Abstract
Antagonistic actinomycete strains isolated from the environment are valuable tools for an eco-friendly, healthy, and safe control of phytopathogenic fungi. We have evaluated the culture filtrate of Streptomyces griseorubens E44G, an actinomycete strain isolated from soil, on the growth and ultrastructure of hyphal cells of the phytopathogenic fungus Fusarium oxysporum f. sp. lycopersici, the causal agent of Fusarium wilt disease of tomato. The effect of the Streptomyces culture filtrate on some of the carbohydrate fractions in the hyphal cell of the pathogen using gold-labeled lectin complexes was also elucidated. Of the concentrations of S. griseorubens E44G culture filtrate tested, the highest (400 μL) had the most potent antifungal effect on the mycelial growth of the fungus. At this concentration, some changes in the morphology of the fungal hyphae were observed by scanning electron microscopy, and a number of dramatic changes in the ultrastructure of the hyphal cells of the fungus were observed by transmission electron microscopy. Ultracytochemical localization of carbohydrate fractions of the hyphal cell of the fungus revealed the presence of a very high quantity of chitin in the cell wall which was digested following exposure to the culture filtrate of S. griseorubens E44G, indicating the presence of a chitinase enzyme in that filtrate. The ultracytochemical investigations also indicated the presence of mannose, glucose, and galactose in the fungal cell wall, as well as the absence of glucosides. Moreover, the fungal cell cytoplasm contained glucosides and galactose but not chitin. These results confirm that the chitinase enzyme was produced by S. griseorubens E44G and that this enzyme may play a role in the potential of this strain as an antifungal agent against F. oxysporum f. sp. lycopersici.
Similar content being viewed by others
Introduction
Fusarium oxysporum f. sp. lycopersici (FOL), the causal agent of Fusarium wilt disease in tomato, is an economically important fungal pathogen that causes serious damage to the plant, leading to significant losses in tomato yield (Suárez-Estrella et al. 2007). Currently, the most effective method of preventing this disease is to treat tomato seeds with chemical fungicides. The efficacy of various chemical fungicides, such as benomyl, carbendazim, prochloraz, fludioxonil, bromuconazole, and azoxystrobin, to control this disease has been tested (Amini and Sidovich 2010). However, the use of chemical fungicides may not always be desirable due to their toxic effects on non-target organisms and the environment (Arcury and Quandt 2003). This has led researchers to focus on an alternative means for fungal disease control that had be implemented in integrated disease management systems, i.e. biological control (Gnanamanickam 2002).
Actinomycetes in general and Streptomycetes in particular are known to include several species that inhibit the growth activities of many fungal phytopathogens in vitro. The genus Streptomyces is considered to be the richest source of microorganisms which produce anti-microbial compounds (Al-Askar et al. 2011, 2013). The antagonistic activity of Streptomyces against fungal phytopathogens may be attributed to the production of bioactive compounds and/or extracellular hydrolytic enzymes (Singh et al. 2008; Sajitha and Florence 2013; Ghorbel et al. 2014). Lysis of the fungal wall by extracellular lytic enzymes secreted by specific microorganisms is one of the important mechanisms involved in the antagonistic activity of biocontrol agents (Haggag and Abdallh 2012; Choudhary et al. 2014). Among these, chitinases have been implicated in plant resistance against fungal phytopathogens because of their inducible nature and antifungal activities in vitro (Dahiya et al. 2006). Chitinases inhibit fungal growth through the lysis of fungal cell walls, hyphal tips, and germ tubes. Among the chitinolytic actinomycetes, Streptomyces species are thought to degrade the chitinous fungal cell wall through the production of chitinases and antibiotics (Thiagarajan et al. 2011; Choudhary 2014). Because of their inhibitory abilities, Streptomyces spp. has been actively studied and utilized as biocontrol agents against various plant pathogens (Srividya et al. 2012; Kanini et al. 2013).
Ultracytochemical studies on the localization of different carbohydrate fractions, particularly chitin, in the fungal cell wall and cell components have been conducted by many authors (Benhamou 1988; Baka and Lösel 1998). This localization may throw light on how the chitinolytic actinomycetes can be used as biocontrol agents against fungal phytopathogens. In this context, the aim of the study reported here was to evaluate the effect of culture filtrate of Streptomyces griseorubens E44G on the growth and ultrastructure of FOL. We also performed ultracytochemical studies of some carbohydrate fractions, particularly chitin in the cell wall of FOL. The results of these studies are reported here.
Materials and methods
Isolation of soil-borne actinomycetes
Twenty random rhizosphere soil samples were collected from different fields cultivated with tomato in Saudi Arabia and immediately placed in labeled, sterile plastic bags. All bagged samples were stored at 4 °C until use. Each soil sample (300 g) was removed carefully from around the roots of tomato plants with a spatula, at a depth of 10 cm. For analysis, 10 g of air-dried soil sample was suspended in 100 mL of basal salt solution (5 g/L KH2PO4 and 5 g/L NaCl) and shaken in a rotary shaker (150 rpm) at 28 °C for 30 min. The soil suspension was then diluted, and 1 mL of diluted soil suspension was spread onto starch nitrate agar plates (Waksman 1961). The medium was adjusted to the initial pH of 7 prior to sterilization, supplemented with 50 μg/mL of filter-sterilized cycloheximide to inhibit fungal growth, and incubated at 28 °C for 1 week. Colonies of actinomycetes on the agar plates were picked on the basis of their morphological characteristics, purified, and then transferred onto starch nitrate/NaCl slants for further use (Shirling and Gottlieb 1966).
Isolation of the seed-borne pathogen
Fusarium oxysporum f. sp. lycopersici was isolated from the seeds of naturally diseased tomato plants exhibiting typical symptoms of Fusarium wilt disease and collected from the same tomato fields from which soil samples were collected. The isolated fungus was grown on potato dextrose agar (PDA) (Difco Laboratories, Detroit, WI, USA) plates and incubated at 28 °C for 4–6 days. Purification of the resulting fungus was done using the single spore technique. The fungus thus isolated and purified was then transferred onto the PDA slant and kept at 4 °C for further studies. Pure cultures of the isolated fungus were identified according to cultural properties and morphological and microscopical characteristics as described by Booth (1977) and Domsch et al. (1980).
Screening for antifungal activity
All actinomycete isolates were screened for their in vitro antifungal activity against FOL. A 7-mm-diameter disk from a 5-day-old culture of the actinomycete isolate being tested was placed in the center of a starch nitrate agar plate inoculated with the tested fungus. Each treatment was done in triplicate. The starch nitrate plates were then incubated at 30 ± 1 °C for 72 h, following which the diameter of the inhibition zone, if any appeared, was measured (in mm) (Waksman 1961).
Identification of isolated antagonist
Molecular identification of the selected actinomycete isolate was based on 16S rRNA gene analysis. The total genomic DNA was extracted according to Sambrook et al. (1989). PCR amplification of the 16S rRNA was carried out in a thermocycler (Cetus Model 480; PerkinElmer, Waltham, MA, USA) using the universal primers 27f (5′-AGA GTT TGA TCC TGG CTC AG -3′) and 1525r (5′-AAG GAG GTG ATC CAG CC-3′) under the following cycling conditions: 94 °C for 5 min, 35 cycles at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 90 s, and a final extension step at 72 °C for 5 min. The product was directly sequenced by a BigDye terminator cycle sequencing kit (PE Applied Biosystems, Foster City, CA, USA) on an ABI 310 automated DNA sequencer (Applied Biosystems). Homology of the 16S rRNA sequence was analyzed using the BLAST algorithm, available in Genbank (http: www.ncbi.nlm.gov/BLAST/).
Effect of culture filtrate of S. griseorubens E44G on the radial growth of FOL
The inhibitory effect of the culture filtrate of S. griseorubens E44G on the radial growth of FOL was investigated on agar plates. The antagonist microorganism S. griseorubens E44G was grown on starch-nitrate broth medium, pH 7, at 30 °C on a rotary shaker (160 rpm) for 5 days. Different concentrations of the culture filtrate (100, 200, and 400 μL) were incorporated into PDA plates by adding the appropriate amounts aseptically to the melted medium just before solidification. Plates containing 20 mL of the medium at each concentration were prepared. Disks (diameter 7 mm) taken from the growing edge of 5-day-old colonies of FOL were used to inoculate the prepared plates. Each treatment was conducted in triplicate. The plates were incubated at 25 ± 2 °C for 6 days.
Scanning electron microscopy
To study the effect of Streptomyces culture filtrate on the hyphae of FOL using scanning electronic microscopy (SEM), fungal hyphae before sporulation were processed as follows. First, hyphal disks (diameter 1 cm) were taken from the actively growing margin of the colonies of both control and treated plates and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 2 h at room temperature. The fixed hyphal disks were then washed twice, 10 min each wash, in the same buffer before passing through a graded ethanol series [70, 80, 90 % (all one time each), 100% (three times; 30 min at each concentration]. The samples were critical point dried in a critical point drying system (Polaron CPD 7501; VG Microtech, East Grinstead, UK) up to the critical point with CO2. The fixed material was then mounted on stubs using double-sided carbon tape and coated with gold/palladium in a sputter coater system in a high-vacuum chamber (Polaron SC7620, VG Microtech) for 150 s at 9 mA. The samples were examined and digital images captured using a JEOL model JSM 5500 scanning electron microscrope (JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 5 KV.
Transmission electron microscopy
Conventional methods
Samples (1 mm3) of fungal culture treated with Streptomyces culture filtrate were processed for observation by transmission electron microscopy (TEM) according to the method of Hayat (2000). The samples were first immersed in 3 % (v/v) glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.0, for 2 h at 4 °C, rinsed in the same buffer, and post-fixed in 1% (w/v) OsO4. They were then dehydrated through a graded series of ethanol solutions and embedded in Spurr’s resin. Ultrathin sections were collected on Formvar-coated copper grids, stained with uranyl acetate (UA) followed by lead citrate (LC), and examined by using a JEOL 100-S transmission electron microscope.
Periodic acid–thiocarbohydrazide–silver proteinate technique
The Thiery (1967) method was applied to detect general carbohydrates in the hyphal cell walls of FLO exposed to Streptomyces culture filtrate. Ultrathin sections of glutaraldehyde–osmium tetroxide-fixed tissue were floated on 1 % periodic acid (PA) for 30 min in a high humidity chamber, washed in distilled water, then floated on 2 % thiocarbohydrazide (TCH) in 20 % acetic acid for 2, 12, or 24 h. After successive washes in 15, 10, and 5% acetic acid over a 30-min period and a final wash in distilled water, the sections were floated on aqueous 1% silver proteinate (SP) for 30 min in the dark. Sections were stained with UA/LC) and examined by TEM (JEOL 100-S; JEOL, Ltd.). As controls, either PA, TCH, or SP was omitted from the procedure (Courtory and Simar 1974).
Ultracytochemical studies
For the study of lectin binding sites, gold particles with a diameter of approximately 14–16 nm were prepared according to Frens (1973) and coated with probes specific to the substrates to be investigated (Table 1) according to the techniques of Horisberger and Rosset (1977) and Benhamou (1988). For the direct labeling with concanavalin A ConA), Ricinus communis agglutinin (RcA1) and β-glucosidase, sections were first incubated for 5 min on a drop of phosphate-buffer saline (PBS) containing 0.02% polyethylene glycol (PEG) 20000, with a pH corresponding to the optimal activity of the protein tested (Table 1). Sections were then transferred to a drop of the protein–gold complex and incubated for 30 min at room temperature in a moist chamber. Finally, grids were washed with PBS, rinsed with distilled water, and stained with UA and LC (UA/LC). For indirect labeling of substances containing N-acetylglucosamine, sections were incubated for 30 min at room temperature on a drop of wheat germ agglutinin (WGA) in PBS (10 μg/mL), rinsed with PBS, and then incubated for 30 min with colloidal gold-labeled ovomucoid, a protein which has a high affinity for WGA. The sections were then washed with PBS and distilled water and collected on formvar-coated nickel grids, stained with UA/LC, and examined by TEM (JEOL 100-S). Control experiments were performed according to Benhamou (1988).
Results
Isolation of actinomycetes and screening for antifungal activity
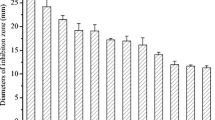
In total, we isolated 250 isolates of actinomycetes from rhizosphere soils, all of which were screened for their antifungal activities against FOL. Among them, 97 actinomycete isolates were found to be antagonistic to FOL to varying extents. Only the isolate with the strongest antagonistic activity, designated E44G, was selected for further study.
The 16S rRNA gene sequence of strain E44G was determined and compared with corresponding sequences in the GenBank database using DNA BLASTn (NCBI website), which revealed that strain E44G is similar to Streptomyces griseorubens (99 % similarity). The strain was then designated S. griseorubens E44G and the 16S rRNA gene sequence was deposited in the GenBank under accession number (KJ605118).
Effect of culture filtrate of S. griseorubens E44G on the radial growth of FOL
The inhibitory effect of different concentrations of the culture filtrate of S. griseorubens E44G on the radial growth of FOL hyphae was investigated on PDA plates. Concentrations of 100, 200, and 400 μL yielded varied degrees of inhibition against FOL (Table 2), with maximum inhibition achieved at 400 μL. However, in the control, the same volume of sodium acetate buffer did not inhibit the pathogen (Fig. 1).
Effect of the culture filtrate of Streptomyces griseorubens E44G on radial growth of Fusarium oxysporum f. sp. lycopersici (FOL) in vitro without the addition of filtrate (control) (a), after the addition of 100 μL of filtrate (b), after the addition of 200 μL of filtrate (c), and after the addition of 400 μL of filtrate (d)
SEM observations
Observations of FOL by SEM revealed that the untreated hyphae (control) appeared to be thin and smooth (Fig. 2a), while the hyphae treated with S. griseorubens E44G culture filtrate (concentration 400 μL) appeared to be much thicker with granulated surfaces (Fig. 2b).
Scanning electron microscopy (SEM) micrographs showing the morphology of FLO hyphae. a FLO hyphae not exposed to S. griseorubens E44G culture filtrate, i.e. untreated control (bar: 1.0 μm), b FLO hyphae treated with S. griseorubens E44G filtrate (concentration 400 μL). Note that the treated hyphae are relatively thicker with a granulated surface (bar: 1.0 μm)
TEM observations
Effect of S. griseorubens E44G culture filtrate on the ultrastructure of FOL hyphal cell
Observations of FOL hyphae by TEM provided a more detailed picture of the cellular disorganization induced by S. griseorubens E44G culture filtrate. Hyphal cells grown under control conditions were regularly septate, with Woronin bodies typically associated with septa. The plasma membrane was closely appressed against the thin cell wall, and the cytoplasm appeared to be metabolically active, based on the amount of polyribosomes and organelles (Fig. 3a). In contrast, collapsed fungal cell cytoplasm and local retraction of the plasma membrane accompanied by cell-wall swelling were typical features of fungal cells grown on PDA amended by 400 μL of S. griseorubens E44G culture filtrate (Fig. 3b).
Transmission electronic microscope (TEM) micrographs of FOL hyphae grown on PDA. a FLO hypha not exposed to S. griseorubens E44G culture filtrate, i.e. untreated control. The regularly septate hyphal cell contains a polyribosome-rich cytoplasm in which numerous organelles, such as mitochondria (M), are embedded; the fungal wall (FW) is thin; a lipid body (L) is present. Bar: 3.0 μm. b FLO hypha exposed to S. griseorubens E44G filtrate (concentration 400 μL). There is increased vacuolation, a thicker FW (compared to a), and collapsed cytoplasm (Cy), In addition, the plasmalemma (PM) is further away from the fungal wall. Note also the septum (S). Bar : 3.0 μm
General localization of carbohydrates
The hyphal wall of FOL showed a greater affinity for periodic acid–thiocarbohydrazide–silver proteinate (PATCHSP) staining, indicating a higher content of carbohydrates (compare Fig. 4a and b).
TEM micrographs of FOL hyphae grown on potato dextrose agar. a Untreated hypha with periodic acid–thiocarbohydrazide–silver proteinate (PATCHSP) (control). Note the electron-lucent cell wall (arrow), small vacuole (V), and large vacuole (arrowhead). Bar : 3.0 μm. b Hypha with PATCHSP staining to localize general carbohydrates in the cell wall. Note the electron-opaque fungal cell wall (FW) indicating the presence of general carbohydrates and in the fungal cytoplasm (Cy). Bar: 3.0 μm
Localization of N-acetylglucosamine (chitin)
To localize N-acetylglucosamine residues (chitin) in the hyphal cell wall of FOL, we applied the WGA–gold-labeled ovomucoid complex to sections of fungal hyphae grown on PDA. We found an intense accumulation of gold particles over the hyphal cell wall, but the cytoplasm and organelles were nearly free of labeling. The labeling pattern over the hyphal cell walls showed that the gold particles accumulated preferentially over the outermost wall layers (Fig. 5a). Moreover, the application of S. griseorubens E44G culture filtrate to sections of fungal hyphae previously treated with the WGA–gold-labeled ovomucoid complex revealed the absence of labeling over the cell wall (Fig. 5b), indicating the presence of a chitinase enzyme in the culture filtrate.
TEM micrographs of FOL hyphae treated with wheat germ agglutinin (WGA)–gold-labeled ovomucoid complex. a Control (untreated) hypha showing strongly labeled fungal cell walls (FW) and septa (S). Labeling of the cytoplasm, mitochondria (M), and Woronin bodies (Wb) is almost non-existent. Bar: 3.5 μm. b Hypha treated with S. griseorubens E44G filtrates (concentration 400 μL) previously treated with the WGA–gold-labeled ovomucoid complex. Note the breakdown of hyphal wall (arrows) and absence of labeling over that wall; also note some labeling over the septum (S). Bar: 2.5 μm
Localization of ß-glucosides
TEM examination of sections of FOL hyphae treated with the ß-glucosidase–gold complex revealed the presence of moderate labeling which was mainly concentrated over the cytoplasm of the hyphal cell (Fig. 6a). The cell wall and septum were free of labeling. Adsorption of the ß-glucosidase–gold complex with S. griseorubens E44G culture filtrate before the incubation gave negative results (Fig. 6b)
TEM micrographs of a hyphal cell of FOL treated with the ß-glucosidase–gold complex. a Control, treated with the ß-glucosidase gold complex. Gold particles can be seen to be abundant in the cytoplasm (Cy), whereas only few occur over the fungal walls (FW) and septa (S). Bar: 2.5 μm. b Labeling is absent in the cytoplasm after treatment with the ß-glucosidase–gold complex which was previously treated with S. griseorubens E44G filterate. WB Woronin body, M mitochondrion, S septum. Bar: 2.0 μm
Localization of D-galactose
Incubation of sections of FOL hyphae with the RcA1-gold complex gave an intense labeling over the cytoplasm of the FOL cells, particularly in the polysome-rich cytoplasmic regions, whereas vacuoles and mitochondria showed almost no labeling (Fig. 7a). Gold particles were also present over the plasma membrane (Fig. 7a). In contrast, walls and septa appeared to be weakly labeled. Adsorption of the RcA1–gold complex with S. griseorubens E44G culture filtrate prior to incubation gave negative results (Fig. 7b)
TEM micrographs of hyphal cell of FOL treated with the Ricinus communis agglutinin (RcA1)–gold complex. a Control, gold particles are mainly associated with polysome-rich cytoplasmic regions whereas vacuoles and mitochondria (M) are almost unlabeled. Walls and septa are weakly labeled, with gold particles being preferentially located in the outer cell layers. The plasma membrane (PM) is labeled by few dispersed gold particles. Bar: 5.0 μm. b Hyphal cell treated with the RcA1–gold complex and previously treated with S. griseorubens E44G filterate. Note that the labeling is persistent. Bar: 2.0 μm
Localization of α-D-mannose and α-D-glucose
TEM examination of sections from FOL hyphae incubated with the Con A–gold complex revealed the presence of numerous gold particles over the cell-wall layers, septa, and the triangular junctions between septa and lateral walls (Fig. 8a). In contrast, cytoplasm, organelles, and vacuoles were nearly devoid of labeling. Labeling was absent, however, in the hyphal section treated with S. griseorubens E44G culture filtrate at the concentration of 400 μL previously adsorbed with the Con A–gold complex (Fig. 8b). The occurrence, localization, and relative amounts of carbohydrate fractions detected in the hyphal cell of FOL are presented in Table 3.
TEM micrographs of hyphal cells of FOL treated with the concanavalin A (Con A)–gold complex. a Gold particles mainly associated with the fungal wall (FW) and septa whereas cytoplasm and vacuoles are almost unlabeled. Bar: 2.5 μm. b Hyphal cells treated with S. griseorubens E44G metabolites (400 μL) and previously adsorbed with (Con A)-gold complex; note absence of labeling over the fungal cell wall (FW). Bar: 2.5 μm
Discussion
In this study, we isolated 250 isolates of actinomycetes from rhizosphere soils and screened them for their antifungal activities against FOL. Among these, 97 actinomycete isolates were found to be antagonistic to FOL to varying extents. Many researchers have reported similar antimicrobial activity of actinomycetes against phytopathogens (Haggag and Abdallh 2012; Sajitha and Florence 2013; Choudhary et al. 2014). Khucharoenphaisan et al. (2013) isolated 83 actinomycete strains from different soil samples, of which 79 % exhibited antifungal activity against the phytopathogenic fungus Colletotrichum gloeosporioides that ranged from 21 to 100%.
The results from our antifungal assay show that FOL was highly sensitive to S. griseorubens E44G culture filtrate at all of the concentrations tested, but maximum inhibition was recorded at 400 μL. It is well known that many species of actinomycetes, particularly those belonging to the genus Streptomyces, are biocontrol agents that inhibit the growth of many phytopathogenic fungi (Al-Askar et al. 2011, 2013). The antagonistic activity of Streptomyces to phytopathogens is usually related to the production of bioactive compounds (Atta 2009; Khamna et al. 2009) and/or extracellular hydrolytic enzymes (Choudhary et al. 2014).
The use of lectins labeled with colloidal gold for identifying specific compounds at the ultrastructural level has enabled researchers to localize carbohydrate residues and enzymes in various fungal phytopathogens and host tissues (Dabour et al. 2005). In our study, the use of gold-labeled lectin–carbohydrate complexes allowed the in vitro localization of various carbohydrate-containing molecules of the cell surface of FOL. The intense labeling observed on the walls of FOL hyphae after treatment with the WGA–gold-labeled ovomucoid complex indicates the presence of N-acetylglucosamine residues (chitin) in the cell wall. These results are in agreement with those obtained by other authors (e.g., Bernard and Latgé 2001; Zamani et al. 2008). Since chitin is a polymer of interlinked N-acetylglucosamine residues, it is reasonable to assume that WGA binding sites are associated with chitin. Following the exposure of sections of FOL hyphae previously treated with the WGA–gold-labeled ovomucoid complex to S. griseorubens E44G culture filtrate, there was no labeling of the cell wall, indicating the presence of a chitinase enzyme in S. griseorubens E44G culture filtrate. The production of lytic enzymes has been shown to be a crucial property of some actinomycetes (Fogliano et al. 2002). Most phytopathogenic fungi have cell walls that contain chitin as a structural backbone arranged in regularly ordered layers, in addition to proteins and lipids (Chernin and Chet 2002). There have been several reports of biocontrol agents which can inhibit the growth and cause deformation of viable hyphae of the phytopathogenic fungi (Di Giambattista et al. 2001; Fogliano et al. 2002).
Our ultracytochemical investigation revealed the relative occurrence of α-D-mannose and α-D-glucose in the cell wall of FOL. Galactose and glucosides were absent from the hyphal cell wall, although these residues were detected in the cytoplasm of the fungal cells. Glucose and glucan have been reported to be present in the fungal cell walls of different species of fungi (Chen and Seviour 2007; Osherov and Yarden 2010) and in the cell wall of Fusarium species (Schoffelmeer et al. 1999). Many authors have confirmed that ß-glucosides are present in the cytoplasm of other phytopathogenic fungi and absent from their cell wall (e.g., Ruiz-Herrera 2012). Fontaine et al. (2000) reported that the central fibrillar core of the cell wall of Aspergillus fumigatus is composed of β-1,3-glucan.
D-galactose was observed on the plasma membranes and in polysome-rich cytoplasmic regions of cells of FOL, whereas nuclei, mitochondria, and vacuoles were free from this residue. D-galactose residues were also very scarce in the cell wall. These results are in agreement with those reported by Benhamou (1988) for Ophiostoma ulmi, this author found the same residue in an insignificant quantity in the cell wall of Verticillium albo-atrum. Baka and Losel (1998) reported that D-galactose was present in the wall of the rust fungus Melampsora euphorbiae. These results may indicate that the presence or absence of this carbohydrate fraction depends on the fungal species. Carbohydrate analysis of the isolated cell walls of three formae speciales of F. oxysporum showed that they contained mannose, galactose, and uronic acids, in addition to glucose and chitin, and that these presumably originate from cell-wall glycoproteins. Ultrastructural studies of gold-labeled lectin complexes indicate that glycoproteins are present in the external layers covering an inner layer composed of chitin and glucan (Ruiz-Herrera 2012).
Application of S. griseorubens E44G culture filtrate to the sections of fungal hyphae previously treated with the enzyme or gold-labeled lectin complexes with the aim to localize β-glucosides revealed the absence of labeling over the cell wall, indicating the presence of glucosidases enzymes in S. griseorubens E44G culture filtrate. Several studies have demonstrated that glucosidases produced from many Streptomyces strains have the potential to inhibit the growth of many phytopathogenic fungi (Di Giambattista et al. 2001; Fogliano et al. 2002).
In conclusion, our results indicate that chitinase and ß-glucosidase were produced by S. griseorubens E44G culture filtrate. This ability provides this strain with the potential to control phytopathogenic fungi. However, more studies need to be conducted in terms of formulation and mass production of this biocontrol agent in order to develop a biofungicide for field application.
References
Al-Askar AA, Abdul Khair WM, Rashad YM (2011) In vitro antifungal activity of Streptomyces spororaveus RDS28 against some phytopathogenic fungi. Afr J Agric Res 6(12):2835–2842
Al-Askar AA, Rashad YM, Abdulkhair WM (2013) Antagonistic activity of an endemic isolate of Streptomyces tendae RDS16 against phytopathogenic fungi. Afr J Microbiol Res 7(6):509–516
Amini J, Sidovich DF (2010) The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J Plant Prot Res 50:172–178
Arcury TA, Quandt SA (2003) Pesticides at work and at home: exposure of migrant farm workers. Lancet 362:2021
Atta HM (2009) An antifungal agent produced by Streptomyces olivaceiscleroticus, AZ-SH514. World Appl Sci J 6:1495–1505
Baka ZAM, Lösel DM (1998) Ultrastructure and lectin-gold cytochemistry of the interaction between the rust fungus Melampsora euphorbiae and its host, Euphorbia peplus. Mycol Res 102:1387–1398
Benhamou N (1988) Ultrastructural localization of carbohydrates in the cell walls of two pathogenic fungi: a comparative study. Mycologia 80:324–337
Bernard M, Latgé JP (2001) Aspergillus fumigatus cell wall: composition and biosynthesis. Med Mycol 39:9–18
Booth C (1977) The genus Fusarium. Commonwealth Mycological Institute, Kew
Chen J, Seviour R (2007) Medicinal importance of fungal β-(1 → 3), (1 → 6)-glucans. Mycol Res 111:635–652
Chernin L, Chet I (2002) Microbial enzymes in the biocontrol of plant pathogens and pests. In: Dick RP, Burns RG (eds) Enzyme in the environment. Marcel Dekker, New York, pp 171–225
Choudhary B, Nagpure A, Gupta RK (2014) Fungal cell-wall lytic enzymes, antifungal metabolite(s) production, and characterization from Streptomyces exfoliatus MT9 for controlling fruit-rotting fungi. J Basic Microbiol 54(12):1295–1309
Courtory R, Simar LJ (1974) Importance of controls for the determination of carbohydrates in electron microscopy with the silver methenamine or the thiocarbohydrazide-silver proteinate methods. J Microsc 100:199–211
Dabour N, LaPointe G, Benhamou N, Fliss I, Kheadr EE (2005) Application of ruthenium red and colloidal gold-labeled lectin for the visualization of bacterial exopolysaccharides in Cheddar cheese matrix using transmission electron microscopy. Int Dairy 15:1044–1055
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of Chitinolytic enzymes: a review. Appl Microbiol Biot 71:773–782
Di Giambattista R, Federici F, Petruccioli M, Fenice M (2001) The chitinolytic activity of Penicillium janthinellum P9: purification, partial characterization and potential application. J Appl Microbiol 91:498–505
Domsch KW, Gams W, Anderson TH (1980) Compendium of soil fungi, vol 1. Academic, London
Fogliano V, Ballio A, Gallo M, Woo S, Scala F, Lorito M (2002) Pseudomonas lipodepsipeptides and fungal cell wall-degrading enzymes act synergistically in biological control. Mol. Plant-Microbe Interact 15:323–333
Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latgé JP (2000) Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem 275:27594–27607
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold solutions. Nat Physiol Sci 241:20–22
Ghorbel S, Kammoun M, Soltana H, Nasri M, Hmidet N (2014) Streptomyces flavogriseus HS1: Isolation and characterization of extracellular proteases and their compatibility with laundry detergents. BioMed Res Int 2014:345980. doi:10.1155/2014/345980
Gnanamanickam SS (2002) Biological control of crop diseases. Marcel Dekker, New York
Haggag WM, Abdallh EG (2012) Purification and characterization of chitinase produced by endophytic Streptomyces hygroscopicus against some phytopathogens. J Microbiol Res 2:145–151
Hayat MA (2000) Principles and techniques of electron microscopy: biological applications, 4th edn. Cambridge University Press, Cambridge
Horisberger M, Rosset J (1977) Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem 25:295–300
Kanini GS, Katsifas EA, Savvides AL, Hatzinikolaou DG, Karagouni AD (2013) Greek indigenous streptomycetes as biocontrol agents against the soil-borne fungal plant pathogen Rhizoctonia solani. J Appl Microbiol 114:1468–1479
Khamna S, Yokota A, Peberdy JF, Lumyong S (2009) Antifungal activity of Streptomyces spp. isolated from rhizosphere of Thai medicinal plants. Int Integr Biol 6:143–147
Khucharoenphaisan K, Sinma K, Lorrungruang C (2013) Efficiency of actinomycetes against phytopathogenic fungus of chili anthracnose. J Appl Sci 13:472–478
Osherov N, Yarden O (2010) The cell wall of filamentous fungi. In: Borkovich KA, Ebbole DJ, Momany M (eds) Cellular and molecular biology of filamentous fungi. American Society for Microbiology Press, Washington DC, pp 224–237
Ruiz-Herrera J (2012) Fungal cell wall: structure, synthesis, and assembly. CRC Press, Boca Raton, p 203
Sajitha KL, Florence EJM (2013) Effects of Streptomyces sp. on growth of rubberwood sapstain fungus Lasiodiplodla theobromae. J Trop For Sci 25:393–399
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. a laboratory manual. vol. 1, 2nd edn. Harbor Laboratory Press, Cold Spring Harbor, p 23
Schoffelmeer EAM, Klis FM, Sietsma JH, Cornelissen BJC (1999) The cell wall of Fusarium oxysporum. Fungal Genet Biol 27:275–282
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Singh V, Tripathi CKM, Bihari V (2008) Production, optimization and purification of an antifungal compound from Streptomyces capoamus MTCC 8123. Med Chem Res 17:94–102
Srividya S, Thapa A, Bhat DV, Golmei K, Dey N (2012) Streptomyces sp. 9p as effective biocontrol against chilli soil-borne fungal phytopathogens. Eur J Exp Biol 2:163–173
Suárez-Estrella F, Vargas-Garcia C, Lopez MJ, Capel C, Moreno J (2007) Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f. sp. melonis. Crop Prot 26:46–53
Thiagarajan V, Revathia R, Aparanjinib K, Sivamanic P, Girilala M, Priyad CS, Kalaichelvan PT (2011) Extracellular chitinase production by Streptomyces sp. PTK19 in submerged fermentation and its lytic activity on Fusarium oxysporum PTK2 cell wall. Int J Curr Sci 1:30–44
Thiery JP (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc 6:987–1018
Waksman SA (1961) The actinomycetes II. classification, identification and descriptions of genera and species. The Williams and Wilkins Company, Baltimore
Zamani A, Jeihanipour A, Edebo L, Niklasson C, Taherzadeh MJ (2008) Determination of Glucosamine and N-acetyl Glucosamine in fungal cell walls. J Agric Food Chem 56:8314–8318
Acknowledgments
This work was supported by NSTIP strategic technologies program number (10-BIO976-02) in the Kingdom of Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Askar, A.A., Baka, Z.A., Rashad, Y.M. et al. Evaluation of Streptomyces griseorubens E44G for the biocontrol of Fusarium oxysporum f. sp. lycopersici: ultrastructural and cytochemical investigations. Ann Microbiol 65, 1815–1824 (2015). https://doi.org/10.1007/s13213-014-1019-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-1019-4