Abstract
Aquaculture is an extremely valuable and rapidly expanding sector worldwide, but concerns exist related to environmental sustainability. The sediment below aquaculture farms receives inputs of antimicrobials, metal-containing products, and organic matter from uneaten food and fecal material. These inputs impact the surrounding marine microbial communities in complex ways; however, functional diversity shifts related to taxonomic composition remain poorly understood. Here, we investigated the effect of pollution from marine fish farms on sediment bacterial communities. We compared the bacterial communities and functional bacterial diversity in surface sediments at salmon aquaculture and reference sites in Chiloé, southern Chile, using Roche 454 pyrosequencing of the 16S ribosomal RNA (rRNA) gene and the predictive metagenomics approach (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States, PICRUSt). Bacterial diversity, measured as the inverse Simpson index, was significantly lower at aquaculture than at reference sites, while species richness, based on Chao’s estimator, was not significantly different. Nevertheless, community composition differed significantly between reference and aquaculture sites. We found that Gammaproteobacteria and several taxa involved in remediating metal contamination and known to have antimicrobial resistances were enriched at aquaculture sites. However, PICRUSt predicted functions indicated a degree of functional redundancy between sites, whereas taxonomic-functional relationships indicated differences in the functional traits of specific taxa at aquaculture sites. This study provides a first step in understanding the bacterial community structure and functional changes due to Chilean salmon aquaculture and has direct implications for using bacterial shifts as indicators of aquaculture perturbations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Salmon aquaculture has rapidly expanded and Chile is the second largest producer of Salmo salar, after Norway (Food and Agriculture Organization of the United Nations, FAO 2016). The world population is expected to reach ~ 9 billion by 2050, with aquaculture activities playing a key role in its growth (Bostock et al. 2010; World Bank 2013). However, intensive salmon production systems require exogenous feed inputs (Buschmann et al. 2008). Uneaten fish feed, fecal matter, and excretory products accumulate in sediments below fish cages (Carroll et al. 2003; Buschmann et al. 2006) and form a layer of soft black sediment (Holmer et al. 2008). These sediments have lower pH (Hargrave et al. 1993), higher concentrations of organic matter, and greater accumulation of nutrients, particularly phosphorus and nitrogen compounds (Karakassis et al. 1998), than reference areas. These organic inputs modify the physical and chemical properties of the sediment and influence biogeochemical processes, which alter the structure of benthic microbial communities (McCaig et al. 1999; Asami et al. 2005; Kawahara et al. 2009; Fodelianakis et al. 2015). Organic inputs from Chilean salmon aquaculture also shift stream ecosystems to a more heterotrophic state, which impairs ecosystem health (Kamjunke et al. 2017).
Bacterial communities present in marine sediments provide the important ecological role of nutrient cycling, mineralization, degradation, and diagenesis of organic matter (Deming and Baross 1993; Gooday 2002; Vezzulli et al. 2002; Buschmann et al. 2008). These communities also play a vital role in the transformation of pollutants (Benoit et al. 2003; Smith and Hollibaugh 1993); however, it remains unknown how inputs from salmon farming in Chile modify these bacterial communities. The use of antimicrobials for preventing and controlling pathogens in salmon aquaculture is common in Chile, and has resulted in an increased antibiotic resistance of bacteria in the environment (Miranda and Zemelman 2002a, b; Buschmann et al. 2012). Fish farmers also use metal-containing products and other pharmaceuticals to prevent fouling, to feed and to treat fish in order to limit the spread of infections (Burridge et al. 2010). This has resulted in elevated copper (O’Brien et al. 2009) and zinc (Simpson and Spadaro 2012; Simpson et al. 2013) concentrations in sediments. Because microbial communities respond rapidly to environmental variation, identifying changes in sediment microbial composition and function represents a useful indicator of aquaculture impacts on coastal environments. However, the changes in sediment bacteria communities in and around aquaculture operations is complex and results from the combination of heavy metals, antibiotics, and organic depositions. Therefore, scientific attention to understand environmental complexities and to characterize impacts is critical.
Most of our knowledge regarding the environmental impact of salmon cage culture has resulted from studies on shifts in benthic macrofaunal communities (Carroll et al. 2003; Macleod and Forbes 2004; Tomassetti and Porrello 2005; Hall-Spencer et al. 2006). Many of these studies used video surveillance and identified macrofauna and meiofauna to individual species level within each sample, which requires large monetary, time, and skill investment to provide insight into impact (Castine et al. 2009). For the successful characterization of impacts, stable macrofauna distributions, low wave and current activity, and low water turbidity are necessary. However, macrofauna communities tend to be highly variable in space and time. In addition, strong tidal currents, high depths (> 60 m) and turbidity associated with the salmon-aquaculture region can inhibit the ability to monitor macrofauna communities. Due to the rapid proliferation of salmon aquaculture in southern Chile, the improvement of monitoring programs in relation to salmon cage culture is important. Changes in bacteria community structure and abundance have been used as a monitoring tool to investigate the impact of fish farms in Tanzania (Bissett et al. 2007) and in the tropics (Castine et al. 2009). Additionally, studies of subsurface sediments below fish cages have demonstrated increases in bacterial abundances compared to reference sites (Mirto et al. 2000; Vezzulli et al. 2002; Bissett et al. 2007; Castine et al. 2009), changes in bacterial community structure (Bissett et al. 2006, 2007, 2009; Garren et al. 2008), and functionality (Christensen et al. 2000; Holmer et al. 2003; Bissett et al. 2009), indicating that identifying bacterial community changes provides valuable insights regarding the ecosystem-wide response to aquaculture pollution and the potential biogeochemical process modifications.
In soft-bottom communities, microbes provide important ecological services such as nutrient cycling and organic matter mineralization, so understanding the effect of pollution from aquaculture is critical to understanding the ecosystem-wide response (Bissett et al. 2006; Castine et al. 2009). The response of bacterial communities to aquaculture inputs has remained unexplored until recent years; however, advances in molecular techniques have enabled in-depth studies of the response of benthic bacterial communities to organic depositions from aquaculture. To our knowledge, studies providing in-depth analyses of microbial community composition and metabolic function of aquaculture-exposed environments in Chile remain scarce and require attention to target more specific and complex ecological studies. Microbes are generally the first organisms to respond to chemical and physical changes in the environment and, due to their low trophic level, can be used as indicators of environmental change (Zak et al. 2011). Sediments under fish farms provide suitable tools to monitor the response of bacterial communities to aquaculture perturbations, because inputs and organic load are constantly monitored and deposited, and the deposition site is known (Fodelianakis et al. 2015). Furthermore, non-impacted sites with similar physicochemical characteristics for comparison are easy to find (Bissett et al. 2007). However, the structure of sediment bacterial communities depends largely on the geographic region (Fodelianakis et al. 2015). In Chile specifically, there are concerns regarding the impacts of fish farming on previously pristine marine environments (Buschmann et al. 2006), and it remains unknown how aquaculture inputs affect these sediment bacterial communities.
The aim of this study was to examine changes in sediment bacterial communities associated with salmon aquaculture and the potential links between functional inferences and community structure variation. We compared sediment bacterial community compositions from reference and salmon aquaculture sites in the coastal waters of Chiloé in southern Chile to gain insight into the natural bacterial community composition, diversity, and metabolic function in southern Pacific coastal sediments and their response to organic loading and pollution resulting from salmon aquaculture. This study provides baseline information on bacterial composition modifications resulting from complex environmental modifications associated with salmon aquaculture; therefore, we discuss important future studies.
Materials and methods
Field site and sampling
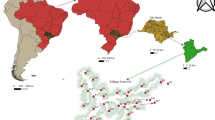
Sediment samples were obtained in November 2012 in the coastal waters of Chiloé, Chile (Traiguén, Quenac North, and Quenac South; 42°32′01.3” S, 073°23′52.8” W) (Fig. 1). This region of Chiloé is characterized by strong tidal currents (up to 18–20 cm s−1), surface temperature ranges of 14–16 °C during spring, and a salinity of 30 ppt (Buschmann, unpublished data). From each of the three locations, sediment was collected from a commercial-scale salmon farm (> 1000 tons of production) site and a reference site. Sediment samples were obtained as close as possible to pens (< 50 m) at a depth of 30–45 m. The organic matter content was > 3.5%, indicating that the sediments were influenced by aquaculture (e.g., Carroll et al. 2003; Soto and Norambuena 2004). Chemical changes in sediments due to aquaculture have been identified in southern Chile (Table 1), and we, thus, infer similar conditions at aquaculture sites. Reference sites were located ca. 2.5 km from salmon aquaculture sites (black symbols; Fig. 1).
At each site, the diver retrieved sediment cores (15 cm inner diameter; N = 3). Immediately after retrieval, the surface sediments of each core (1–2 cm) were collected using a sterile sampling device. Collected samples were placed in tagged plastic bags, stored in a cooler with gel packs, and brought to the laboratory (Centro i-mar in Puerto Montt) within 6 h, where they were frozen at − 80 °C until further analysis. Thus, we collected nine samples from reference sites and nine samples from aquaculture sites, for a total of 18 samples from both sites.
Laboratory and sequencing analysis
DNA was extracted using a modified version of the hot detergent/CTAB DNA extraction protocol (Zhou et al. 1996), using 5 g of sediment, 13.5 mL of extraction buffer, 100 μL Protease K, incubating with 1.5 mL of 20% SDS, then re-extracting with 4.5 mL of extraction buffer and 0.5 mL of 20% SDS. Extracted DNA was verified and quantified by 1% agarose gel electrophoresis with DNA marker (100–1000 pb, 500 ng/mL Winkler). A second quantification and quality check was performed by spectrophotometry using a Tecan Infinite® 200 PRO (Gene X-Press, Santiago, Chile). A Labconco CentriVap® Vacuum Concentrator was used to dry DNA extracts.
Dried samples were sent to Macrogen, Ltd. (Seoul, Korea) for bacterial tag-encoded FLX-Titanium amplicon pyrosequencing (TEFAP) on the 454 GS FLX System (Roche) using standard protocols (Sun et al. 2011). Bacterial 16S ribosomal RNA (rRNA) gene amplicons were sequenced on 1/8th of a plate using Roche 454 sequencing technology with Titanium chemistry.
The sequence processing was performed using the program mothur v.1.31.2 (Schloss et al. 2009) with default command parameters, unless specified. Raw sequences were processed by barcode, primers, length, and quality, and were denoised with the PyroNoise algorithm (Quince et al. 2009). The sequences were checked for chimeras using Perseus (Quince et al. 2011) and UCHIME (Edgar et al. 2011) sequentially, and were aligned to the Greengenes reference (gg_13_8_99). The cluster command was used to assign sequences to operational taxonomic units (OTUs) using the nearest-neighbor algorithm. All subsequent OTU-based analyses were performed using a 97% sequence similarity cutoff. Taxonomic analysis of representative OTUs was conducted in mothur using the Greengenes 16S rRNA gene database (DeSantis et al. 2006). Sequences are available via the NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra) under accession number (PRJNA302218).
We used the bioinformatics tool Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) v.1.1.0 (Langille et al. 2013) to gain further insight into the putative metabolic functions of bacteria enriched at aquaculture sites. This program uses marker genes, in this case, 16S rRNA, to predict metagenome functional content. The metagenome gene functional content predictions are precalculated for genes in databases including the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa and Goto 2000) and Clusters of Orthologous Groups of proteins (COG). In the present study, we used the KEGG database and functional predictions were assigned up to KEGG Orthology (KO) tier 3 for all genes. To simplify analysis, only tier 1 functions of “metabolism”, “genetic information processing”, “environmental information processing”, and “cellular processes” were analyzed further, as the categories of “organismal systems” and “human disease” were thought to be poorly relevant to environmental samples. The accuracy of the metagenome predictions was evaluated using weighted nearest sequenced taxon index (weighted NSTI) scores (Langille et al. 2013).
Data analysis and statistics
Differences of community richness were assessed using the Chao estimator (Chao 1984). Community diversity was examined using the inverse Simpson index (Simpson 1949), which takes species richness and species abundance into account. To compare samples on an equal basis, all samples were rarefied to even sampling depths prior to statistical analysis.
The metastats command in mothur was used to detect differentially abundant OTUs from aquaculture and reference sites. The thetayc calculator (Yue and Clayton 2005) was used to calculate the dendrogram describing the similarity between the structures of reference and aquaculture communities based on the OTUs in mothur. Non-metric multidimensional scaling (NMDS) was performed in mothur using the Bray–Curtis distances between samples and the resulting ordination was visualized using ggplot2 (Wickham 2009) in the statistical software R 3.1.1 (R Core Team 2016). Bray–Cutis OTU-based analysis of similarity (ANOSIM) (Clarke 1993) was performed to test for significant differences between reference and aquaculture samples using 1000 Monte Carlo permutation tests in the vegan R package (Oksanen et al. 2013). t-Tests were used to determine the minimum significant difference in relative abundance species richness (Chao1) and diversity (inverse Simpson) of bacteria between reference and aquaculture samples in R. We tested for statistical significance between aquaculture and reference functional predictions using linear discriminant analysis effect size (LEfSe) (Segata et al. 2011). Spearman correlations relating inferred functional abundance from PICRUSt and taxonomic class abundances were performed for aquaculture and reference sites using R. All statistical analyses were evaluated at α = 0.05.
Results
Bacterial community composition, richness, and diversity
The final OTU dataset consisted of 43,267 reads, with a mean of 7212 sequences per sample (Table 2). The number of randomly subsampled sequences used for normalization from each replicate sample was 1195, for a total of 21,510 sequences, respectively. At 97% sequence similarity, the rarefaction curves were relatively saturated, indicating that sequencing effort captured a large proportion of the taxa present in each sample (Fig. S1). The total number of OTUs observed in each site is shown in Table 2 and the number of OTUs per replicate is shown in Table S1. By using the most recent Greengenes database, we demonstrated that more than 97% of tags could be unambiguously mapped at the genus level.
The OTUs were classified into 47 bacterial classes and the majority of these classes made up less than 1% of the bacterial community at each site. Gammaproteobacteria was the most abundant class at reference (29.1%) and aquaculture (51.3%) sites and represented a greater proportion of the bacterial community at the aquaculture sites (Table 3). The relative abundance of Bacilli decreased at all aquaculture sites. Pairwise comparisons between respective reference and aquaculture sites revealed differences (Fig. 2). Gammaproteobacteria was enriched at AQN and AQS, whereas it slightly decreased in ATR. The relative decrease of Bacilli (28.9% to 3.1%), Gammaproteobacteria (10.7% to 24.2%), and Alphaproteobacteria (36.8% to 8.2%) was compensated by the relative increase in Flavobacteriia (18.8% to 35.3%), Epsilonproteobacteria (0% to 11.1%), and Actinobacteria (0.5% to 8.7%) at AQN (Fig. 2). The relative decrease of Bacilli (37% to 8.7%) and Alphaproteobacteria (23%% to 21.9%) was compensated by the relative increase in Gammaproteobacteria (18.2% to 48.7%), Planctomycetacia (0.5% to 1.2%), and Acidimicrobiia (0.6% to 1.2%) at AQS (Fig. 2). The differences between ATR and RTR were less severe and the only phyla that were slightly enriched at ATR were Deltaproteobacteria (1.5% to 1.7%) and Actinobacteria (0.4% to 0.8%). Within the class Gammaproteobacteria, Psychrobacter was enriched at all aquaculture sites, Shewanella was enriched at AQS, and Glaciecola was enriched at RTR (Fig. 2). Within Bacilli, Exiguobacterium decreased at aquaculture sites and within Alphaproteobacteria, Loktanella decreased at all aquaculture sites. Within Flavobacteriia, Olleya was enriched at AQN, AQS, and RTR (Fig. 2).
The average relative abundances of bacterial classes in reference (RQN, RQS, RTR) and aquaculture (AQN, AQS, ATR) sites (a). Groups accounting for < 1% of all sequences in all sites are summarized in the group “Others”. The relative abundances of bacterial genera in reference (RQN, RQS, RTR) and aquaculture (AQN, AQS, ATR) sites (b). Groups accounting for < 1.5% of sequences at a site are summarized in the group “Others”
Bacterial communities were dissimilar between reference and aquaculture sites (ANOSIM, R = 1.5, P = 0.03). Additionally, when the bacterial community composition of the 18 sediment samples from reference and aquaculture sites was compared using an OTU-based approach, the results revealed differences between sample types (Fig. 3, Fig. S2). The bacteria community structure of Quenac South aquaculture 2 (AQS2) and Traiguén reference 3 (RT3) differed from all other samples and sites. All reference samples clustered together, with the aquaculture site Quenac South 3 (AQS3) present. Similarly, Traiguén reference 1 and 2 (RT1, RT2) were the only reference sites present in the second cluster among aquaculture sites (Fig. S2). NMDS cluster analysis revealed similar results, with RT1 clustered among aquaculture samples and AQN3 clustered among reference samples (Fig. 3).
Bacterial diversity (inverse Simpson) differed significantly (P = 0.04) between aquaculture and reference sites, with higher diversity in reference (9.48 ± 6.25) than in aquaculture (5.32 ± 2.07) sites (Table 2). The species richness (Chao1) ranged from 275.4 to 331.9, with no statistical differences (P > 0.05) between salmon aquaculture and reference sites, indicating that it is less sensitive than diversity to pressures caused by pollution from aquaculture.
Functional characterization of bacterial communities
The PICRUSt metagenome predictions had NSTI scores ranging from 0.13 to 0.14, with an overall mean of 0.14 ± 0.003, which was lower than that reported for sediment bacterial communities (0.17 ± 0.02; Langille et al. 2013). Lower NSTI values indicate that microbes in each sample are more closely related to sequenced genomes (Langille et al. 2013). Notably, PICRUSt showed little variation in the abundances of second- and third-tier KO functional gene annotations in aquaculture and reference sites (Table 4 and Table S3). The highest standard deviation observed within the tier 2 functional category was 0.08% of sequence reads in references sites and 0.004% of sequence reads in aquaculture sites. When third-tier functional categories were compared, the maximum standard deviation for a category was 0.04% of sequence reads in reference sites and 0.003% of sequence reads in aquaculture sites, suggesting similarities in the distribution and abundance of functional traits within treatments.
To determine which bacterial classes may be contributing to differences in functional traits among sites, correlation analyses were performed using PICRUSt inferences relating bacterial classes and genera (occurring at > 0.01% abundance) with second-tier functional classifications (Table S3). A greater number of correlations were observed between functional and taxonomic abundance in aquaculture sites. Overall, the majority of the correlations were negative at reference sites, and about half were negative and half were positive at aquaculture sites. Positive taxonomic-functional correlations among the PICRUSt data, however, are likely a result of autocorrelations, as functional traits were predicted from taxonomic information (Staley et al. 2014). In aquaculture sites, the abundances of almost all second-tier functions were also correlated with the abundances of at least one of the most abundant classes identified in either dataset (Table S2), most notably the Gammaproteobacteria and Flavobacteriia. At the genus level, there were the greatest number of correlations at aquaculture sites with the second-tier function of amino acid metabolism and, notably, Shewanella was correlated with a number of second-tier functions (Table S3). In reference sites, only one second-tier function, “carbohydrate metabolism”, was correlated with an abundant class (Gammaproteobacteria), while the rare Verrucomicrobiae was the only other order with significant correlations. At the genus level, Oceanibulbus was correlated with several second-tier functions, but reference sites had less correlations overall. Interestingly, none of the functional trait abundances differed significantly between sites.
Discussion
We found a significant difference in the bacterial community structure between aquaculture and reference sites. Several authors have reported that bacterial community changes and respiration processes in sediments below fish farms reflect impacts from nutrient enrichment (Christensen et al. 2000; Holmer et al. 2003; Bissett et al. 2007, 2009; Kawahara et al. 2009). Nutrient enrichment from fish cages causes a significant increase in bacterioplankton abundance and heterotrophic production (Sakami et al. 2003; Sarà 2007; Garren et al. 2008; Navarro et al. 2008; Nogales et al. 2011), as well as in the abundance of virus-like particles (Garren et al. 2008). In addition, the bactericidal action of antibiotics can lead to changes in the composition of microbial communities by selectively inhibiting susceptible bacteria (Nogales et al. 2011). Heavy metals are highly persistent and several authors have demonstrated that heavy metal contamination significantly shapes bacterial community composition (Quero et al. 2015; Yao et al. 2017). Therefore, it is possible that the chemical changes in the aquaculture sediments (Table 1) were reflected by the changes in bacterial groups identified in this study; however, more research is necessary to test this hypothesis, and to separate the importance of the different components that may affect these bacterial communities.
We found a significant decrease in bacterial biodiversity in aquaculture sediments compared to reference sediments. This conclusion was also supported by bacterial composition analysis, which indicated a significant difference in the community structure between sites. However, pairwise comparisons demonstrated that bacterial composition varied between sites, which we assume results from differences in the combinations of antimicrobials, heavy metals, and organic depositions that provide the sediment an increased amount of carbon, nitrogen, and phosphorus (Hargrave et al. 1997; Ruohonen et al. 1999; Storebakken et al. 2000). Unfortunately, we were unable to obtain data on the fecal microbiotas of salmon reared at the farms and recognize that this could explain pairwise differences between sites; therefore, future studies should incorporate this into analyses.
The number of OTUs was higher in aquaculture sites. Many studies have shown that impacted sites (from different sources of pollution) and reference sites are rich in bacterial OTUs (Fodelianakis et al. 2014, 2015; Sanz-Lázaro et al. 2015). In addition, some have demonstrated that impacted sites are richer in bacterial OTUs than reference sites (McCaig et al. 1999; Powell et al. 2003; Bissett et al. 2007; Marcial Gomes et al. 2008), while others have found no difference between the two (Torsvik et al. 1996; Bissett et al. 2006; Zhang et al. 2008; Kawahara et al. 2009; Fodelianakis et al. 2015). The differences in these studies may be attributed to the differing dynamics of microbial communities in varying locations, differing geological and chemical characteristics (e.g., Bissett et al. 2006; Tamminen et al. 2011), or due to the varying types or severity of the disturbance in each case.
Effects of salmon farming on bacterial community structure
The NMDS showed a difference in the bacterial community at aquaculture sites, a trend also noted in previous studies (Bissett et al. 2006). Although recent studies use the UniFrac-based distance calculation (Lozupone et al. 2011) for comparing microbial communities, we found that the Bray–Curtis index provided sharp contrasts on the differences in the microbial community composition of aquaculture sites studied. One of the most notable differences among sites was the high abundance of Gammaproteobacteria at aquaculture sites. This result corresponds with previous research of fish farm sediments (Asami et al. 2005); however, those within this class were related to potential sulfate-reducing bacteria (SRB), whereas ours were not. PICRUSt inferences indicated that Gammaproteobacteria could potentially be involved with several functions at aquaculture sites, but not reference sites. Additionally, Gammaproteobacteria has been found to be the most significant clade present in most marine sediments (Li et al. 1999; Bowman and McCuaig 2003; Inagaki et al. 2003; Polymenakou et al. 2005), irrespective of pollution levels. In general, many of these studies on aquaculture-impacted sediments (e.g., Bissett et al. 2006; Kawahara et al. 2009) have shown varying bacterial community composition among sites of organic enrichment, highlighting the need for more localized studies focusing on the various inputs from aquaculture. Microbes are at the bottom of the food chain; thus, changes in their taxonomic structure and diversity would influence higher trophic levels in coastal sediment communities. The enrichment of Gammaproteobacteria and the difference in specific microbial diversities at the different aquaculture sites may result from biological traits to adapt, survive, and replenish under the predation pressure, inter- and intraspecific competition resulting from temporal and spatial environmental changes driven by salmon aquaculture.
The presence of metals at these sites is likely, as specific taxa involved in remediating metal contamination were enriched at aquaculture sites. Microbacterium, Psychrobacter, and Shewanella were significantly enriched at aquaculture sites and this enrichment could be explained by several reasons. Several Microbacterium strains are tolerant to heavy metals such as nickel, cobalt, and cadmium (Brown et al. 2012; Iyer et al. 2017). Psychrobacter strains can resist and accumulate several metals, specifically cadmium, lead, zinc, and copper (Abd-Elnaby et al. 2016). Interestingly, Psychrobacter has been isolated from the kidney of salmonids at several aquaculture sites in Scotland (McCarthy et al. 2013) and has previously been isolated from Chilean salmon farms (Roberts et al. 2014). Shewanella are anaerobic metal reducers and have been identified as a commonly occurring intestinal bacterium for salmon (Navarrete et al. 2009). High concentrations of metals, specifically copper and zinc, have been found under cages at fish farms treated with anti-fouling paints (Simpson et al. 2013; Nikolaou et al. 2014). These paints are used in Chilean salmon aquaculture to prevent biofouling on nets, which is critical to maintaining good water flow, ensuring high dissolved oxygen concentrations, and maintaining fish health. Zinc is also a lesser component of paint formulation and is also a dietary additive in salmon feed (Maage et al. 2001). Interestingly, copper and zinc concentrations in sediments did not change when they were monitored over a 12-month fallowing period (Macleod et al. 2014), which suggests that, even if we sampled during a recovery period, the potential presence of metals in the sediment is likely, thus affecting the bacterial communities. However, further research is necessary to characterize potential microbial remediation of metal contamination at aquaculture sites and the various environmental and anthropogenic pressures at each aquaculture site.
The presence of residual antimicrobials in aquaculture sediments is likely, as several genera resistant to antibiotics were enriched at these sites. Antimicrobials used in aquaculture are administered to fish mostly in food (Cabello 2006), which results in increased antimicrobial concentrations in sediments below cages (Armstrong et al. 2005), from where they can be carried by currents to sediments at distant sites (Buschmann et al. 2012). These antimicrobials are the principal selective pressure for antibiotic resistance in sediment bacteria (e.g., Dang et al. 2007; Tomova et al. 2015), and the impact of this process leads to changes in sediment bacteria diversity by replacing susceptible communities of bacteria with resistant ones (e.g., Miranda and Zemelman 2002a; Kim et al. 2004). Tomova et al. (2015) found that bacteria from the genera Cobetia, Pseudoalteromonas, Psychrobacter, and Shewanella at Chilean aquaculture sites harbored multiple antibiotic-resistant genes. These genera were enriched at aquaculture sites, specifically, AQS, AQN, all aquaculture sites, and AQS, respectively. Pseudoalteromonas was also enriched at RTR, which may indicate that these antimicrobials are affecting distant sites; however, further research is necessary to test this hypothesis and measure antimicrobial concentrations in sediments at Chilean aquaculture and reference sites.
One of the anomalies in our dataset was the low abundance of Desulfobacterales at aquaculture sites (0.17% mean abundance). Desulfobacterales are an order of strictly anaerobic SRB and have been found in bacterial communities at aquaculture sites (Bissett et al. 2006; Dowle et al. 2015). SRB play a significant role in the mineralization of organic matter in anaerobic environments and in the biogeochemical cycling of sulfur. Kawahara et al. (2009) reported a high abundance of SRB in sediment around torafugu (Takifugu rubripes) farm sediments (< 100 sequences per sample). Other researchers have used quantitative polymerase chain reaction (PCR) approaches and have shown that organic enrichment associated with marine fish farms influences the abundance of SRB (Kawahara et al. 2008; Kondo et al. 2008, 2012). We did not carry out chemical analysis and are unable conclude if the high organic matter content in the sediments led to the formation of anaerobic sediment. Additionally, we do not understand temporal bacterial community changes associated with salmon aquaculture fallowing strategies, which complicates comprehension of these results. Since 2010, new regulations in Chile require area-specific fallowing periods, and this subject requires further attention in order to understand how bacterial community modifications affect ecosystem functioning. Macleod et al. (2008) reported that the main ecological functions in affected benthic habitats were re-established after 12 months under Australian salmon farms, but there was no evidence regarding bacterial community changes. Additionally, due to logistical constraints, samples were stored on ice for several hours prior to freezing. The possibility that changes in bacterial community composition occurred during this period cannot be excluded, and we recommend that direct freezing in liquid nitrogen is used for future studies. Thus, we suggest further collection, isolation, sequencing, and characterization, including detailed chemical analysis of sediments, due to the importance of this group to sulfur cycling in sediments under fish farms.
Functional changes resulting from salmon aquaculture
Many of the predicted functional profile abundances were redundant between bacterial communities in reference and aquaculture sites. It is useful to supplement 16S rRNA analyses with metagenome studies, especially for broad surveys with microbial ecology applications. Several studies have demonstrated that this stable profile is due to the existence of functional gene redundancy among bacterial communities (Fernandez et al. 2000; Schimel et al. 2007; Fierer et al. 2012). Additionally, despite the quality of the functional predictions by PICRUSt, they are largely dependent on the availability of annotated reference genomes. These results should be interpreted cautiously, as PICRUSt may not be useful for high-resolution studies of functional ecology in diverse and especially perturbed ecosystems until their accuracy is better evaluated and/or databases are improved. A recent study found differences in the relative abundance between PICRUSt inferences and shotgun metagenomic data for every second-tier functional trait (Staley et al. 2014). Although we obtained relevant information on the functions of specific groups through PICRUSt analysis, a shotgun metagenomic study would be valuable to allow for an accurate quantitative assessment of the distribution of functional traits in this ecosystem.
Comparisons of taxonomic-functional correlations between bacteria communities in aquaculture and reference sites suggest less functional redundancy among specific members at each site. Several abundant taxa were correlated with functional traits in aquaculture sites, notably Gammaproteobacteria, Flavobacteriia, and Alphaproteobacteria, but Synechococcophycideae also presented a high number of correlations that do not belong to one of the most abundant groups. In reference sites, the rare Verrucomicrobiae presented a high number of correlations. These data point to an important role of relatively rare groups in the community, by keeping important connections on a larger scale with other groups and displaying important functional traits. A previous study characterizing functional and taxonomic diversity of marine sediments found that the organic carbon content of sediments may be important in structuring communities, more so than geography (Kimes et al. 2013). Another recent study suggested that the responses of functional traits to heavy metal contamination depended more on environmental changes, while stochasticity played an important role in the formation and succession of phylogenetic composition for microbial communities (Ren et al. 2016). Similarly, a previous study showed that stochastic processes played important roles in controlling the assembly and succession of the groundwater microbial community (Zhou et al. 2014). In this study, we provide a snapshot of bacterial communities at aquaculture and reference sites. We speculated that selection strength, mainly changes induced by aquaculture, shaped and directed the functional shift pattern of sediment bacterial communities, but their taxonomic composition had various shift patterns to achieve the same functional shift because similar functional genes are widely distributed. For example, various taxa, including those enriched at aquaculture sites, were correlated to several functional traits at aquaculture sites, but not at reference sites. So, each microbial population correlated with a specific function at only aquaculture sites had a chance to become more abundant at these sites, in theory. However, more research is necessary to test this hypothesis using environmental data and considering time variation of these communities.
Marine sediments are affected by the interaction of geological, hydrological, physicochemical, and biological factors, and function as reservoirs of absorbed nutrients, pesticides, toxic materials, and heavy metals (Köster and Meyer-Reil 2001). The structure of bacterial communities is sensitive to changes in environmental conditions (Danovaro et al. 2000), especially when subjected to nutrient input related to anthropogenic activity (Hansen and Blackburn 1992), such as aquaculture. Aquaculture affects these communities in complex ways as a result of organic inputs, and through the use of antimicrobials, pesticides, and anti-fouling agents. Unfortunately, we did not measure environmental parameters and simply provide a snapshot of these bacterial communities at one time as a first step in characterizing changes resulting from aquaculture. The seasonal variability in marine bacterial communities (e.g., Fuhrman et al. 2006), their ability to respond rapidly to environmental changes, the patterns they exhibit in distribution and abundance as a result of environmental variables (e.g., Du et al. 2013), and the complexities of impacts due to aquaculture make it difficult to accurately assess changes in relation to environmental variables at a single time point. We also note the spatial and temporal variability in environmental parameters at these locations and, therefore, suggest that future studies incorporate multiple time points to characterize impacts both spatially and temporally.
Bacterial communities as indicators of biotic integrity
The impacts of salmon cage farming on the surrounding microbial environments has been studied using various approaches, including physicochemical changes to sediments (Buschmann 2002; Soto and Norambuena 2004), phytoplankton and macrobenthos communities (Buschmann 2002; Buschmann et al. 1994, 2008; Soto and Norambuena 2004; Buschmann and Fortt 2005; Mulsow et al. 2006), and antibiotic resistance (Cabello et al. 2013). In Chile, research on the environmental effects of salmon aquaculture and the impact on adjacent environments remain scarce, especially in relation to production level (Buschmann et al. 2009). Although valuable, the power of macrofaunal analyses is hindered because these organisms vary within and among different patches at one time, especially in temperate seasonal environments (Zajac et al. 2013). Therefore, such analysis would require rigorous sampling to understand changes resulting from culture cages (Fernandes et al. 2001). As microbial communities rapidly respond to environmental changes, using differences in benthic microbial community composition and function as indicators of environmental perturbations represents a powerful monitoring tool. However, the benefits of this monitoring tool are limited without a proper understanding of the specific drivers of such shifts.
Bacteria offer many advantages over other current or proposed bioindicator species, as they are highly ubiquitous, highly abundant in all sediment types (Wang et al. 2012), small, and respond rapidly to environmental changes (Zak et al. 2011). Thus, a minimal amount of sediment is required for analysis. Current benthic macrofaunal techniques require 500–1000 g of sediment. In this study, we used 5 g of sediment, but this could likely be reduced further. We acknowledge that this study only encompassed a small dataset and is only representative of one type of condition, i.e., high water flow. However, based on these preliminary findings and the advantages conferred by working on bacteria using high-throughput sequencing, a further spatio-temporal investigation over large sample sets to explore their potential as bioindicators is warranted.
Although previous studies in Chile have addressed the microbial composition of bacterial mats proliferating in the surface of impacted sediments (Aranda et al. 2010, 2015), studies addressing changes in the microbial community composition of sediments below and within proximity to salmon farms remain absent. The physicochemical and subsequent sediment bacteria community changes resulting from aquaculture are complex, and it remains difficult to characterize which environmental factors are driving such changes, as they may vary temporally or spatially. Further investigations are needed to elucidate how specific abiotic factors might explain variance both temporally and spatially in bacterial community composition between aquaculture and reference sites, as well as between aquaculture sites. Additionally, a whole-genome shotgun (metagenomics) sequencing study would be valuable for characterizing functional diversity and identifying rare species. Such thorough characterization will allow for insight into how these changes can influence other components of biogeochemical processes. However, these results depend largely on the geographic region and season, so more in-depth long-term studies are necessary. Finally, with this understanding, the application of this technique could be used as a monitoring tool to understand the effects and changes and to reduce the impact of aquaculture on coastal marine ecosystems.
Conclusion
The results of our investigation indicate that Chilean salmon aquaculture affects taxonomic diversity, composition, and function of sediment bacteria communities, indicating the importance of understanding microbial communities and their relevance to ecosystem functioning. This study offers a better understanding of the relationships of salmon aquaculture and bacteria in Pacific coastal ecosystems, and provides fundamental knowledge for remediating aquaculture-impacted areas. Bacteria may be better indices of biotic integrity than the laborious task of identifying sediment microbes, especially with the advent of high-throughput sequencing, which enables environmental bacterial diversity to be determined rapidly and cost-effectively.
References
Abd-Elnaby HM, Abou-Elela GM, Ghozlan HA, Hussein H, Sabry SA (2016) Characterization and bioremediation potential of marine Psychrobacter species. Egypt J Aquat Res 42:193–203. https://doi.org/10.1016/j.ejar.2016.04.003
Aranda C, Paredes J, Valenzuela C, Lam P, Guillou L (2010) 16S rRNA gene-based molecular analysis of mat-forming and accompanying bacteria covering organically-enriched marine sediments underlying a salmon farm in Southern Chile (Calbuco Island). Gayana 74:125–135
Aranda CP, Valenzuela C, Matamala Y, Godoy FA, Aranda N (2015) Sulphur-cycling bacteria and ciliated protozoans in a Beggiatoaceae mat covering organically enriched sediments beneath a salmon farm in a southern Chilean fjord. Mar Pollut Bull 100:270–278. https://doi.org/10.1016/j.marpolbul.2015.08.040
Armstrong SM, Hargrave BT, Haya K (2005) Antibiotic use in finfish aquaculture: modes of action, environmental fate, and microbial resistance. In: Hargrave BT (ed) Environmental effects of marine finfish aquaculture, vol 5M. Springer, Berlin, pp 341–357. https://doi.org/10.1007/b136017
Asami H, Aida M, Watanabe K (2005) Accelerated sulfur cycle in coastal marine sediment beneath areas of intensive shellfish aquaculture. Appl Environ Microbiol 71:2925–2933. https://doi.org/10.1128/AEM.71.6.2925-2933.2005
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. ACS Symp Ser 835:262–297
Bissett A, Bowman J, Burke C (2006) Bacterial diversity in organically-enriched fish farm sediments. FEMS Microbiol Ecol 55:48–56. https://doi.org/10.1111/j.1574-6941.2005.00012.x
Bissett A, Burke C, Cook PLM, Bowman JP (2007) Bacterial community shifts in organically perturbed sediments. Environ Microbiol 9:46–60. https://doi.org/10.1111/j.1462-2920.2006.01110.x
Bissett A, Cook PLM, Macleod C, Bowman JP, Burke C (2009) Effects of organic perturbation on marine sediment betaproteobacterial ammonia oxidizers and on benthic nitrogen biogeochemistry. Mar Ecol Prog Ser 392:17–32. https://doi.org/10.3354/meps08244
Bostock J, McAndrew B, Richards R, Jauncey K, Telfer T, Lorenzen K et al (2010) Aquaculture: global status and trends. Philos Trans R Soc B 365:2897–2912. https://doi.org/10.1098/rstb.2010.0170
Bowman JP, McCuaig RD (2003) Biodiversity, community structural shifts, and biogeography of prokaryotes within Antarctic continental shelf sediment. Appl Environ Microbiol 69:2463–2483. https://doi.org/10.1128/AEM.69.5.2463-2483.2003
Brown SD, Palumbo AV, Panikov N, Ariyawansa T, Klingeman DM, Johnson CM et al (2012) Draft genome sequence for Microbacterium laevaniformans strain OR221, a bacterium tolerant to metals, nitrate, and low pH. J Bacteriol 194:3279–3280. https://doi.org/10.1128/JB.00474-12
Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K (2010) Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 306:7–23
Buschmann AH (2002) Impacto ambiental de la salmonicultura en Chile: la situación de la Xa Región de Los Lagos. Análisis Políticas Públicas 16:1–11
Buschmann AH, Fortt A (2005) Efectos ambientales de la acuicultura intensiva y alternativas para un desarrollo sustentable. Rev Ambiente Desarrollo 21:58–64
Buschmann AH, Mora OA, Gómez P, Böttger M, Buitano S, Retamales C et al (1994) Gracilaria chilensis outdoor tank cultivation in Chile: use of land-based salmon culture effluents. Aquacult Eng 13:283–300. https://doi.org/10.1016/0144-8609(94)90016-7
Buschmann AH, Riquelme VA, Hernández-González MC, Varela D, Jiménez JE, Henríquez LA et al (2006) A review of the impacts of salmonid farming on marine coastal ecosystems in the southeast Pacific. ICES J Mar Sci 63:338–1345. https://doi.org/10.1016/j.icesjms.2006.04.021
Buschmann AH, Hernández-González MC, Aranda C, Chopin T, Neori A, Halling C et al (2008) Mariculture waste management. In: Jørgensen SE, Fath BD (eds) Encyclopedia of ecology, vol 3. Ecological engineering. Elsevier, Oxford, pp 2211–2217
Buschmann AH, Cabello F, Young K, Carvajal J, Varela DA, Henríquez L (2009) Salmon aquaculture and coastal ecosystem health in Chile: analysis of regulations, environmental impacts and bioremediation systems. Ocean Coast Manage 52:243–249. https://doi.org/10.1016/j.ocecoaman.2009.03.002
Buschmann AH, Tomova A, López A, Maldonado MA, Henríquez LA, Ivanova L et al (2012) Salmon aquaculture and antimicrobial resistance in the marine environment. PLoS One 7:e42724
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8:1137–1144
Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A et al (2013) Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ Microbiol 15:1917–1942. https://doi.org/10.1111/1462-2920.12134
Carroll ML, Cochrane S, Fieler R, Velvin R, White P (2003) Organic enrichment of sediments from salmon farming in Norway: environmental factors, management practices, and monitoring techniques. Aquaculture 226:165–180. https://doi.org/10.1016/S0044-8486(03)00475-7
Castine SA, Bourne DG, Trott LA, McKinnon DA (2009) Sediment microbial community analysis: establishing impacts of aquaculture on a tropical mangrove ecosystem. Aquaculture 297:91–98. https://doi.org/10.1016/j.aquaculture.2009.09.013
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270. https://doi.org/10.2307/4615964
Christensen PB, Rysgaard S, Sloth NP, Dalsgaard T, Schwærter S (2000) Sediment mineralization, nutrient fluxes, denitrification and dissimilatory nitrate reduction to ammonium in an estuarine fjord with sea cage trout farms. Aquat Microb Ecol 21:73−84. https://doi.org/10.3354/ame021073
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Dang H, Zhang X, Song L, Chang Y, Yang G (2007) Molecular determination of oxytetracycline-resistant bacteria and their resistance genes from mariculture environments of China. J Appl Microbiol 103:2580–2592. https://doi.org/10.1111/j.1365-2672.2007.03494.x
Danovaro R, Tselepides A, Otegui A, Della Croce N (2000) Dynamics of meiofaunal assemblages on the continental shelf and deep-sea sediments of the Cretan Sea (NE Mediterranean): relationships with seasonal changes in food supply. Prog Oceanogr 46:367–400. https://doi.org/10.1016/S0079-6611(00)00026-4
Deming JW, Baross JA (1993) The early diagenesis of organic matter: bacterial activity. In: Engel MH, Macko SA (eds) Organic geochemistry. Plenum Press, New York, pp 119–144
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K et al (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. https://doi.org/10.1128/AEM.03006-05
Dowle E, Pochon X, Keeley N, Wood SA (2015) Assessing the effects of salmon farming seabed enrichment using bacterial community diversity and high-throughput sequencing. FEMS Microbiol Ecol 91:fiv089. https://doi.org/10.1093/femsec/fiv089
Du J, Xiao K, Li L, Ding X, Liu H, Lu Y et al (2013) Temporal and spatial diversity of bacterial communities in coastal waters of the South China Sea. PLoS One 8:e66968. https://doi.org/10.1371/journal.pone.0066968
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Fernandes TF, Eleftheriou A, Ackefors H, Eleftheriou M, Ervik A, Sanchez-Mata A et al (2001) The scientific principles underlying the monitoring of the environmental impacts of aquaculture. J Appl Ichthyol 17:181–193. https://doi.org/10.1046/j.1439-0426.2001.00315.x
Fernandez AS, Hashsham SA, Dollhopf SL, Raskin L, Glagoleva O, Dazzo FB et al (2000) Flexible community structure correlates with stable community function in methanogenic bioreactor communities perturbed by glucose. Appl Environ Microbiol 66:4058–4067. https://doi.org/10.1128/AEM.66.9.4058-4067.2000
Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL et al (2012) Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci U S A 109:21390–21395. https://doi.org/10.1073/pnas.1215210110
Fodelianakis S, Papageorgiou N, Pitta P, Kasapidis P, Karakassis I, Ladoukakis ED (2014) The pattern of change in the abundances of specific bacterioplankton groups is consistent across different nutrient-enriched habitats in Crete. Appl Environ Microbiol 80:3784–3792. https://doi.org/10.1128/AEM.00088-14
Fodelianakis S, Papageorgiou N, Karakassis I, Ladoukakis ED (2015) Community structure changes in sediment bacterial communities along an organic enrichment gradient associated with fish farming. Ann Microbiol 65:331–338. https://doi.org/10.1007/s13213-014-0865-4
Food and Agriculture Organization of the United Nations (FAO) (2016) The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. FAO, Rome, 200 pp. Available online at: http://www.fao.org/3/a-i5555e.pdf
Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV et al (2006) Annually reoccurring bacterial communities are predictable from ocean conditions. Proc Natl Acad Sci U S A 103:13104–13109. https://doi.org/10.1073/pnas.0602399103
Garren M, Smriga S, Azam F (2008) Gradients of coastal fish farm effluents and their effect on coral reef microbes. Environ Microbiol 10:2299–2312. https://doi.org/10.1111/j.1462-2920.2008.01654.x
Gooday AJ (2002) Biological responses to seasonally varying fluxes of organic matter to the ocean floor: a review. J Oceanogr 58:305–322. https://doi.org/10.1023/A:1015865826379
Hall-Spencer J, White N, Gillespie E, Gillham K, Foggo A (2006) Impact of fish farms on maerl beds in strongly tidal areas. Mar Ecol Prog Ser 326:1–9. https://doi.org/10.3354/meps326001
Hansen LS, Blackburn TH (1992) Effect of algal bloom deposition on sediment respiration and fluxes. Mar Biol 112:147–152. https://doi.org/10.1007/BF00349738
Hargrave BT, Duplisea DE, Pfeiffer E, Wildish DJ (1993) Seasonal changes in benthic fluxes of dissolved oxygen and ammonium associated with marine cultured Atlantic salmon. Mar Ecol Prog Ser 96:249–257. https://doi.org/10.3354/meps096249
Hargrave BT, Phillips GA, Doucette LI, White MJ, Milligan TG, Wildish DJ et al (1997) Assessing benthic impacts of organic enrichment from marine aquaculture. Water Air Soil Pollut 99:641–650. https://doi.org/10.1007/BF02406903
Holmer M, Duarte CM, Heilskov A, Olesen B, Terrados J (2003) Biogeochemical conditions in sediments enriched by organic matter from net-pen fish farms in the Bolinao area, Philippines. Mar Pollut Bull 46:1470–1479. https://doi.org/10.1016/S0025-326X(03)00281-9
Holmer M, Argyrou M, Dalsgaard T, Danovaro R, Diaz-Almela E, Duarte CM et al (2008) Effects of fish farm waste on Posidonia oceanica meadows: synthesis and provision of monitoring and management tools. Mar Pollut Bull 56:1618–1629. https://doi.org/10.1016/j.marpolbul.2008.05.020
Inagaki F, Suzuki M, Takai K, Oida H, Sakamoto T, Aoki K et al (2003) Microbial communities associated with geological horizons in coastal subseafloor sediments from the Sea of Okhotsk. Appl Environ Microbiol 69:7224–7235. https://doi.org/10.1128/AEM.69.12.7224-7235.2003
Iyer R, Damania A, Iken B (2017) Whole genome sequencing of microbacterium sp. AISO3 from polluted San Jacinto River sediment reveals high bacterial mobility, metabolic versatility and heavy metal resistance. Genom Data 14:10–13. https://doi.org/10.1016/j.gdata.2017.07.009
Kamjunke N, Nimptsch J, Harir M, Herzsprung P, Schmitt-Kopplin P, Neu TR et al (2017) Land-based salmon aquacultures change the quality and bacterial degradation of riverine dissolved organic matter. Sci Rep 7:43739. https://doi.org/10.1038/srep43739
Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30. https://doi.org/10.1093/nar/28.1.27
Karakassis I, Tsapakis M, Hatziyanni E (1998) Seasonal variability in sediment profiles beneath fish farm cages in the Mediterranean. Mar Ecol Prog Ser 162:243–252. https://doi.org/10.3354/meps162243
Kawahara N, Shigematsu K, Miura S, Miyadai T, Kondo R (2008) Distribution of sulfate-reducing bacteria in fish farm sediments on the coast of southern Fukui Prefecture, Japan. Plankton Benthos Res 3:42–45. https://doi.org/10.3800/pbr.3.42
Kawahara N, Shigematsu K, Miyadai T, Kondo R (2009) Comparison of bacterial communities in fish farm sediments along an organic enrichment gradient. Aquaculture 287:107–113. https://doi.org/10.1016/j.aquaculture.2008.10.003
Kim SR, Nonaka L, Suzuki S (2004) Occurrence of tetracycline resistance genes tet(M) and tet(S) in bacteria from marine aquaculture sites. FEMS Microbiol Lett 237:147–156. https://doi.org/10.1111/j.1574-6968.2004.tb09690.x
Kimes NE, Callaghan AV, Aktas DF, Smith WL, Sunner J, Golding B, Drozdowska M et al (2013) Metagenomic analysis and metabolite profiling of deep-sea sediments from the Gulf of Mexico following the Deepwater Horizon oil spill. Front Microbiol 4:50. https://doi.org/10.3389/fmicb.2013.00050
Kondo R, Shigematsu K, Butani J (2008) Rapid enumeration of sulphate-reducing bacteria from aquatic environments using real-time PCR. Plankton Benthos Res 3(3):180–183. https://doi.org/10.3800/pbr.3.180
Kondo R, Shigematsu K, Kawahara N, Okamura T, Yoon YH, Sakami T et al (2012) Abundance of sulphate-reducing bacteria in fish farm sediments along the coast of Japan and South Korea. Fish Sci 78:123–131. https://doi.org/10.1007/s12562-011-0439-3
Köster M, Meyer-Reil LA (2001) Characterization of carbon and microbial biomass pools in shallow water coastal sediments of the southern Baltic Sea (Nordrügensche Bodden). Mar Ecol Prog Ser 214:25–41. https://doi.org/10.3354/meps214025
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. https://doi.org/10.1038/nbt.2676
Li L, Kato C, Horikoshi K (1999) Microbial diversity in sediments collected from the deepest cold-seep area, the Japan Trench. Mar Biotechnol 1:391–400. https://doi.org/10.1007/PL00011793
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5:169–172. https://doi.org/10.1038/ismej.2010.133
Maage A, Julshamn K, Berge GE (2001) Zinc gluconate and zinc sulphate as dietary zinc sources for Atlantic salmon. Aquacult Nutr 7:183–187. https://doi.org/10.1046/j.1365-2095.2001.00170.x
Macleod C, Forbes S (2004) Guide to the assessment of sediment condition at marine finfish farms in Tasmania. Aquafin CRC Project 4.1. Tasmanian Aquaculture and Fisheries Institute, Australia
Macleod CK, Moltschaniwskyj NA, Crawford CM (2008) Ecological and functional changes associated with long-term recovery from organic enrichment. Mar Ecol Prog Ser 365:17–24. https://doi.org/10.3354/meps07534
Macleod C, Eriksen R, Simpson S, Davey A, Ross J (2014) Assessment of the environmental impacts and sediment remediation potential associated with copper contamination from antifouling paint (and associated recommendations for management). FRDC Project No. 2011-041 (University of Tasmania, CSIRO), Australia
Marcial Gomes NC, Borges LR, Paranhos R, Pinto FN, Mendonça-Hagler LCS, Smalla K (2008) Exploring the diversity of bacterial communities in sediments of urban mangrove forests. FEMS Microbiol Ecol 66:96–109. https://doi.org/10.1111/j.1574-6941.2008.00519.x
McCaig AE, Phillips CJ, Stephen JR, Kowalchuk GA, Harvey SM, Herbert RA et al (1999) Nitrogen cycling and community structure of proteobacterial beta-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments. Appl Environ Microbiol 65:213–220
McCarthy Ú, Stagg H, Donald K, Garden A, Weir SJ (2013) Psychrobacter sp. isolated from the kidney of salmonids at a number of aquaculture sites in Scotland. Bull Eur Assoc Fish Pathol 33:67–72
Miranda CD, Zemelman R (2002a) Bacterial resistance to oxytetracycline in Chilean salmon farming. Aquaculture 212:31–47. https://doi.org/10.1016/S0044-8486(02)00124-2
Miranda CD, Zemelman R (2002b) Antimicrobial multiresistance in bacteria isolated from freshwater Chilean salmon farms. Sci Total Environ 293:207–218. https://doi.org/10.1016/S0048-9697(02)00022-0
Mirto S, La Rosa T, Danovaro R, Mazzola A (2000) Microbial and meiofaunal response to intensive mussel-farm biodeposition in coastal sediments of the western Mediterranean. Mar Pollut Bull 40:244–252. https://doi.org/10.1016/S0025-326X(99)00209-X
Mulsow S, Krieger Y, Kennedy R (2006) Sediment profile imaging (SPI) and micro-electrode technologies in impact assessment studies: example from two fjords in Southern Chile used for fish farming. J Mar Syst 62:152–163. https://doi.org/10.1016/j.jmarsys.2005.09.012
Navarrete P, Magne F, Mardones P, Riveros M, Opazo R, Suau A et al (2009) Molecular analysis of intestinal microbiota of rainbow trout (Oncorhynchus mykiss). FEMS Microbiol Ecol 71:148–156. https://doi.org/10.1111/j.1574-6941.2009.00769.x
Navarro N, Leakey RJG, Black KD (2008) Effect of salmon cage aquaculture on the pelagic environment of temperate coastal waters: seasonal changes in nutrients and microbial community. Mar Ecol Prog Ser 361:47–58. https://doi.org/10.3354/meps07357
Nikolaou M, Neofitou N, Skordas K, Castritsi-Catharios I, Tziantziou L (2014) Fish farming and anti-fouling paints: a potential source of cu and Zn in farmed fish. Aquacult Environ Interact 5:163–171. https://doi.org/10.3354/aei00101
Nogales B, Lanfranconi MP, Piña-Villalonga JM, Bosch R (2011) Anthropogenic perturbations in marine microbial communities. FEMS Microbiol Rev 35:275–298. https://doi.org/10.1111/j.1574-6976.2010.00248.x
O’Brien DP, Simpson SL, Spadaro DA (2009) Ecological effects due to contamination of sediments with copper-based antifoulants. Part 1: FRDC Project No. 2008/226, 55 pp. Part 2: FRDC Project No. 2009/218, 49 pp. Fisheries Research and Development Corporation and CSIRO
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB et al (2013) vegan: community ecology package. R package version 2.0-8. Available online at: http://CRAN.R-project.org/package=vegan
Polymenakou PN, Bertilsson S, Tselepides A, Stephanou EG (2005) Bacterial community composition in different sediments from the Eastern Mediterranean Sea: a comparison of four 16S ribosomal DNA clone libraries. Microb Ecol 50:447–462. https://doi.org/10.1007/s00248-005-0005-6
Powell SM, Bowman JP, Snape I, Stark JS (2003) Microbial community variation in pristine and polluted nearshore Antarctic sediments. FEMS Microbiol Ecol 45:135–145. https://doi.org/10.1016/S0168-6496(03)00135-1
Quero GM, Cassin D, Botter M, Perini L, Luna GM (2015) Patterns of benthic bacterial diversity in coastal areas contaminated by heavy metals, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Front Microbiol 6:1053. https://doi.org/10.3389/fmicb.2015.01053
Quince C, Lanzén A, Curtis TP, Davenport RJ, Hall N, Head IM et al (2009) Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Methods 6:639–641. https://doi.org/10.1038/nmeth.1361
Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ (2011) Removing noise from pyrosequenced amplicons. BMC Bioinform 12:38. https://doi.org/10.1186/1471-2105-12-38
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Home page at: https://www.R-project.org/
Ren Y, Niu J, Huang W, Peng D, Xiao Y, Zhang X et al (2016) Comparison of microbial taxonomic and functional shift pattern along contamination gradient. BMC Microbiol 16:110. https://doi.org/10.1186/s12866-016-0731-6
Roberts MC, No D, Kuchmiy E, Miranda CD (2014) Tetracycline resistance gene tet(39) identified in three new genera of bacteria isolated in 1999 from Chilean salmon farms. J Antimicrob Chemother 70:619–621. https://doi.org/10.1093/jac/dku412
Ruohonen K, Vielma J, Grove DJ (1999) Low-protein supplement increases protein retention and reduces the amounts of nitrogen and phosphorus wasted by rainbow trout fed on low-fat herring. Aquacult Nutr 5:83–91. https://doi.org/10.1046/j.1365-2095.1999.00090.x
Sakami T, Abo K, Takayanagi K, Toda S (2003) Effects of water mass exchange on bacterial communities in an aquaculture area during summer. Estuar Coast Shelf Sci 56:111–118. https://doi.org/10.1016/S0272-7714(02)00126-9
Sanz-Lázaro C, Fodelianakis S, Guerrero-Meseguer L, Marín A, Karakassis I (2015) Effects of organic pollution on biological communities of marine biofilm on hard substrata. Environ Pollut 201:17–25. https://doi.org/10.1016/j.envpol.2015.02.032
Sarà G (2007) Ecological effects of aquaculture on living and non-living suspended fractions of the water column: a meta-analysis. Water Res 41:3187–3200. https://doi.org/10.1016/j.watres.2007.05.013
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394. https://doi.org/10.1890/06-0219
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Simpson EH (1949) Measurement of diversity. Nature 163:688. https://doi.org/10.1038/163688a0
Simpson SL Spadaro DA (2012) Metal management limits for copper in sediments contaminated through the use of copper-based antifouling paints in aquaculture. CSIRO Wealth from Oceans Flagship, Technical Report
Simpson SL, Spadaro DA, O’Brien D (2013) Incorporating bioavailability into management limits for copper in sediments contaminated by antifouling paint used in aquaculture. Chemosphere 93:2499–2506. https://doi.org/10.1016/j.chemosphere.2013.08.100
Smith SV, Hollibaugh JT (1993) Coastal metabolism and the oceanic organic carbon balance. Rev Geophys 31:75–89
Soto D, Norambuena F (2004) Evaluation of salmon farming effects on marine systems in the inner seas of southern Chile: a large-scale mensurative experiment. J Appl Ichthyol 20:493–501. https://doi.org/10.1111/j.1439-0426.2004.00602.x
Staley C, Gould TJ, Wang P, Phillips J, Cotner JB, Sadowsky MJ (2014) Core functional traits of bacterial communities in the Upper Mississippi River show limited variation in response to land cover. Front Microbiol 5:414. https://doi.org/10.3389/fmicb.2014.00414
Storebakken T, Shearer KD, Roem AJ (2000) Growth, uptake and retention of nitrogen and phosphorus, and absorption of other minerals in Atlantic salmon Salmo salar fed diets with fish meal and soy-protein concentrate as the main sources of protein. Aquacult Nutr 6:103–108. https://doi.org/10.1046/j.1365-2095.2000.00135.x
Sun Y, Wolcott RD, Dowd SE (2011) Tag-Encoded FLX amplicon pyrosequencing for the elucidation of microbial and functional gene diversity in any environment. In: Kwon YM, Ricke SC (eds) High-throughput next generation sequencing: Methods and applications. Humana Press, Totowa, pp 129–141
Tamminen M, Karkman A, Corander J, Paulin L, Virta M (2011) Differences in bacterial community composition in Baltic Sea sediment in response to fish farming. Aquaculture 313:15–23. https://doi.org/10.1016/j.aquaculture.2011.01.020
Tomassetti P, Porrello S (2005) Polychaetes as indicators of marine fish farm organic enrichment. Aquacult Int 13:109–128. https://doi.org/10.1007/s10499-004-9026-2
Tomova A, Ivanova L, Buschmann AH, Rioseco ML, Kalsi RK, Godfrey HP et al (2015) Antimicrobial resistance genes in marine bacteria and human uropathogenic Escherichia coli from a region of intensive aquaculture. Environ Microbiol Rep 7:803–809. https://doi.org/10.1111/1758-2229.12327
Torsvik V, Sørheim R, Goksøyr J (1996) Total bacterial diversity in soil and sediment communities—a review. J Ind Microbiol Biotechnol 17:170–178. https://doi.org/10.1007/BF01574690
Vezzulli L, Chelossi E, Riccardi G, Fabiano M (2002) Bacterial community structure and activity in fish farm sediments of the Ligurian sea (Western Mediterranean). Aquacult Int 10:123–141. https://doi.org/10.1023/A:1021365829687
Wang Y, Sheng H-F, He Y, Wu J-Y, Jiang Y-X, Tam NF-Y et al (2012) Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of Illumina tags. Appl Environ Microbiol 78:8264–8271. https://doi.org/10.1128/AEM.01821-12
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
World Bank (2013) Fish to 2030: prospects for fisheries and aquaculture. Agriculture and environmental services discussion paper 03. World Bank report number 83177-GLB, 102 pp
Yao X-F, Zhang J-M, Tian L, Guo J-H (2017) The effect of heavy metal contamination on the bacterial community structure at Jiaozhou Bay, China. Braz J Microbiol 48:71–78. https://doi.org/10.1016/j.bjm.2016.09.007
Yue JC, Clayton MK (2005) A similarity measure based on species proportions. Commun Stat Theory Methods 34:2123–2131. https://doi.org/10.1080/STA-200066418
Zajac RN, Vozarik JM, Gibbons BR (2013) Spatial and temporal patterns in macrofaunal diversity components relative to sea floor landscape structure. PLoS One 8:e65823. https://doi.org/10.1371/journal.pone.0065823
Zak DR, Pregitzer KS, Burton AJ, Edwards IP, Kellner H (2011) Microbial responses to a changing environment: implications for the future functioning of terrestrial ecosystems. Fungal Ecol 4:386–395. https://doi.org/10.1016/j.funeco.2011.04.001
Zhang W, Ki J-S, Qian P-Y (2008) Microbial diversity in polluted harbor sediments I: bacterial community assessment based on four clone libraries of 16S rDNA. Estuar Coast Shelf Sci 76:668–681. https://doi.org/10.1016/j.ecss.2007.07.040
Zhou J, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322
Zhou J, Deng Y, Zhang P, Xue K, Liang Y, Van Nostrand JD et al (2014) Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc Natl Acad Sci U S A 111:E836–E845. https://doi.org/10.1073/pnas.1324044111
Acknowledgements
Katherine Hornick would like the acknowledge Cristian Valenzuela and Dr. Daniel Varela for their assistance on specific protocols and methodological suggestions, as well as Dr. Edwin Niklitschek for his help and direction with the statistical analyses during her stay in Chile. She would also like to thank Dr. Carlos Aranda for his help with the bioinformatics analysis. AHB acknowledges the support of the Lenfest Ocean Program/Pew Charitable Trusts, FONDECYT nr. 1110845, and CONICYT Basal Program (FB-001). The help in the field by Adrián Villarroel and Juan Maulén is also specially acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 720 kb)
Rights and permissions
About this article
Cite this article
Hornick, K.M., Buschmann, A.H. Insights into the diversity and metabolic function of bacterial communities in sediments from Chilean salmon aquaculture sites. Ann Microbiol 68, 63–77 (2018). https://doi.org/10.1007/s13213-017-1317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-017-1317-8