Abstract
Cassava waste pulp (CWP)–enzymatic hydrolysate was co-fermented with molasses (CWP-EH/molasses mixture) with the aim to optimize ethanol production by Saccharomyces cerevisiae TISTR 5606 (SC 90). The optimal fermentation conditions for ethanol production using this mixture were 245 g/L initial total sugar supplemented with KH2PO4 (8 g/L), at 30 °C for 48 h of fermentation under an oxygen-limited condition with agitation at 100 rpm, producing an ethanol concentration of 70.60 g/L (0.31 g ethanol/g total sugar). The addition of cassava tuber fiber (solid residue of CWP after enzymatic hydrolysis) at 30 g/L dry weight to the CWP-EH/molasses mixture increased ethanol production to 74.36 g/L (0.32 g ethanol/g total sugar). Co-fermentation of CWP-EH with molasses had the advantage of not requiring any supplementation of the fermentation mixture with reduced nitrogen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Cassava waste pulp (CWP), a waste product from the cassava starch manufacturing industry, is produced at approximately 7 million tons annually in Thailand (Office of the National Economic and Social Development Board 2006). Although the predominant component of CWP is starch (67.8% w/w, dry weight; Akaracharanya et al. 2011), this waste product is not suitable as an animal feed due to its low nitrogen content and is not an economically viable substrate for ethanol production on its own because its saccharified sugar content is too dilute. Despite efforts to improve the technology to extract as much starch from cassava tubers as possible, at the present time a considerable amount of starch remains in CWP. The dumping of CWP leads to environmental problems, including air pollution due to offensive odor and smells due to microbial deterioration of the residual starch in CWP.
Molasses, a waste product from the sugar industry, is a major substrate for ethanol production in Thailand. Because it contains a high concentration of sucrose (30–40%, w/w) and other nutrients necessary for microbial growth and ethanol production (Chotineeranat et al. 2010), pretreatment and saccharification steps are not required for fermentation with molasses. However, it has been reported that supplementation of molasses with urea, MgSO4, MnCl2 or soybean powder improves the production of ethanol from molasses. Specifically, urea and soybean powder supplement a reduced nitrogen for improvement of yeast growth, Mg2+ is a cofactor of some enzymes in the ethanol fermentation pathway and Mn2+ increases the activity of invertase, an enzyme that hydrolyzes sucrose to glucose and fructose, which is necessary for sucrose fermentation by Saccharomyces cerevisiae (Takeshige and Ouchi 1995; Pradeep and Reddy 2010).
In the study reported here we studied the value addition of CWP by co-fermentation of its saccharified starch with molasses to ethanol using S. cerevisiae. The composition and optimal concentrations of supplemented nutrients required for maximizing ethanol production were determined.
Materials and methods
Materials
The CWP was collected from the Sa-nguan Wong Industry Co., Ltd., Nakhon Ratchasima province, Thailand and kept at −20 °C until use. The chemical composition of the CWP is shown in Table 1. Just before use the CWP was thawed to room temperature. The molasses sample was collected from the Khonburi Sugar Co., Ltd., Nakhon Ratchasima province, Thailand and kept at 4 °C until use.
The enzymes cellulase [Accellerase™ 1500; 2500 carboxymethylcellulose (CMC) units (U)/g], 650 p-nitrophenyl-glucoside, α-amylase (Spezyme alpha; 13,775 U/g) and the glucoamylase (GC 147; 580 U/g) were obtained from Genencor, Danisco US Inc., Rochester, NY).
Saccharomyces cerevisiae TISTR 5606 (SC 90) was obtained from the Thailand Institute of Science and Technology and was maintained on YPD agar slant (in g/L: yeast extract, 10; peptone, 20; glucose, 20; agar, 20; pH 5.5) at 4 °C.
Hydrolysis of CWP
Acid hydrolysis
The CWP at the designated substrate loading level of 100 g/L was hydrolyzed at the optimized condition as described by Thongchul et al. (2010). Specifically, 1 g CWP was added to 9 mL of 1 N HCl (or 1 g CWP/0.33 g HCl) and the mixture autoclaved at 121 °C, 15 lb/in2 (0.1034 MPa) for 15 min and then filtered. The filtrate was harvested and is hereafter defined as the CWP-acid hydrolysate (CWP-AH); its reducing sugar content was analyzed by the Somogyi–Nelson method (Somogyi 1952).
Enzymatic hydrolysis
The CWP at a substrate loading level of 250 g/L was hydrolyzed at the optimized condition (Thongchul et al. 2010) by suspension in deionized water (1 g CWP/4 mL deionized water) and autoclaving at 121 °C, 15 lb/in2 for 15 min. The suspension was then sequentially hydrolyzed by cellulase (1.41 CMC U/g) at 50 °C for 24 h, α-amylase (48 U/g) at 85 °C for 1 h and GC (4.8 TGA U/g) at 60 °C for 3 h. The resultant CWP slurry was filtered, and the filtrate is hereafter defined as the CWP-enzymatic hydrolysate (CWP-EH), and its reducing sugar content was analyzed.
All experiments described above were performed based on wet weight. In some experiments the solid residue of the CWP separated from the filtrate. This solid residue, designated as cassava tuber fibers (CTF), was dried at 65 °C and remixed into CWP-EH at concentrations of 0, 25, 30, and 35 g/L.
Ethanol production from CWP-AH and CWP-EH mixtures
Inoculum preparation
A single colony of S. cerevisiae TISTR 5606 grown on YPD agar at 30 °C for 48 h was inoculated into 50 mL YPD broth in a 250-mL Erlenmeyer flask and incubated at 30 °C on a gyrotary shaker (100 rpm) for 24 h. The culture was then transferred into 100 mL of fresh YPD broth in a 500-mL Erlenmeyer flask at 1% (v/v) and incubated at the same condition until the late log phase (18 h). The cell number per milliliter of culture was determined using a haemacytometer under a light microscope prior to harvesting by centrifugation (4 °C, 8000 rpm, 15 min), and the culture was used as the inoculum by suspension in fermentation medium.
Fermentation medium preparation
The CWP fermentation medium was prepared by adding 2 g/L (NH4)2SO4 to 35 mL of the CWP-AH or the CWP-EH mixture in a 50-mL Erlenmeyer flask, adjusting the medium to pH 5.5. The medium was sterilized by autoclaving at 110 °C (10 min).
The CWP-EH/molasses fermentation medium was prepared by mixing molasses (26.1 g) into CWP-EH (100 mL). The initial total sugar content of the resultant supernatant, determined using the phenol sulfuric acid method (Dubois et al. 1956), was 265 g/L. The CWP-EH/molasses mixture was supplemented with 2 g/L (NH4)2SO4, 2 g/L KH2PO4, 0.75 g/L MgSO4 7H2O and 10 g/l yeast extract and then sterilized by autoclaving (110 °C, 10 min); this mixture was used to determine nutrient requirements for maximization of ethanol production.
Ethanol fermentation
The different fermentation media [CWP-AH, CWP-EH, CWP-EH/molasses (standard or modified)] were inoculated with the inoculum cells to a final concentration of 108 cells/mL and incubated at 30 °C with agitation at 100 rpm for 48 h under an oxygen-limited condition. The oxygen-limited condition was obtained by sealing the 50-mL Erlenmeyer flask with a rubber stopper connected to an airlock containing saturated copper sulfate solution. At the end of the 48-h fermentation period the culture medium was clarified by centrifugation and the ethanol concentration in the supernatant analyzed by gas chromatography as described by Jutakanoke et al. (2012).
Nutrient requirements for maximum ethanol production
Fermentation of the CWP-EH/molasses mixture to produce ethanol was studied by varying the composition of the CWP-EH/molasses fermentation medium by adding or not adding the nutrient supplements shown in Table 2 and analyzing the supernatant for ethanol content following the ethanol fermentation process. The CWP-EH/molasses fermentation media was then further optimized for highest ethanol production by univariate sequential analysis of the optimal concentration of KH2PO4, initial total sugar and CTF in the fermentation medium. Specifically, the CWP-EH/molasses medium was supplemented with various concentrations of KH2PO4 (0, 2, 4, 6, 8 and 10 g/L final) and then fermented for 48 h prior to the analysis of the ethanol level. Then the initial concentration of total sugar in the CWP-EH/molasses mixture supplemented with the determined optimal level of KH2PO4 was varied at 205, 225, 245 and 265 g/L and fermented for 48 h prior to the analysis of the ethanol level. Finally, the CWP-EH/molasses medium containing the determined optimal level of KH2PO4 and molasses for the optimal initial total sugar level was supplemented with CTF at 0, 25, 30 or 35 g/L and fermented for 48 h prior to the analysis of the ethanol level.
Analytical procedure
Sugar composition of CWP-EH was analyzed by high-performance liquid chromatography (Agilent 1100 Series; Agilent Technology, Santa Clara, CA). Sugars were identified and quantified by resolution through a Microsorb column 100–5 NH2 (250 x 4.6 mm; Agilent Technology). Samples (20 μL) of each sample were injected and eluted with acetonitrile:H2O (75:25) at a flow rate of 1.5 mL/min using a refractive index detector.
Results and discussion
Acid and enzymatic hydrolysis of CWP
Hydrolysis of CWP at a 100 g/L substrate loading by HCl yielded 31.6 g/L reducing sugar or 0.42 g reducing sugar/g [dry weight (DW)] CWP. When the CWP was hydrolyzed by cellulase, α-amylase or GC at a substrate loading level of 250 g/L, 34.9 g/L reducing sugar (0.28 g reducing sugar/g CWP DW) was obtained. CWP comprises 11.2% (w/w) lignocellulose (Akaracharanya et al. 2011), and these fibers efficiently both protect the starch from enzymes and bind the enzymes, leading to a non-productive situation of reduced hydrolytic efficiency.
Ethanol production from the CWP-AH and CWP-EH
Ethanol fermentation using the CWP-AH or the CWP-EH fermentation medium was assessed by inoculating the respective medium with S. cerevisiae TISTR 5606 (final concentration 108 cells/mL) and incubating the inoculated media under an oxygen-limited condition at 30 °C for 48 h with agitation at 100 rpm. The ethanol yield was lower with CWP-AH medium (0.149 g ethanol/g reducing sugar) than with CWP-EH medium (0.242 g ethanol/g reducing sugar). Based on this result, we selected the CWP-EH medium for further study. Saccharomyces cerevisiae can not ferment the pentose sugars xylose and arabinose which are generally found in lignocellulosic hydrolysate, and it noteworthy that CWP-EH contains glucose (2.84%), xylose and arabinose (<0.1%). The same ethanol yield was obtained from CWP-AH and CWP-EH when Rhizopus oryzae was used (Thongchul et al. 2010). However, R. oryzae gave a higher ethanol yield and productivity from CWP-EH than with glucose at the same carbon content, which may reflect the promotional effect of the organic nitrogen in the CWP-EH on R. oryzae population growth, causing a more rapid onset of oxygen limitation in the medium and thereby an increase in the ethanol production (Thongchul et al. 2010)..
The potential of CWP, which contains 67.8% w/w (DW) starch, as a raw material for ethanol production was evaluated. Akaracharanya et al. (2011) reported that fermentation of the starch hydrolysate (22.6 g/L glucose) obtained from 30 g CWP (DW) resulted in the production of 9.9 g ethanol (0.43 g ethanol/g glucose) at 48 h. Due to the low concentration of reducing sugar of CWP-EH, it is not an economically viable ethanol-producing fermentation substrate on its own. In our study, the CWP-EH was co-fermented with molasses, another abundant, sustainable and renewable industrial waste product in Thailand.
Ethanol production from CWP- EH/molasses mixture
The levels of some nutrients present in the CWP-EH and in molasses that are known to potentially influence ethanol production by S. cerevisiae (Maiorella et al. 1983; Stehlik-Tomas et al. 2004; Chotineeranat et al. 2010) are shown in Tables 3 and 4, respectively.
Nutrient requirement for optimal ethanol production level
Various modified CWP-EH/molasses media were prepared (Table 2) and then fermented to ethanol for 48 h as described in the “Methods” section. The lowest ethanol concentration was obtained in the media containing all four supplements (media no. 1), at approximately 1.05-fold less than that in the unsupplemented CWP-EH/molasses (Fig. 1). A slight reduction in the ethanol concentration was also noted with media supplemented with (NH4)2SO4 + KH2PO4, (NH4)2SO4 + yeast extract and KH2PO4 + MgSO4, respectively,, suggesting that there was no clear single inhibitory nutrient. High ethanol concentrations were obtained with media no. 2, 10–12 and 15, with the highest ethanol level (62.67 g/L) obtained in the modified CWP-EH/molasses medium that was supplemented with only 2 g/L KH2PO4 (medium no.15), again showing no clear pattern of a single optimal nutrient supplement.
Effect of nutrient supplementation on net ethanol production level in the 48-h fermentation of the cassava waste pulp-enzymatic hydrolysate (CWP-EH)/molasses mixture. Medium number refers to composition shown in Table 2. Data are shown as the mean ± standard deviation (SD), derived from three independent replicates. Means with a different lowercase letter are significantly different at p < 0.05 (Duncan’s multiple means test)
Optimal concentration of KH2PO4 in CWP-EH/molasses mixture for ethanol fermentation
Various concentrations of KH2PO4 were then added into the CWP-EH/molasses mixture and fermented for 48 h to ethanol as described in the “Methods” section. The highest ethanol concentration (70.92 g/L) after 48 h of fermentation was found when the CWP-EH/molasses mixture was supplemented with 8 g/L KH2PO4 (Fig. 2), although this was not significantly greater than ethanol production at 6 or 10 g/L KH2PO4; it was, however, 1.12-fold higher than that at 2 g/L KH2PO4.
Optimal initial total sugar concentration in CWP-EH/molasses mixture for ethanol fermentation
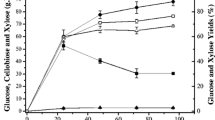
The level of molasses in the CWP-EH/molasses mixture supplemented with 8 g/L KH2PO4 was varied to give a final initial total sugar concentration that ranged from 205 to 265 g/L, following which the mixture was fermented for ethanol production for 48 h. At an initial total sugar content of 245 and 265 g/L a similar ethanol concentration was obtained after 48 h—70.6 and 70.92 g/L, respectively—which was higher than that obtained with the two lower (225 and 205 g/L) total sugar levels (Fig. 3). Thus, an initial total sugar concentration of 245 g/L was used in subsequent trials.
Effect of the initial total sugar concentration on net ethanol production in the CWP-EH/molasses mixture supplemented with 8 g/L KH2PO4. Data are shown as the mean ± SD, derived from three independent replicates. Means with a different lowercase letter are significantly different at p < 0.05 (Duncan’s multiple means test)
There was no marked effect on ethanol production level when the 8 g/L KH2PO4 was replaced by 8 g/L NaH2PO4 (data not shown). The depletion of phosphate in the CWP-EH/molasses fermentation system, and therefore the need for its supplementation, might be caused by the precipitation of an insoluble calcium phosphate. Molasses contains a high concentration of Ca2+ because calcium oxide is used to clarify the sugarcane juice in the sugar production process. The molasses used in this study contained 6.8 g/L of Ca 2+ (Table 3), a level similar to the 5.47 and 6.6 g/L Ca 2+ reported in molasses by Takeshige and Ouchi (1995) and Chotineeranat et al. (2010), respectively. Thus, the requirement for phosphate supplementation would depend on the concentration of Ca2+ions in the molasses.
Effect of addition of CTF on ethanol production from CWP-EH/molasses
The inclusion of CTF at 0–35 g/L in the CWP-EH/molasses mixture increased net ethanol production level after 48 h of fermentation by 1.05-fold (Fig. 4), with the highest ethanol concentration (74.36 g/L) being found with the addition of 30 g DW/L CTF. However, further increases in the amount of CTF supplemented to the CWP-EH/molasses mixture from 30 to 35 g/L DW caused a decrease (1.04-fold) in the amount of ethanol produced.
Effect of the addition of cassava tuber fiber (CTF) in the CWP-EH/molasses mixture on ethanol production after 48 h of fermentation. The CWP-EH/molasses mixture contained 8 g/L KH2PO4 at an initial total sugar level of 245 g/L. Data are shown as the mean ± SD, derived from three independent replicates. Means with a different lowercase letter are significantly different p < 0.05 (Duncan’s multiple means test)
The reason that CTF enhanced the net amount of ethanol produced during the fermentation is unclear, but several low-cost plant materials have been reported to be biomaterials for cell immobilization by natural adsorption in ethanol fermentation, including sugar beet pulp (Razmovski and Pejin 1996), sugarcane bagasse (Santos et al. 2008), corn cob and grape pomace (Genisheva et al. 2011). During the fermentation process yeast cells are exposed to several stresses, such as ethanol, CO2 and oxidative stresses, among others (Tesfaw and Assefa 2014). Thus, the CTF might protect yeast cells from these stresses, resulting in an increased of ethanol production. Regardless, the advantages of the natural adsorption technique are that the yeast population growth is less affected and there is adsorption of new cells and wash out of old cells (Bai et al. 2008). In addition, the adsorption technique is simple and easy to run (El-Latif et al. 2010).
Conclusion
Based on the results of this study, co-fermentation of CWP-EH and molasses for ethanol production had an advantage of not requiring any reduced nitrogen supplementation. The addition of CTF in the CWP-EH/molasses mixture increased net ethanol production.
References
Akaracharanya A, Kesornsit J, Leepipatpiboon N, Srinorakutara T, Kitpreechavanich V, Tolieng V (2011) Evaluation of the waste from cassava starch production as a substrate for ethanol fermentation by Sacharomyces cerevisiae. Ann Microbiol 61:431–436
Association of Official Analytical Chemists (AOAC) (2010) Official methods of analysis. 19th ed. AOAC International, Gaithersburg, MD
Association of Official Analytical Chemists (AOAC) (2012) Official methods of analysis. 18th ed. AOAC International, Gaithersburg, MD
Bai FW, Anderson WA, Moo-Young M (2008) Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv 26:89–105
Chotineeranat S, Wansuksri R, Piyachomkwan K, Chatakanonda P, Weerathaworn P, Sriroth K (2010) Effect of calcium ions on ethanol production from molasses by Saccharomyces cerevisiae. Sugar Tech 12(2):120–124
El-Latif MM, Ibrahim AM, El-Kady MF (2010) Adsorption Equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J Am Sci 6(6):267–283
Genisheva Z, Mussatto SI, Oliveira JM, Teixeria JA (2011) Evaluating the potential of wine-making residues and corn cobs as support materials for cells immobilization for ethanol production. Indian Crops Prod 34:979–985
Jutakanoke R, Leepipatpiboon N, Tolieng V, Kitpreechavanich V, Srinorakutara T, Akaracharanya A (2012) Sugacane leaves: pretreatment and ethanol fermentation by Saccharomyces cerevisiae. Biomass Bioenergy 39:283–289
Maiorella B, Blanch HW, Wilke CR (1983) By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 25:103–121
Office of the National Economic and Social Development Board (2006) Final report: Zero waste industry project. Department of Industrial Economic, Ministry of Industry, Thailand
Pradeep P, Reddy OVS (2010) High gravity fermentation of sugarcane molasses to produce ethanol: Effect of nutrients. Indian J Microbiol 50:S82–S87
Puwastien P, Siong TE, Kantasubrata J, Craven G, Feliciano RR, Judprasong K (2011) ASEAN manual of food analysis regional center of ASEAN network of food data system. Institute of Nutrition, Mahidol University, Putthamonthon
Razmovski R, Pejin D (1996) Immobilization of Saccharomyces diastaticus on wood chips for ethanol production. Folia Microbiol 41:201–207
Santos DT, Sarrouh BF, Rivaldi JD, Converti A, Silva SS (2008) Use of sugarcane bagasse as biomaterial for cell immobilization for xylitol production. J Food Eng 86:542–548
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Stehlik-Tomas V, Zetic VG, Stanzer D, Grba S, Vahcic N (2004) Zinc, copper and manganese enrichment in yeast Saccharomyces cerevisiae. Food Technol Biotech 42(2):115–120
Takeshige K, Ouchi K (1995) Factors affecting the ethanol productivity of yeast in molasses. J Ferment Bioeng 79:449–452
Tesfaw A, Assefa F (2014) Current trends in bioethanol production by Saccharomyces cerevisiae: substrate, inhibitor reduction, growth variables, coculture, and immobilization. Int Sch Res Notices. doi:10.1155/2014/532852
Thongchul N, Navankasattusas S, Yang ST (2010) Production of lactic acid and ethanol by Rhizopus oryzae integrated with cassava pulp hydrolysis. Bioprocess Biosyst Eng 30:407–416
Acknowledgments
The authors thank Dr. Robert Butcher for critical reading of this manuscript and the Thai Alcohol Public Company, Thailand for providing the α-amylase and glucoamylase enzymes. This study was financially supported by the Thai Government budget (fiscal year 2015) and Graduate school, Chulalongkorn University to commemorate the 72nd Birthday Anniversary of His Majesty the King Bhumibol Aduladej.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wattanagonniyom, T., Lee, WC., Tolieng, V. et al. Co-fermentation of cassava waste pulp hydrolysate with molasses to ethanol for economic optimization. Ann Microbiol 67, 157–163 (2017). https://doi.org/10.1007/s13213-016-1245-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-016-1245-z