Abstract
In India, propiconazole, a triazole group fungicide, is broadly used against powdery mildew, rusts, and leaf spot diseases of cereals and coffee. The toxicity of this fungicide is known to affect the quality of the soil. Hence, in the present study, a bacterium isolated from contaminated paddy soil was used to study the degradation of propiconazole under in vitro conditions. The isolated bacterium was confirmed as Pseudomonas aeruginosa strain (PS-4) based on morphological and biochemical characteristics, and 16S rRNA gene sequencing. When the isolated bacterium was grown in mineral salt medium amended with 10 μg/l propiconazole as a sole carbon source, culture filtrates of the bacterium utilized up to 8 μg/L of propiconazole after 72 h of incubation at 30 °C and pH 7, as analyzed by HPLC. Degradation of propiconazole by the bacterium was also aided by the secretion of three metabolites—1,2,4-triazole; 2,4-dichlorobenzoic acid; and 1-chlorobenzene—as determined by their mass spectra. Furthermore, induction of monooxygenase activity and the CYP450 gene was observed in the culture filtrate of strain PS-4, showing evidence of their role in the degradation of propiconazole. These results revealed that PS-4 is an efficient candidate for the reduction of contaminants present in the soil, thereby contributing to soil health and crop improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Propiconazole (1-[[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole) belongs to the triazole group of fungicides that inhibit demethylation. In India, propiconazole is used extensively as a popular agrochemical due to its wide spectrum of triazole action. This fungicide is used as a foliar spray, and thus will drift and reach the soil during application (Colson et al. 2003; Kim et al. 2003; Z.H. Li et al. 2013). Triazole fungicides are toxic and persist in the soil for long periods of time, thus affecting soil fertility and microflora (Elmholt 1992; Munier and Borde 2000). Remediation of fungicide toxicity has been a major research concern, and application of traditional methods to reduce toxicity has many environmental side effects. Therefore, ecofriendly and feasible approaches such as microbial biodegradation are gaining importance.
Microorganisms are most desirable biological tools, because of their ability to resist various pesticides, and their metabolic capacity to degrade toxic compounds into non-toxic forms. Hence, soil microorganisms are considered a key reservoir of biological activity with the potential to significantly enhance environmental cleanup (Dong et al. 2008; Satapute et al. 2012; Kulkarni and Kaliwal 2014). Many pesticide-degrading microorganisms have been reported belonging to various species of bacteria, fungi, algae and yeast. However, bacterial bio-remediation studies have been more successful because of the diversity of their metabolism and their ability to grow on complex carbon substrates. In addition, many genes involved in the metabolism of toxic compounds have been identified. Additionally, cytochrome P450 monooxygenase, which constitutes a huge family of protein haem thiolates capable of degradation of wide range of toxic compounds, are extremely well characterised in bacteria (Degtyarenko 1999). Therefore, the purpose of present investigation was to isolate and identify propiconazole-metabolising bacteria from contaminated paddy fields, and to study the degradation mechanism of propiconazole under in vitro conditions.
Materials and methods
Chemicals, media and soil sample
Propiconazole of 94 % purity was obtained from the Nagarjuna Agrichem Co. (Srikakullam, India). Ethyl acetate and acetonitrile used were of highest analytical and HPLC grades, respectively. Seubert’s mineral salts medium (MSM) (Seubert 1960) containing 10 μg/L propiconazole was used in the study. Soil was collected from a fungicide (upper layer 0–10 cm)-contaminated paddy field in Dharwad, Karnataka, India (15° 27′ 29 N, 75° 0′ 36E, 764 m altitude, reddish black soil). The physicochemical properties of the collected soil sample were recorded.
Isolation and screening of propiconazole-degrading bacteria
The soil was serially diluted up to 10−7 with sterile saline solution using 1 g sieved soil; 100 μL suspension of appropriate dilutions (10−5 and 10−6) was inoculated on mineral salts agar (MSA) medium containing 10 μg/L propiconazole as a sole source of carbon. After 7 days of incubation at 30 °C, all the colonies that appeared on the plates were purified by the quadrant streaking method on nutrient agar plates. All strains were screened for their tolerance level to propiconazole with different concentrations (10 μg/L, 20 μg/L and 30 μg/L) in mineral salt medium, and controls without propiconazole were maintained for all concentrations. All flasks were incubated at 30 °C on a rotary shaker at 120 rpm. The growth of all strains was observed regularly using a spectrophotometer (Hitachi U2900) at 600 nm. Strains that showed luxuriant growth at all concentrations of propiconazole were selected for further study.
Characterization of propiconazole-degrading bacterium
The bacterial isolate with highest tolerance to the different concentrations of propiconazole was identified based on its colony morphology, gram staining and biochemically by the bioMérieux vitek2 (bioMérieux, Marcy-l’Étoile, France) system. Further, 16S rRNA sequencing was done at Xcelris genomics (Ahmedabad, India). The selected bacterial DNA was isolated using an Xcelgen kit, and DNA stock samples were quantified using a nanodrop spectrophotometer at 260 and 280 nm. Simultaneously, DAN purity was checked by agarose gel electrophoresis (Sambrook and Russell 2001). Bacterial 16S RNA gene fragments were amplified by PCR from genomic DNA using 16S gene universal primers: 8 F and 1492R. Conditions of thermal cycling for PCR were, initial denaturation at 95 °C for 2 min in one cycle and final denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s and extension at 72 °C for 90 s. The number of cycles for all three steps was 30, with a final extension at 72 °C for 10 min in one cycle. Further, the nucleotide sequence of the isolate was checked by BLAST analysis using the NCBI server (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and a phylogenetic tree was constructed by the neighbor joining method using MEGA 6 software (Tamura et al. 2013).
Degradation of propiconazole in soil
The physicochemical properties of soil were studied following the method of Tandon (2005). To determine the degradation rate of propiconazole in soil samples, two different sets of experiments were conducted under in vitro conditions using different concentrations of propiconazole [commercial grade fungicide Tilt 25 EC (http://www3.syngenta.com), at 0.05, 0.1 μg/kg of technical grade]. All three concentrations of propiconazole were applied to soil as follows: one set of sterile (controlled) and non-sterile (not controlled) soil samples (1000 g) were placed in 30 × 25 cm tray and kept at 22 ± 2 °C and 64 ± 4 % humidity under laboratory conditions. Simultaneously, a similar set of sterile and not-sterilized soil samples were kept outside the laboratory (25 ± 2 °C, 68 ± 4 % relative humidity). The degradation rate of propiconazole in soil samples was monitored immediately after the treatment, and was repeated at 10-, 20-, 30- and 40-day intervals; the half life of propiconazole (DT50) was also recorded.
Degradation of propiconazole in liquid medium by soil isolate PS-4 strain
To investigate the biodegradation of propiconazole, 100 mL MSM with the propiconazole (10 μg/L) as sole carbon source was placed in a 500 mL conical flask and inoculated with 1 mL of PS-4 strain containing 3 × 10−6 cfu/mL; MSM with the same propiconazole concentration but without the bacterial culture was used as a control. Treated and control flasks were incubated at 30 °C on a rotary shaker at 120 rpm. In addition, DT50 of propiconazole was calculated according to the obtained results.
Effect of temperature and pH on biodegradation of propiconazole
The effect of different temperatures (20 °C, 25 °C, 30 °C, 35 °C, 40 °C, 45 °C and 50 °C) and pH (5, 6, 7, 8 and 9) on fungicide degradation was determined. The optimum temperature and pH for the degradation of fungicide was determined spectrophotometrically at 220 nm, and bacterial cell density was measured at 600 nm after 72 h of incubation.
Cytochrome P450 gene identification in PS-4 strain
Genomic DNA of strain PS-4 was isolated, and CYP P450 gene amplified using the primers F: 5′-ACCACATGCTCAACCTCGAC-3′ and R: 5′-TCATTGGGCGATCCTCTCGAT-3′, which were designed from the CYP P450 gene of Pseudomonas aeruginosa (LOCUS AP014839, 1426914 bp and accession AP014839). The 50 μL reaction mixture contained 1× DNA polymerase buffer, 0.2 mM dNTP mix, 25 μM each forward and reverse primer (final concentration 0.5 μM), 1 U Taq DNA polymerase, and 50 ng template DNA. The reaction mixture was subjected to the following PCR program (Applied Biosystems Life Technologies Veriti Thermal Cycler, 96 wells; https://www.thermofisher.com): initial denaturation for 5 min followed by the denaturation at 98 °C for 1 min, followed by 20 cycles (98 °C for 30 s, 65 °C for 30 s and 72 °C for 90 s) and final extension at 72 °C for 5 min. The final amplified product was fractionated on a 1 % agarose gel using gel documentation (Uvitech, Firereader-v4; http://www.uvitec.co.uk), analyzed using a 5-kb ladder (Amnion Biosciences, Bangalore, India) and sequencing was carried out using Applied Biosystems 3010XL capillary sequencer. The ABI’s BigDye® Terminator v3.1 sequencing chemistry was used. In order to carry out pairwise/multiple sequence alignment, the ClustalW2 Tool (http://www.ebi.ac.uk/Tools/msa/clustalw2/) was used.
Preparation of cell-free extracts for enzyme assay
For extraction of cell-free filtrates, the method described by Talwar et al. (2014) was followed. Briefly, P. aeruginosa PS-4 cells were washed and grown in Tris–HCl buffer pH 6.8 amended with 10 μg/L propiconazole, sonicated (Sonics vibra cell) for 5 min, and centrifuged. The supernatant was used for enzyme assay and protein estimation. The activity of propiconazole monooxygenase was measured spectrophotometrically, and determination of protein concentration was done using NanoDrop (http://www.nanodrop.com/).
Chemical analyses
Propiconazole extraction from soil
To analyze the degradation of propiconazole in the soil, 1 g soil was taken and mixed with 10 mL distilled water, and centrifuged at 10,000 g for 10 min (Eppendorf centrifuge); the supernatant was extracted twice using ethyl acetate (1:1), and the residue dissolved in acetonitrile. Further, the percentage of degradation was determined spectrophotometrically at 220 nm. In addition, sterile soil with applied Tilt fungicide was examined by LC/MS-MS for its propiconazole degradation capacity . A sample aliquot of 10 μL was injected into an Agilent 1290 Infinity UHPLC system (http://www.agilent.com); 10 mM ammonium acetate in water (0.1 % FA) was used as mobile phase A and acetonitrile (0.1%FA) as mobile phase B in a Shimpak ODS column 2.0 x 150 mm in size (http://www.shimadzu.com). The column flow rate was adjusted to 0.2 mL/min for both the standard and samples. Identification and quantification of the propiconazole was based on its retention time and area. Mass spectra (Thermo Fisher-TSQ Vantage) conditions were Spray Voltage (positive) 4000 V,Spray Voltage (negative) 2800 V, Vaporizer temp 300 °C, sheath gas flow rate 20 Arb and Aux gas flow rate 10 Arb.
Propiconazole extraction from biomass
To follow degradation of propiconazole by strain PS-4, 5 mL culture filtrate was withdrawn aseptically from liquid medium cultures at 12-h intervals and bacterial cell growth was measured at 600 nm. To obtain culture filtrates from bacterial suspensions, all samples were subjected to centrifugation at 10,000 g for 15 min at 4 °C. The filtrate was extracted twice with ethyl acetate (1:1) using the rotorflash evaporator (Buchirotavapor R 210), and residues were dissolved in 3 mL HPLC grade acetonitrile. The percentage degradation of propiconazole was monitored with a UV spectrophotometer (Hitachi U 2900) at 220 nm, and the degradation rate was confirmed by HPLC analysis. Further, to confirm the chemical data, a 40-μL aliquot was injected into an HPLC Agilent 1260 device equipped with quarternary pump, auto sampler and variable wavelength UV detector with a C18 column (diameter 150 × 4.6 mm) with a particle size of 5 μm, and samples were eluted at 1.2 min/mL with the mobile phase acetonitrile:water (80:20). Identification of metabolites of propiconazole was based on the molecular weight of the compound as determined by their mass spectra (Shimadzu); the flow rate was 1 mL/min, injection temperature 250 °C—the temperature was programmed by the DI probe from 100 °C to 250 °C.
Statistical analysis
All experimental data were determined in triplicate and expressed as means ± standard error. Statistical analyses of the data were performed using one-way ANOVA with SPSS version 20.0 software with advanced models (SPSS Japan, Tokyo, Japan). Differences between means were located using Tukey’s test (P < 0.05).
Results
Isolation and screening of propiconazole-degrading bacteria
Twenty-seven (PS-1 to PS-27) strains were isolated from paddy soil and the bacterium with most potential for utilizing propiconazole was screened in MSM with the propiconazole as sole carbon source. Based on the growth of the isolated strains, PS-4 was found to be the most promising strain in terms of its ability to grow on MSM, and was used for further biodegradation studies.
Characterization of propiconazole-degrading bacterium by bioMérieux Vitek2 analysis and 16S rRNA sequencing
PS-4 strain is an aerobic Gram negative bacterium. Fully grown propiconazole-resistant colonies of strain PS-4 were circular in shape with raised elevation with an undulating margin. Microbial identification was by bioMérieux Vitek2 (biochemical analysis) tests (Table 1), and showed positive reactions for catalase, oxidase and citrate. The 16S rRNA sequence obtained was 850 bp in length, and was identified as P. aeruginosa PS-4 strain. The closest relative were first determined based on the similarity of their 16S rRNA sequences obtained by a direct blast search of NCBI GenBank. The results shown that PS-4 sequence showed closest matches with those of soil microorganisms that play a vital role in pesticide degradation. The sequence of this organism was deposited in the NCBI GenBank under the accession number KM923901. A phylogenetic tree constructed with microbes classified as potent agents in bioremediation is presented in Fig. 1.
Degradation of propiconazole in soil
The physicochemical properties of paddy soil revealed reddish black soil, with pH 7.9 and electric conductivity of 291 dS/m. It was also noted that the soil contained organic carbon (9500 mg/kg), nitrogen (90.21 mg/kg), phosphorus (63.44 mg/kg), potassium (476.8 mg/kg), sulfur (15.465 mg/kg), calcium (11,880 mg/kg), magnesium (1032 mg/kg) zinc (1.368 mg/kg), iron (1.15 mg/kg), manganese (1.15 mg/kg) and copper (1.718 mg/kg) (Table 2). The rate of degradation of propiconazole in sterile soil placed outside the laboratory was found to be 21.95 % to 49.85 % (Fig. 2a), whereas, 21.29 % to 39.65 % (Fig. 2b) of propiconazole was degraded in controlled soil kept inside the laboratory for 40 days. In contrast to this, the rate of loss of fungicidal activity in the non-sterile soil is low, at 25.37 % to 41.02 % (Fig. 2c) in soil placed outside the laboratory, and 16.33 % to 32.27 % (Fig. 2d) degradation was observed in not controlled soil placed inside the laboratory for 40 days, respectively. A high (27°C) and low (22°C) temperature was recorded during the experimental set up. The optimum half life of DT50 was occurred on day 40 in the sterile soil placed outside the laboratory.
Degradation of propiconazole in soil. a Sterile soil placed out side the laboratory. b Sterile soil (controlled) placed under laboratory conditions. c Non-sterile soil situated outside the laboratory. d Non-sterile soil (not controlled) placed inside the laboratory. Values are means ± SE of three independent replicates for each incubation period. Means followed by the different letter(s) are significantly different from each other according to Tukey’s test (P < 0.05)
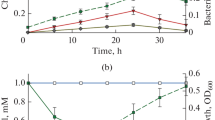
Degradation of propiconazole in liquid medium by soil isolate strain PS-4
The isolated P. aeruginosa PS-4 was investigated for its propiconazole degradation ability. The results show that 8 μg/L propiconazole was degraded after 3 days of incubation when compared to its respective control without PS-4 strain. (Fig. 3); the half life DT50 of propiconazole in MSM was found to be 34 h.
Effect of temperature and pH on biodegradation of propiconazole
It was observed that 80 % of degradation was achieved at 30 °C and pH 7, which indicates the mesophillic nature of bacterium and hydrogen ion balance during the degradation of propiconazole by PS-4 strain. However, 52.44 % degradation was found at 20 °C and 27.14 % at 50 °C (Fig. 4). Similarly, degradation of propiconazole was found to be 44.6 % at pH 5 and 26 % at pH 9 (Fig. 5).
Effect of temperature on the degradation of propiconazole; data are from 72 h samples from different degradation experiments at different temperatures. Values are means ± SE of three independent replicates. Means with different letters are significantly different from each other according to Tukey’s test (P < 0.05)
Cytochrome P450 gene identification in strain PS-4
The presence of the CYP P450 gene in P. aeruginosa PS-4 was confirmed and identified with a product size of 1.3 kb (Fig. 6). Blast analysis showed that the sequence of the amplified gene had high homology with cytochrome P450 genes identified in other strains of P. aeruginosa and the resulting phyllogram and dendrogram showed that the gene segment (amplicon) was most closely related to P. aeruginosa VRFPA04, complete genome (Fig. 7).
Extraction of cell-free filtrates for enzyme assay
The extracts of cell-free solution showed monooxygenase activity and catalyse propiconazole, yielding major three metabolites: 1,2,4-triazole; 2,4-dichlorobenzoic acid; and 1-chlorobenzene. The enzyme and specific activity was found to be 0.241 ± 12 μmol min−1 and 0.310 ± 0.3 μmol min −1 mg protein−1, respectively. The concentration of protein in the cell free extracts was found to be 0.969 mg mL−1.
Chemical analyses
Based on the liquid chromatography mass spectroscopy selected reaction monitoring (LC/MS/SRM), propiconazole was detected at m/z 342.13 (Fig. 8a). The degradation rate of propiconazole in Tilt-applied soil was measured based on the area count, the compound eluted at retention time 8.69 min, and it was confirmed that 49 % of degradation was observed (Fig. 8b). Note that culture filtrates of PS-4 strain analyzed by HPLC expressed different peak patterns. Whereas the propiconazole was eluted at retention time 2.811 min, there was a significant decrease in the propiconazole peak (Fig. 9), indicating degradation of propiconazole. The results obtained from mass spectra were used to predict possible metabolites accumulated in the culture filtrate. Three possible products of propiconazole were identified based on molecular weight of the compounds, namely 1,2,4-triazole; 2,4-dichlorobenzoic acid; and 1-chlorobenzene, which are possible metabolites involved in the propiconazole degradative pathway (Fig. 10).
Discussion
Superior, safe and affordable food for a constantly increasing population is the basic agricultural target of any nation (Babu et al. 2015). Pesticides are used widely as crop protection products to combat losses caused by pests and diseases. However, the harmful effects of these pesticides on human health and the environment is well known. In the last few decades, researchers have established that microbial degradation can have beneficial effects on soil fertility and crop growth. Several degradation studies have shown that bacteria metabolise toxic compounds under in vitro conditions (Cain and Mitchell 1996; Mitchell and Cain 1996; Shetti and Kaliwal 2012; Abraham and Silambarasan 2013).
In the current study, a low rate of degradation and persistence of propiconazole was observed after 30 days under all conditions tested. Interestingly, soil samples placed outside the laboratory showed a good rate of degradation compared to soil placed inside the laboratory. Therefore, microbial degradation of propiconazole was carried out for complete remediation by isolating the resistant bacteria. The population of isolated Pseudomonas aeruginosa (PS-4) strain from paddy fields was found to be most predominant in the propiconazole-contaminated paddy soil. Moreover, the isolated PS-4 strain utilized propiconazole as a sole source of carbon and energy for growth in MSM, resulting degradation of 8 μg/L propiconazole under the optimal conditions of pH 7 and 30 °C within 3 days. This is in good agreement with the findings of earlier researchers who demonstrated the degradation of triazole fungicides; the latter reported that degradation of fungicides under different environmental conditions was found to be more eco-friendly, efficient and useful for cleaning up of polluted areas (Nelson et al. 1973; Bailey and Coffey 1985; Oltmanns et al. 1989).
In our study, Pseudomonas aeruginosa PS-4 strain was found to be the most efficient while utilising propiconazole as the sole substrate. The results also show that CYP P450 monooxygenase metabolizes propiconazole by yielding (1-[[2-(2,4-dichlorophenyl)-4-methyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole) (m/z = 313). Further, monooxygenase activities give rise to 2,4-dichlorobenzoic acid (m/z = 193) by the partial fragmentation of dioxolane ring and complete cleavage of the1,2,4-triazole ring. Also, a carboxylic group and one chlorine atom was fragmented from 2,4-dichlorobenzoic acid by the action of propiconazole CYP P450 monooxygenase activity by yielding 1-chlorobenzene (m/z = 113). Thus, the metabolism of propiconazole was confirmed based on the formation of the above-mentioned metabolites and CYP P450 monooxygenase activity. Also, the results obtained can be used to construct a pathway of propiconazole degradation by the P. aeruginosa PS-4 strain. These results show for the first time that a degradative pathway for the propiconazole by P. aeruginosa PS-4 acts mainly via propiconazole CYP P450 monooxygenase activity.
The previous experiment showed varying degrees of degradation of propiconazole, yielding 1-[[2(2,4-dichlorophenyl)-2-(1,2,4-triazole-1-yl) ketone, 1-(2,4-dichlorophenyl)-2-(1,2,4-triazole-1-yl) ethanol and 1[[2(2,4-dichlorophenyl)-4-hydroxypropyl-1,3-dioxolane-2-yl]methyl]1H-1,2,4-triazole. Also, propiconazole dissolution in paddy soil under anaerobic conditions was found to be minimal, which indicates that temperature and aeration will play an important role in the degradation of propiconazole (Kim et al. 2002). Similarly, the degradation rate of flutriafol, epoxiconazole, propiconazole, triadimefon and triadimenol fungicides also increases with increased temperature (Bromilow et al. 1999). Similarly, Chlorpyrifos degradation in soil was enhanced by an increase in temperature (Racke et al. 1994). Even though the application of triazole fungicides in agriculture is extensive, very few studies have been conducted on microbial degradation of these pesticides. Previously, propiconazole biodegradation was undertaken and was achieved successfully with amendment of glucose in the degradation medium by Pseudomonas putida. However, the degradation products have not been reported as is the case in our study (Sarkar et al. 2009). Interestingly, basidiomycete fungi were found to be efficient for the degradation of propiconazole and tubeconazole, but the degradation pathway has not been studied (Woo et al. 2010). Chlorobenzene degradation by P. putida through 3-chlorocatechol by the meta cleavage pathway involving the activity of catechol 2,3-dioxygenase (Mars et al. 1997) strongly supports our experimental results. Some investigations specified that microorganisms will not degrade propiconazole, because of its strong adsorption to soil organic matter (Kloskowski et al. 1987; Ekler 1988; Taylor and Spencer 1990). Although several reports on the degradation of triazoles in soil are available, microbial degradation studies have been vastly under represented. A few reports on tubaconazole and other fungicide degradation by bacteria isolated from contaminated soil have also been reported (Nicole et al. 2009; Megadi et al. 2010; Elhussein et al. 2011). Moreover, Pseudomonas fluorescence was found to degrade 10 % to 70 % tubeconazole in the culture medium with a time gap of 6–21 days. In addition, Pseudomonas chrysosporium also showed some tubeconazole degradation (Obanda and Shupe 2009). Recently Vaz et al. (2015) reported the efficacy of a Pseudomonas sp. that has the ability to degrade Paclobutrazol fungicide, a trizole fungicide well known for its longer persistence in soil.
Here, we have demonstrated for the first time that P. aeruginosa PS-4 strain degrades propiconazole via the metabolic activity of CYP P450, involving three metabolites namely 1,2,4-triazole; 2,4-dichlorobenzoic acid; and 1-chlorobenzene. Microbial degradation via CYP P450 monooxygenase is efficient and acts as a precursor for the degradation of many toxic compounds. CYP P450 monooxygenase has been shown to be responsible for the degradation of ketoconazole, which consists of dioxolane ring (Rodriguez and Acosta 1997). Earlier reports also demonstrated that metabolism of the carboxyl group in tazarotenic acid was initiated by cytochrome P450 monooxygenase activity (Attar et al. 2003). Further studies on the aerobic degradation of chlorine also indicated a role for monooxygenase in the elimination of chlorine (Shim and Wood 2000). It has been reported that CYP P450 catalysed the oxidation of chlorinated ethenes to yield chloroacetaldehydes (Meunier et al. 2004) and that monooxygenase activity in culture filtrate of Pseudomonas sp. strain DCA1 is responsible for the degradation of 1,2-dichloroethane (Hage and Hartmans 1999). In spite of above mentioned application of CYP P450 monooxygense, in the last decade, a number of researchers have reported that CYP P450 monooxygenase is a multipurpose product useful for drug development and bioremediation processes for the detoxification of many toxic compounds. Other in vitro reports suggest that CYP P450 monooxygenase is the main route for the production of metabolites from any toxic substrate (Miners 2002; Guengerich 2002; Larkin et al. 2005; Urlacher and Eiben 2006). Azole fungicides are also reported to share this usual mode of action (Y. Li et al. 2013). Therefore, P. aeruginosa strain PS-4 may utilize other azole fungicides and may thus be used for cleaning up contaminated soil. The present study provided evidence that propiconazole degradation by P. aeruginosa PS-4 strain is associated with cytochrome P450 monooxygenase. The results have yielded important new information on how isolated bacteria are able to degrade toxic pesticides that prevail in agricultural soil. The uniqueness of our study lies in the fact that the degradation pathway for the propiconazole metabolism by the bacteria is proposed for the first time.
References
Abraham J, Silambarasan S (2013) Biodegradation of chlorpyrifos and its hydrolyzing metabolite 3,5,6-trichloro-2-pyridinol by Sphingobacterium sp. JAS3. Process Biochem 48:1559–1564
Attar M, Dong D, John Ling JK, Tang-Liu DSD (2003) Cytochrome p450 2c8 and flavin-containing monooxygenases are involved in the metabolism of tazarotenic acid in humans. Drug Metab Dispos 31(4):476–481
Babu NA, Jogaiah S, Ichi IS, Nagaraj KA, Tran PSL (2015) Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci 231:62–73
Bailey AM, Coffey MD (1985) Biodegradation of metalaxyl in avocado soils. Phytopathology 75:135–137
Bromilow RH, Evans AA, Nicholls PH (1999) Factors affecting degradation rate of triazole fungicides in two soil types: II. Field studies. Pest Manag Sci 55:1135–1142
Cain RB, Mitchell JA (1996) Enhanced degradation of the fungicide vinclozolin: isolation and characterisation of a responsible organism. Pest Manag Sci 48:13–23
Colson ES, Platz GJ, Usher TR (2003) Fungicidal control of Pyrenophoratritici-repentis in wheat. Australas Plant Pathol 32:241–246
Degtyarenko KN (1999) Structural domain of P450 containing monooxygenase systems. Protein Eng 8:737–747
Dong X, Hong Q, He L, Jiang X, Li S (2008) Characterization of phenol-degrading bacterial strains isolated from natural soil. Int Biodeterior Biodegrad 62:257–262
Ekler Z (1988) Behaviour of thiophanate herbicides in soil: adsorption and volatilization. Pestic Sci 22:145–157
Elhussein AA, Osman AG, Sherif AM (2011) Isolation, characterization, identification and potentiality of fungicide thiram (TMTD) degraders under laboratory conditions. Int J Appl Environ Sci 6(2):193–199
Elmholt E (1992) Effect of propiconazole on substrate amended soil respiration following laboratory and field application. Pest Manag Sci 34:139–146
Vaz F, Santos-Filho E, Silva S, Araújo S, Stamford-Arnaud T, Bandeira A, Brasileiro-Vidal AC, Pereira Stamford N, Aparecida Mouco M, Gouveia E (2015) Biodegradation of paclobutrazol—a plant growth regulator used in irrigated mango orchard soil. In: Rolando C (ed) Biodegradation and bioremediation of polluted systems. InTech, Croatia, pp85–107
Guengerich FP (2002) Cytochrome P450 enzymes in the generation of commercial products. Nat Rev Drug Discov 1:359–366
Hage JC, Hartmans S (1999) Monooxygenase-mediated 1,2-ichloroethane degradation by Pseudomonas sp. strain DCA1. Appl Environ Microbiol 65:2466–2470
Kim IS, Beaudette LA, Shim JH, Trevors JT, Suh YT (2002) Environmental fate of the triazole fungicide propiconazole in a rice-paddy-soil lysimeter. Plant Soil 239:321–331
Kim IS, Shim JH, Suh YT (2003) Laboratory studies on formation of bound residues and degradation of propiconazole in soils. Pest Manag Sci 59:324–330
Kloskowski R, Führ F, Mittelstaedt W (1987) The uptake of nonextractable soil-bound pesticide residues by roots-standardized experiments with four pesticides. In: Greenhalgh R, Roberts TR (eds) Pesticide science and biotechnology. Blackwell, Oxford, pp 405–410
Kulkarni AG, Kaliwal BB (2014) Bioremediation of methomyl by soil isolate- Pseudomonas aeruginosa. J Environ Sci Toxicol Food Technol 8(12):1–10
Larkin MJ, Kulakov LK, Allen CCR (2005) Biodegradation and Rhodococcus masters of catabolic versatility. Curr Opin Biotechnol 12:564–573
Li Y, Dong F, Liu X, Xu J, Chen X, Han Y, Liang X, Zheng Y (2013a) Studies of enantiomeric degradation of the triazole fungicide hexaconazole in tomato, cucumber, and field soil by chiral liquid chromatography-tandem mass spectrometry. Chirality 25:160–169
Li ZH, Zlabek V, Velisek J, Grabic R, Machova J, Kolarova J (2013b) Multiple biomarkers responses in juvenile rainbow trout, Oncorhynchus mykiss, after acute exposure to a fungicide propiconazole. Environ Toxicol 28:119–126
Mars EA, Kasberg T, Kaschabek RS, Van Agteren HM, Janssen BD, Reineke W (1997) Microbial degradation of chloroaromatics: use of the meta-cleavage pathway for mineralization of chlorobenzene. J Bacteriol 179:4530–4537
Megadi BV, Tallur NP, Mulla IS, Ninnekar HZ (2010) Bacterial degradation of fungicide captan. J Agric Food Chem 58:12863–12868
Meunier B, De-Visser SP, Shaik S (2004) Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem Rev 104:3947–3980
Miners JO (2002) Evolution of drug metabolism: hitchhiking the technology bandwagon. Clin Exp Pharmacol Physiol 29:1040–1044
Mitchell JA, Cain RB (1996) Rapid onset of the accelerated degradation of dicarboximide fungicides in a UK soil with a long history of agrochemical exclusion. Pestic Sci 48:1–11
Munier LC, Borde O (2000) Effect of a triazole fungicide on the cellulose decomposition by the soil microflora. Chemosphere 41:1029–1035
Nelson JD, Blair W, Brinckman FE, Colwell RR, Iverson WP (1973) Biodegradation of phenylmercuric acetate by mercury-resistant bacteria. Appl Microbiol 26(3):321–326
Nicole TS, Priscila SC, Maria DCR, Peralbaand Marco AZA (2009) Biodegradation of Tebuconazole by bacteria isolated from contaminated soils. J Environ Sci Health B 45:67–72
Obanda DN, Shupe TF (2009) Biotransformation of tebuconazole by microorganisms: evidence of a common mechanism. Wood Fiber Sci 41:157–167
Oltmanns RH, Muller R, Otto MK, Lingens F (1989) Evidence for a new pathway in the bacterial degradation of 4-fluorobenzoate. Appl Environ Microbiol 55:2499–2504
Racke KD, Fontaine DD, Yoder RN, Miller JR (1994) Chlorpyrifos degradation in soil at termiticidal application rates. Pest management sciece. Pest Manag Sci 42:43–53
Rodriguez RJ, Acosta D (1997) N-deacetyl ketoconazole-induced hepatotoxicity in a primary culture system of rat hepatocytes. Toxicology 117:123–131
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sarkar S, Seenivasan S, Premkumar R (2009) Biodegradation of propiconazole by Pseudomonas putida isolated from tea rhizosphere. Plant Soil Environ 55:196–201
Satapute PP, Olekar HS, Shetti AA, Kulkarni AG, Hiremath GB, Patagundi BI, Shivsharan CT, Kaliwal BB (2012) Isolation and characterization of nitrogen fixing Bacillus subtilis strain as-4 from agricultural soil. Int J Recent Sci Res 3:762–765
Seubert NYW (1960) Determination of isoprenoid compounds by microorganisms. Isolation and characterization of an isoprenoid degrading bacterium. Pseudomonas citronellolis, new species. J Bacteriol 79:426–434
Shetti AA, Kaliwal BB (2012) Biodegradation of imidacloprid by soil isolates Brevundimonas sp. MJ15. I. J Curr Res 4(10):100–106
Shim H, Wood KT (2000) Aerobic degradation of mixtures of chlorinated aliphatics by cloned toluene-o-xylene monooxygenase and toluene o-monooxygenase in resting Cells. Biotechnol Bioeng 70:693–698
Talwar MP, Mulla SI, Ninnekar HZ (2014) Biodegradation of organophosphate pesticide quinalphosby Ochrobactrum sp. strain HZM. J Appl Microbiol 117:1283–1292
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA: molecular evolutionary genentics analysis version 6.0. Mol Biol Evol 30:2727–2729
Tandon HLS (2005) Methods of analysis of soils, plants, waters and fertilizers, edn. New Delhi, FDCO
Taylor AW, Spencer WF (1990) Process, impacts and modeling. In: Cheng HH (ed) Pesticides in the soil environment. Soil Science Society of America, Madison, WI, pp 214–244
Urlacher VB, Eiben S (2006) Cytochrome P450 monooxygenases: perspectives for synthetic application. Trends Biotechnol 24:324–330
Woo C, Daniels B, Stirling R, Morris P (2010) Tebuconazole and propiconazole tolerance and possible degradation by Basidiomycetes: a wood-based bioassay. Int Biodeterior Biodegrad 64:403–408
Acknowledgements
The authors are thankful to the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, Delhi, for providing the instrumentation facility (Centrifuge, UV spectrophotometer and rotorflash evaporator) (BT/PR/4555/INF/22/126/2010 dated 30 September 2010). The authors are also thankful to the UGC-UPE fellowships and Post Graduate Department of Studies in Microbiology and Biotechnology, Karnatak University Dharwad for providing the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest connected to the manuscript.
Ethical approval
This article does not contain any studies related to human participants or animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 112 kb)
Rights and permissions
About this article
Cite this article
Satapute, P., Kaliwal, B. Biodegradation of the fungicide propiconazole by Pseudomonas aeruginosa PS-4 strain isolated from a paddy soil. Ann Microbiol 66, 1355–1365 (2016). https://doi.org/10.1007/s13213-016-1222-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-016-1222-6