transformation events were replicated ten times. For callus induction, ten explants of each stage (four stages) were inoculated in a flask and cumulative results of 3 flasks (10 × 3 = 30 explants) were considered as one replicate, for shoot regeneration, ~ 100 mg of embryogenic calli were cultured as one unit and five to seven units of calli were incubated in one flask. One set of readings was taken from five flasks and the cumulative response was taken as one replicate. For the gene gun experiment, one plate was bombarded once and each treatment (L1, L2, and L3) were repeated ten times. Similarly, for Agrobacterium-mediated transformation, data was collected from ten different events/replicates for statistical analysis. One way analysis of variance (ANOVA) was performed using IBM SPSS Statistics 21 offline software.
Results
Induction and maintenance of embryogenic calli
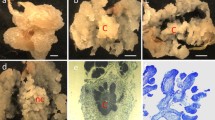
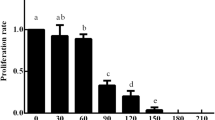
Callus induction was observed from different parts of the floret explants after 10–13 days of inoculation. The induction of the calli was not uniform from the different parts of the floret. Out of the four developmental stages (stages I, II, III and IV) of immature inflorescences, stage IV showed an optimal level of callus induction (86.00 ± 0.83%) (Table 3, Fig. 1). Initially, both embryogenic and non-embryogenic calli were generated on the explant, the embryogenic calli were compact, fragile, and white but the non-embryogenic calli were soft and sticky (Fig. 2). Only embryogenic calli were selected and cultured on maintenance media containing a lower concentration of 2,4-D (1 mg/l) for further multiplication and subcultured after every 21 days. Before performing the genetic transformation experiments, the regenerative response of the calli in the subsequent subculture cycles was tested and found to be declining in the subsequent subcultures (Fig. 3). Accordingly, for genetic transformation, the calli were used from second and third subcultures when their regeneration response was very high. The regeneration frequency (%) was calculated from the number of regenerated calli and the total number of embryogenic calli inoculated.
Different steps in the induction of embryogenic calli. a pieces of immature inflorescence inoculated on the callus induction medium, b initiation of calli formation on the explant after 10–13 days of inoculation, c embryogenic (EC, black arrow) and non-embryogenic (NEC, white arrow) calli induced from the explant at second subculture (6 weeks)
Effect of the age of embryogenic calli on shoot regeneration frequency (%). The X axis represents the subculture cycles with 1st sub (3 weeks), 2nd sub (6 weeks), 3rd sub (9 weeks) and 4th sub (12 weeks). The regeneration frequency (%) was calculated using the number of calli showing shoot regeneration to the total number of calli inoculated on the regeneration medium. Different lowercase letter on the bar indicate the significant difference (p ≤ 0.05)
Biolistic genetic transformation
Examination of bombarded calli for transient and stable GUS expression after three and thirty days, respectively (Fig. 4), indicated the distance of bombardment to be an important parameter. Among the three different distances of bombardment, L1 showed least (46.00 ± 2.15) frequency (%) of transient GUS expression but the highest (69.33 ± 2.93) stable expression. Bombardment from L2 showed the maximum frequency (%) in transient expression; however, stable GUS expression decreased (Fig. 5).
The bombarded calli were selected on hygromycin containing MS medium, and the surviving calli were subcultured for multiplication. About 25% (625 out of 2500) of the calli survived on the selection medium which was transferred to maintenance medium containing hygromycin for the second round of selection and ultimately 100 positive calli had survived. Supplementing the maintenance medium with adjuvants like casein hydrolysate (300 mg/l), two amino acids: l-proline (400 mg/l) and l-arginine (400 mg/l) showed a better multiplication of embryogenic calli and subsequent development of somatic embryos which was also reported by Shashi [32]. Transformation efficiency (%), as evaluated in terms of transient (after 3 days) and stable (30 days after bombardment) GUS expression of the bombarded calli (average of the three different lengths), was observed to be 56.33 ± 2.36 and 4.96 ± 0.21 respectively. The selected 100 GUS positive calli were used for plantlet regeneration. Initially, during shoot development, the selected calli formed globular somatic embryo-like structures just after a week of culture on the regeneration medium (Fig. 6a). Leaflets started emerging from the calli after 21 days (Fig. 6b) and grown further into fully grown shoots (Fig. 6c). A total of 40 plants could be regenerated from the 100 selected positive calli, which were further screened for stable integration of the GUS gene by PCR using GUS-specific primers. Among the 40 regenerated plants, only 6 could be confirmed to be PCR positive for the GUS gene (Fig. 7a). Leaves from the PCR positive plants were also analyzed through histochemical GUS assay, and all of them were found to be GUS positive (Fig. 7b). Thus, in our study, six transgenic shoots could be regenerated and validated using histochemical GUS assay as well as by PCR analysis.
Molecular and histochemical analyses of the regenerated plants. a PCR amplification of GUS gene in regenerated plants (1–6), a positive (pCAMBIA1301 DNA) control, and negative (non-transgenic) plant using GUS-specific primers, b GUS expression in leaf of the regenerated negative (non-transgenic) and positive (transgenic) control plants
Agrobacterium-mediated transformation
Co-cultivation was continued for three days on maintenance medium after transformation. Transformation efficiency was estimated after three days of co-cultivation in terms of the % transient GUS expressing calli by counting the number of GUS positive calli out of the total number of calli selected for the assay (Fig. 8). Agrobacterium-mediated transient GUS expression frequency (%) was observed to be 11.16 ± 1.41. The average number of GUS spots per callus was 3.21 ± 0.17. When we transferred the co-cultivated calli on the selection medium (MS containing 30 mg/l hygromycin, 1 mg/l 2,4-D and 1 mg/l BAP; Table 2) the calli failed to multiply indicating the need for optimizing the conditions required for selection and regeneration of transgenic plants. Therefore, only transient GUS expression (%) and GUS spots per callus were evaluated in this case.
Comparative analysis of the transformation methods
For both the methods (biolistic- and Agrobacterium-mediated), the frequency (%) of transformation was calculated by counting the total number of calli with GUS stain per total number of calli selected for analysis by selecting randomly from the transformed calli for each transformation event. The GUS spot per callus was calculated by counting the number of GUS spots present in one positive callus to the total number of GUS positive calli. Comparative analysis of two transformation methods of embryogenic calli of C. ciliaris using pCAMBIA1301 binary vector revealed that transient GUS expression frequency (%) is significantly higher in the case of biolistic gun-mediated transformation. The transformation efficiency in terms of the mean percentage of GUS expressing calli was observed to be 56.33 ± 2.36 in gene gun as compared to merely 11.17 ± 1.41 in the case of the Agrobacterium-mediated transformation method. However, the frequency of GUS spots per callus for both the transformation method was comparable (Fig. 9).
Discussion
Many researchers have reported immature inflorescence to be an excellent explant for embryogenic callus induction [2, 16, 22, 23, 32, 35] however; no mention was made about the stage of its collection for better response in terms of induction of embryogenic calli. The failure of callus induction at early stages (I and II) of inflorescence development (Fig. 1a, b) might be attributed to the negative impact of phytohormones on younger tissues, change in the environmental condition from in vivo to in vitro conditions. Browning of explants or cultured tissues is one of the major problems in tissue culture, which is mainly caused by phenolic compounds and might also depend on the sampling season [8, 9]. The age-dependent response of immature inflorescence was also reported in pearl millet [15]. At a lower concentration of 2,4-D, the embryogenic calli could be maintained for a longer period through subculturing which was also reported in other grass species [15, 35]. Another limitation in tissue culture is the loss of regenerability in calli over repeated subculturing. For tissue culture mediated genetic transformation, a larger quantity of calli with high regenerable capacity is obligatory which also requires a repeated cycle of subculturing. Therefore, the regeneration ability of the embryogenic calli was tested after every subculture till the fourth at 21 days interval and found to be declined in repeated subcultures. Tissue culture studies on cereal crop plants demonstrated the negative impact of prolonging the culture of calli on 2,4-D containing medium on their regeneration potential [5, 12, 36].
Biolistic transformation is a steady and efficient method of genetic transformation regardless of the plant species, genotype, or tissues compared to the Agrobacterium-mediated genetic transformation which is a cost-effective method for selected plant species. The Agrobacterium-mediated method, being a biological process of transformation, has many limitations but the gene gun method, being a physical process, can be applied, at least theoretically, to any plant tissue or species [7, 33]. Irrespective of its efficiency, many physical and biological parameters such as the concentration of DNA, velocity of bombardment, osmoticum, precipitation procedure, and type of explant used for transformation are critical for enhancing transformation efficiency in biolistic transformation [10, 31]. In addition to these factors, our study indicates the importance of the distance of bombardment for better transformation frequency in buffelgrass. Maximum transient GUS expression was observed at 9 cm distance which did not indicate completely integrated DNA into the cells. Accomplishment of maximum transient expression from a medium distance due to the lesser damage to the bombarded cells as well as GUS expression from the introduced, non-integrated DNA fragments/gene cassettes was reported in the earlier studies [20]. Our results are corroborated by Bhat et al. [4] who compared similar distances with similar results so that bombardment at 10 cm distance gave the highest transient expression, albeit with a different explant, promoters and osmotic medium. But, in the present study, the stable GUS expression was higher when the calli were bombarded at a shorter distance (6 cm) which could be ascribed to the greater force at 6 cm that delivered DNA more deeply into the callus, resulting in higher stable expression. A higher stable expression is one of the prerequisites for a successful genetic transformation of any plant. As the biolistic gene gun directly delivers the foreign gene into the target cells/tissues, its transformation efficiency in terms of transient GUS expression was higher compared to that of the Agrobacterium-mediated method. For efficient Agrobacterium-mediated transformation, embryogenic calli were co-infected for 30 min [3] with the application of vacuum, acetosyringone [32], and a surfactant (Silwet-77 0.05%). Although Agrobacterium-mediated transient GUS expression in buffelgrass was first reported by Batra and Kumar [3], till now, there is no evidence of any stable transformation using Agrobacterium which appears to be a problem with the transformation protocol or callus development under selection rather than with regeneration. The transient expression results observed in our study were not as high as that achieved by Batra and Kumar [3] using the same construct and genotype, but a different explant, Agrobacterium strain and the protocol and stronger selection. Variation in the level of transient GUS expression between these two studies could be ascribed to the age of the callus used in our study, the high pH used during infection, and the prolonged period under the vacuum which may also have affected the results. Shoot development could be induced from the bombarded embryogenic calli but not from the calli transformed by Agrobacterium-mediated method, which indicated the requirement of further optimization of transformation protocol using the later method. Validation of six transgenic shoots using histochemical GUS assay and PCR analysis confirmed the establishment of a proper genetic transformation method for C. ciliaris. However, the copy number of inserted fragments in six transgenic plants could not be determined due to technical limitations. Still, generally Cenchrus ciliaris is difficult to regenerate via somatic embryogenesis which is further complicated by genotype specificity. Hence, our new results represent the “fine tuning” of the regeneration protocol while optimizing a transformation protocol, to be regarded as the major incremental advancement. A large number of regenerated plants were observed to be GUS negative probably because of fragmentation of GUS gene cassette from the hygromycin gene cassette present in the selected calli or the calli somehow escaped selection pressure and survived on the hygromycin containing MS medium. Regeneration of escape plants has been reported with a significantly higher (13%) frequency earlier in rice [19].
Conclusion
This study presents a critical staging of the immature inflorescences as explant for the induction of embryogenic calli in buffelgrass. Comparative analysis of the biolistic- and Agrobacterium-mediated transformation methods indicated a significantly higher transformation efficiency of the biolistic method compared to that of the Agrobacterium-mediated method. However, the average number of GUS spots per callus was observed to be the same by both the methods. Transgenic shoots were regenerated from the bombarded calli which is a significant advancement towards genetic transformation of C. ciliaris, however, further optimization of protocol for plantlet regeneration from the Agrobacterium-mediated transformed calli is required.
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxy acetic acid
- BAP:
-
6-Benzylaminopurine
- GUS:
-
β-Glucuronidase
- psi:
-
Pound per square inch
- kPa:
-
Kilo pascal
References
Ayerza R. Buffelgrass: utilisation and productivity of a promising grass. Buenos Aires: Hemisferio Sur; 1981.
Batra S, Kumar S. In vitro high frequency plant regeneration in buffelgrass (Cenchrus ciliaris L.). J Plant Biol. 2002;29:191–4.
Batra S, Kumar S. Agrobacterium-mediated transient GUS gene expression in buffel grass (Cenchrus ciliaris L.). J Appl Genet. 2003;44(4):449–58.
Bhat V, Dalton SJ, Kumar S, Bhat BV, Gupta MG, Morris P. Particle-inflow gun-mediated genetic transformation of buffel grass (Cenchrus ciliaris L.): optimizing biological and physical parameters. J Appl Genet. 2001;42:405–12.
Bregitzer P, Campbell RD, Wu Y. Plant regeneration from barley callus: effects of 2,4-dichlorophenoxyacetic acid and phenylacetic acid. Plant Cell Tiss Org. 1995;43:229–35. https://doi.org/10.1007/BF00039949.
Burson BL, Hussey MA, Actkinson JM, Shafer GS. Effect of pollination time on the frequency of 2n+n fertilization in apomictic buffelgrass. Crop Sci. 2002;42(4):1075–80. https://doi.org/10.2135/cropsci2002.1075.
Christou P. Particle bombardment for genetic engineering of plants. Biotechnology Intelligence Unit. UK: AcademicPress; 1996.
Chuanjun X, Zhiwei R, Ling L, Biyu Z, Junmei H, Wen H, Ou H. The effects of polyphenol oxidase and cycloheximide on the early stage of browning in Phalaenopsis explants. Hortic Plant J. 2015;1(3):172–80. https://doi.org/10.16420/j.issn.2095-9885.2015-0030.
El-Dengawy ERFA, Elyazid DMA, Attia AAM. Studies on In vitro surface sterilization and control of phenolics on guava (Psidium guajava L.) nodal explants. Pak J Biotechnol. 2017;43(1):105–14.
Finner JJ, Vain P, Jones MW, Mcmullen MD. Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep. 1992;11:323–8. https://doi.org/10.1007/BF00233358.
Fisher WD, Bashaw EC, Holt EC. Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus. Agron J. 1954;46:401–4.
Green CE, Phillips RL. Plant regeneration from tissue cultures of maize. Crop Sci. 1975;15:417–21.
Ishida Y, Hiei Y, Komari T (2007) Agrobacterium-mediated transformation of maize. Nat Protoc 2(7):1614–1621. https://doi.org/10.1038/nprot.2007.241
Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. https://doi.org/10.2135/cropsci1975.0011183X001500030040x.
Jha P, Yadav CB, Anjaiah V, Bhat V. In vitro plant regeneration through somatic embryogenesis and direct shoot organogenesis in Pennisetum glaucum (L.) R. Br. In Vitro Cell Dev Biol Plant. 2009;45:145–54. https://doi.org/10.1007/s11627-009-9198-6.
Kackar A, Shekhawat NS. Plant regeneration from cultured immature inflorescences of Cenchrus setigerus and C ciliaris. Indian J Exp Biol. 1991;29:62–4.
Kumar J, Shukla SM, Bhat V, Gupta S, Gupta MG. In vitro plant regeneration and genetic transformation of Dichantium annulatum. DNA Cell Biol. 2005;24:670–9. https://doi.org/10.1089/dna.2005.24.670.
Kumar S. Epigenetic control of apomixis: A new perspective of an old enigma. Adv Plants Agric Res. 2017;7(1):227–33. https://doi.org/10.15406/apar.2017.07.00243.
Kumar S, Chandra A. In vitro plantlet regeneration in Stylosanthes spp via callus induction from cotyledonary and hypocotyl explants. Natl Acad Sci Lett. 2010;33:289–97. https://doi.org/10.1016/S2221-1691(14)60206-9.
Kumar S, Arul L, Talwar D, Raina SK. PCR amplification of minimal gene expression cassette: an alternative, low cost and easier approach to clean DNA transformation. Curr Sci. 2006;91:930–4.
Kumar S, Bhat V, Bhat BV, Gupta MG. Agrobacterium-mediated Transformation of lucerne (Medicago sativa Linn): optimizing biological and physical parameters. Indian J Biotechnol. 2002;1:298–300.
Kumar S, Bhat V. High frequency direct plant regeneration via multiple shoot induction in the apomictic forage grass Cenchrusciliaris L. In Vitro Cell Dev Biol Plant. 2012;48:241–8. https://doi.org/10.1007/s11627-012-9428-1.
Kumar S, Sahu N, Singh A. High-frequency in vitro plant regeneration via callus induction in a rare sexual plant of Cenchrusciliaris L. Vitro Cell Dev Biol Plant. 2015;51:28–34. https://doi.org/10.1007/s11627-015-9664-2.
Kumar S, Saxena S. Sequence characterized amplified regions linked with apomictic mode of reproduction in four different apomictic Cenchrus species. Mol Plant Breed. 2016;7:1–14. https://doi.org/10.5376/mpb.2016.07.0008.
Kumar S, Saxena S, Rai A, Radhakrishna A, Kaushal P. Ecological, genetic, and reproductive features of Cenchrus species indicate evolutionary superiority of apomixis under environmental stresses. Ecol Indic. 2019;105:126–36. https://doi.org/10.1016/j.ecolind.2019.05.036.
Martin MH, Cox JR, Ibarra-F F. Climatic effects on buffelgrass productivity in the Sonoram Desert. J Range Manage. 1995;48:60–3.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x.
Nishimura A, Aichi I, Matsuoka M. A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc. 2006;1(6):2796–802. https://doi.org/10.1038/nprot.2006.469.
Rao AS, Singh K, Wight JR. Productivity of Cenchrus ciliarisin relation to rainfall and fertilization. J Range Manage. 1996;49:143–6.
Saha P, Blumwald E (2016) Spike-dip transformation of Setaria viridis. Plant J 86(1):89–101. https://doi.org/10.1111/tpj.13148
Sailaja M, Tarakeswari M, Sujatha M. Stable genetic transformation of castor (Ricinus communis L) via particle gun-mediated gene transfer using embryo axes from mature seeds. Plant Cell Rep. 2008;27:1509–19. https://doi.org/10.1007/s00299-004-0898-4.
Shashi. Developmental morphogenesis and in vitro genetic manipulation of Cenchrus ciliaris L. Thesis. University of Delhi. 2013. https://hdl.handle.net/10603/26730
Sharma KK, Bhatnagar-Mathur P, Thorpe TA. Genetic transformation technology: status and problems. In Vitro Cell Dev Biol plant. 2005;41:102–12. https://doi.org/10.1079/IVP2004618.
Visser NC, Spies JJ, Venter HJT. Apomictic embryo sac development in Cenchrus ciliaris (Panicoideae). Bothalia. 2000;28:83–90.
Yadav CB, Jha P, Mahalakshmi C, Anjaiah V, Bhat V. Somatic embryogenesis and regeneration of Cenchrus ciliaris genotypes from immature inflorescences explants. Biol Plantarum. 2009;53(4):603–9. https://doi.org/10.1007/s10535-009-0111-2.
Ziauddin A, Kasha KJ. Long-term callus cultures of diploid barley (Hordeum vulgare). II. Effect of auxins on chromosomal status of cultures and regeneration of plants. Euphytica. 1990;48:279–86. https://doi.org/10.1007/BF00023662.
Acknowledgements
Laishram Sundari Devi would like to thank University Grants Commission (UGC), India for providing financial assistance in the form of J.R.F and S.R.F. The authors express sincere gratitude to the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), Mumbai (37(1)/14/10/2016-BRNS/37024) for sponsoring this research work by providing financial assistance. We are also grateful to the University of Delhi for Research and Development grant at the initial stage of research work. We would like to thank Director, National Institute of Genome Research (NIPGR), New Delhi and Prof. Rajesh Tandon, Department of Botany, University of Delhi, for providing access to the CIF and laboratory facilities respectively, to perform particle bombardment experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Manoj Prasad.
Rights and permissions
About this article
Cite this article
Laishram, S.D., Goyal, S., Shashi et al. Assessment of biolistic and Agrobacterium-mediated genetic transformation methods in Cenchrus ciliaris. Nucleus 63, 303–312 (2020). https://doi.org/10.1007/s13237-020-00332-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-020-00332-1