Abstract
Increasing global concerns over plastic waste disposal and environmental awareness has already highlighted Polyhydroxyalkanoates (PHA’s) as an increasingly attractive bioplastic option. In this regard, the present investigation aims to highlight the production of polyhydroxyalkanoate by Pseudomonas aeruginosa BPC2 (GeneBank entry: JQ866912) using a glycerol by-product as an inexpensive carbon source. The glycerol by-product was generated via the production of biodiesel from kitchen chimney dump lard (KCDL). The strain was also cultured in media comprising other carbon sources like glycerol (commercial), sugar cane molasses and glucose for comparative PHA yield. An appreciable PHA accumulation up to 22.5 % of cell dry weight was found when the bacterium was cultured in media comprised of glycerol by-product. The extracted bacterial biopolymer was further characterized by FTIR, GC-MS, GPC and TGA. The experimental results of the study warrant the feasibility of bacterial biopolymer production using glycerol by-product as an inexpensive carbon source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Polyhydroxyalkanoates (PHA’s) are natural, renewable and biocompatible biopolymers that can be synthesized in many microorganisms from almost all genera of the microbial kingdom. Many microorganisms synthesize polyhydroxyalkanoates (PHA’s) as intracellular carbon and energy reserve materials (Anderson and Dawes 1990; Doi 1990), which accumulate as water insoluble inclusions for storage of carbon and energy during imbalanced growth by various microorganisms (i.e., in the presence of an excess of a carbon source as well as nutrient limiting conditions) (Reddy et al. 2003). PHA’s can be processed into plastic materials with properties that are very similar to petrochemical plastics, besides they possess the ability to replace the later in numerous applications (Philip et al. 2006). With the global trend gradually shifting towards a bio-based economy the use of PHA’s as a substitute for conventional petrochemical based plastics seems to be attractive.
With a view to make PHA production economically attractive, the use of inexpensive substrates has been investigated thoroughly over the years (Lemos et al. 2006; Castilho et al. 2009). In the wake of advancements in biopolymer research, PHA’s offer significant candidature as a class of bio-based and biodegradable polymers that can be produced using renewable resources such as sugars and vegetable oils (Matsumoto et al. 2001; Taguchi et al. 2002; Akiyama et al. 2003; Kahar et al. 2004). Waste streams from oil mills or used oils, which are even less expensive than purified oils too, can be used for the production of PHA’s (Fernández et al. 2005; Mumtaz et al. 2010). Additionally, the ability of many microorganism to produce PHA’s from industrial and domestic wastes is also gaining much importance as this approach can minimize waste disposal problems while at the same time reduce the economics of PHA production. Among the various fermentation feedstocks studied for the production of PHA, vegetable oils are reported to have the best yield owing to high carbon content in fatty acids (Akiyama et al. 2003). Recently, because of the blooming biofuel industry, glycerol (obtainable as a waste from biodiesel production) is also becoming an attractive feedstock for fermentation. Microorganisms that possess the ability to utilize triglycerides and glycerol for cell growth and subsequent PHA production would be of much use for biopolymer research. Increases in biodiesel demand, while providing an additional outlet for fats and oils, will result in a large co-product stream that is composed primarily of glycerol, free fatty acids (FFA) and fatty acid methyl esters (FAME), the composition of which depends on the fat or oil feedstock, the transesterification process, and recovery efficiency of alkyl ester production.
The current investigation is an endeavor to investigate the feasibility of PHA production using glycerol by-product (generated via KCDL based biodiesel production) as an inexpensive carbon source by an indigenously isolated bacterial strain, Pseudomonas aeruginosa BPC2. KCDL is primarily a kitchen bio-waste with a light brown color and murky odor formed as a result of frying processes where the vapors of the cooking oil after condensation get collected in semisolid form in the collecting ducts of a kitchen chimney and can be collected from the chimney ducts. KCDL had a density of 2.01 g/mL, average molecular weight of 825.02 and an acid value of 28 mg KOH/g (Phukan et al. 2013). The study also attempts to establish KCDL based biodiesel waste glycerol (from here onwards only glycerol by-product for brevity) in the development of value added products as an economically and environmentally benign alternative. The production of PHA using glycerol by-product has also been compared with PHA production from glycerol (commercial), sugar cane molasses and glucose as a carbon source. FTIR, GC-MS, GPC and TGA of the extracted PHA have further been done as a part of biopolymer characterization.
Materials and methods
Chemicals
All chemicals were obtained from Sigma–Aldrich Company Ltd. except nutrient agar and commercial glycerol, which were from Hi-media, India. FT-IR grade KBr was used for the FTIR analysis. NMR analysis was performed using analytical grade reagents. Commercial polymer used as a standard for FTIR and TGA analysis was polyhydroxybutyrate, (PHB, 95 %).
Transesterified product of KCDL
The carbon source used for this study was the glycerol by-product obtained from transesterification of kitchen chimney dump lard (KCDL) which has been reported in our previous work (Phukan et al. 2013). This glycerol by-product obtained as a result of the transesterification process was directly used as the carbon source for bacterial growth and subsequent biopolymer production.
Bacterial strain and culture conditions
Pseudomonas aeruginosa was isolated from crude oil contaminated soil of Assam, Assam Arkan Basin, ONGCL, India. The PHA detection medium (Rehman et al. 2007) was used with modifications for the production of PHA. The medium consisted of (g/L): (NH4)2SO4 (2.0), KH2PO4 (13.3), MgSO4 (1.3), citric acid (1.7), the trace element (mg/L), Na2MoO7·2H2O (0.39), FeSO4·7H2O (10.0), ZnSO4·7H2O (0.22), CuSO4·5H2O(0.08), MnSO4·5H2O (1.81), Na2CO3 (20.0), H3BO3 (2.86), and HCl (10 mL). The pH was adjusted to 7.0 prior to autoclaving. Glycerol by-product, glucose, sugarcane molasses and glycerol (commercial) were supplemented at 1 % (w/v) in PHA medium for growth and PHA accumulation.
The bacterial culture was grown in 50 ml sterile Nutrient Broth, in 250 ml Erlenmeyer flasks, which were incubated on a rotary shaker at 180 rpm at 37 °C as a pre-culture. An aliquot of 10 mL of the above pre-culture inoculums was transferred to a 250 mL Erlenmeyer flask in an orbital shaker at 37 °C, for 72 h at 180 rpm. Culture media for PHA accumulation included a 1 % carbon source from glycerol by-product, glucose, sugarcane molasses and glycerol (commercial) and the same concentration of the media as already described above. The increase in turbidity of the culture medium signified bacterial growth. The shaken flask cultures were harvested and assayed for PHA production.
The broth culture obtained after 72 h culture was centrifuged (8,000 × g) for 15 min at 4 °C. The cell pellet was washed twice with deionized water and washed with a small volume of acetone. Soxhlet extraction was done to extract the polymer from lyophilized biomass using chloroform as the solvent for 24 h. The dissolved PHA was concentrated by vacuum rotary evaporation and precipitated with the drop wise addition of ten volumes of ice-cold methanol. The process was repeated thrice for obtaining the purified polymer (Ashby et al. 2002a). The polymer precipitated as a sticky substance. The precipitated polymer was collected by air drying and kept for further analysis.
Identification of the bacterial isolate by PCR amplification of 16S r DNA
Isolation of genomic DNA was carried out using the bacterial genomic DNA Isolation Kit (RKT09). Pure bacterial isolate of Pseudomonas spp. was identified by PCR amplification. The amplification was performed in a 100 μL reaction mixture containing 2.5 mM each of dNTP, 400 ng each of oligonucleotide primer, 10× PCR buffer [200 mM Tris–HCl (pH 8.4), 500 mM KCl] and 3 U Taq DNA polymerase (Invitrogen, USA). The PCR product was sequenced bi-directionally using the forward, reverse and internal primer of the 16S rDNA. The primers used were: 16 s Forward Primer (27 F): (5′- GAGTRTGATCMTYGCTWAC-3′) and 16 s Reverse Primer (1544R): (5′-CGYTAMCTTWTTACGRCT-3′).
The PCR amplification program consisted of initial denaturation at 94 °C for 5 min, 94 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 2 min, and then 5 min final elongation at 72 °C was performed at the end of the 35 cycling steps, following which the samples were maintained at 4 °C. The PCR products (16S rDNA gene) were purified using a gel extraction kit (Bangalore Gene) and sequenced with the Big Dye Terminator version 3.1” Cycle sequencing kit and an ABI 3500 XL Genetic Analyzer. The sequence data were aligned and compared with published sequences obtained from the Gen Bank database using Seq Scape v 5.2. A phylogenetic tree was constructed using ClustalW by distance matrix analysis and the neighbour-joining method (Saitou and Nei 1987). Phylogenetic trees were displayed using TREEVIEW (Page 1996).
Polymer analysis
FTIR spectroscopic analysis
The infrared spectrum of the extracted polymer sample was recorded using a Perkin Elmer, Spectrum 100 Fourier transform infrared (FTIR) spectrometer. The extracted pure polymer sample was mixed with potassium bromide (KBr) and squeezed under pressure to form a KBr pellet. The spectra were recorded in the range 4000–400 cm−1. The functional groups were concluded by comparing the transmittance picks with available literature (Misra et al. 2000; Khardenavis et al. 2009).
GC-MS analysis
The composition of PHA was analyzed by gas chromatography using the lyophilized cells containing polymer. To determine the polymer content of the bacterial cells, freeze dried cell samples were subjected to methanolysis in the presence of sulphuric acid according to Brandl et al. (1988). In this method approximately 4.0 mg of the bacterial cells was reacted in a screw cap test tube with a solution containing 1.0 mL chloroform, 0.85 mL methanol and 0.15 mL sulphuric acid for 140 min at 100 °C in a thermostat-equipped oil bath. Under this condition, the intracellular PHA was degraded to its constituent 3-hydroxyalkanoic acid methyl esters. After the reaction, 0.5 mL distilled water was added and the tube was vigorously shaken for 1 min. After phase separation, the bottom organic phase was collected, dried over anhydrous sodium sulphate, filtered and analysed (Huijberts et al. 1994). The methyl esters were analyzed by gas chromatography with a Perkin Elmer, GC Varian 3800 model and Saturn 2,200 MS spectroscopy equipped with a CP-Sil 8 CB and CP-Sil 5 CB capillary column and a flame ionization GC detector and quadruple ion trap MS detector. A 2.0 μL portion of the organic phase was analyzed after split injection (spilt ratio 1:40) and helium (35 cm/min) was used as a carrier gas. The temperature of the injector and the detector were maintained at 230 °C and 275 °C, respectively. For efficient separation of the methyl esters, the following temperature program was used: 120 °C for 5 min and temperature rise of 8 °C/min, 180 °C for 12 min. P (HB) and P (HB-CO-HV) were used as standards. Benzoic acid was used as an internal standard.
Determination of molecular mass by GPC
The molecular mass of the extracted polymer was determined by gel permeation chromatography (GPC) using teh Waters GPC system. The PHA was dissolved in tetrahydofurane (THF), and analyzed by running at room temperature. Polystyrene standards with a low polydispersity were used to generate a calibration curve equipped with a serially connected RI detector. The product molecular mass was determined with no further corrections and tetrahydofurane (THF) was used as an eluent at a flow rate of 0.5 mL/min and 40 °C.
Thermal property study by TGA
The thermal stability and decomposition profile of the polymer sample was determined by using a Shimadzu TGA-50 thermogravimetric analyzer, which operated at a nitrogen flow rate of 10 mL/min. The sample was weighed to approximately 10 mg and placed in a platinum pan and heated from 30 to 600 °C at the heating rate of 10 °C/min under a nitrogen atmosphere. The decomposition temperature was recorded from the thermograms to the maximum degradation temperature and determined by analysis of the derivative weight loss curve over temperature.
Results and discussion
The bacterial strain used in the present study was isolated from the crude oil contaminated soil site of Assam and Assam Arakan Basin, ONGC, Jorhat, Assam, India. Jiun-Yee et al. (2010) hypothesized that the presence of oil would favor the growth and multiplication of microbes that have the ability to metabolize oil, fatty acids and/or glycerol. Therefore, it may be presumed that the isolated bacteria can synthesize PHA biopolymer by using glycerol by-product as a carbon source.
The identification of the isolate was performed by 16S rDNA analysis. The genomic DNA isolated from the Pseudomonas sp. was used for the amplification of 16S rDNA by PCR. As shown in Fig. 1, phylogenetic analysis indicated a comparative search for the sequences and revealed 99.7 % homology to the Pseudomonas aeruginosa MTH8 (GenBank entry: HQ202541 )16S rDNA gene sequence. Further, a BLAST (NCBI) search showed 99 % homology to other known P. aeruginosa 16S rDNA gene sequences and accordingly it was named as Pseudomonas aeruginosa BPC2 (GeneBank entry: JQ866912).
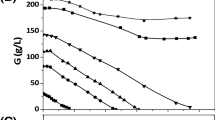
The biomass yield of bacterial cells in fermentation with shake flask culture, using different carbon sources like glycerol by-product, glucose, sugarcane molasses and glycerol (commercial), is shown in Table 1. The results indicated that higher cell densities were obtained from glycerol by-product. Cell growth up to a dry mass of 7.8 g/L was obtained when using media comprising glycerol by-product with shake flask culture. PHA accumulation up to 22.5 % of cell dry weight was obtained for culturing with glycerol by-product. PHA-content for all the cultures has an optimum at 72 h. The resulting curves for the PHA concentrations in fermentations with all the carbon sources are shown in Table 1. Fermentations of Pseudomonas aeruginosa BPC2 with glycerol by-product was obtained with a significantly higher PHA content than with glycerol (commercial).
The production of PHA was found to be better with glycerol by-product in contrast to the other tested carbon sources. It has been reported that genetically engineered Pseudomonas putida GPp104 synthesized poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) using gluconate and glucose, rather than fatty acids (Qiu et al. 2005). Solaiman et al. (2002) reported that the cellular yields were higher than those obtained with Pseudomonas corrugata grown on E medium supplemented with glucose [1.52 g CDW (cell dry weight)/L] or oleic acid [1.62 g CDW/L]. But the PHA yields from the strains were lower than PHA yields of 31 and 61 % CDW from P. corrugata grown on glucose or oleic acid. This value can also be compared with the mcl-PHA accumulation from the bacterial species Pseudomonas oleovorans NRRL B-778 grown on glucose, octanoic acid and oleic acid (Ashby et al. 2002b) and are found to be higher. The composition of polyester synthesized was determined mainly by the specificity of the enzymes involved in the synthesis of PHAs, genetically encoded availability of metabolic links of fatty acid metabolism to PHA synthesizing system, and also on the composition of the substrate. Ashby et al. (2004) reported that P. oleovorans NRRL B-14682 and P. corrugata 388 synthesized mcl-PHA upto 13-27 % and 42 % and cellular growth up to 1.3 ± 0.1 g/L and 1.7–2.1 g/L, respectively, when cultured in soy-based biodiesel production containing glycerol, fatty acid soaps and residual fatty acid methyl esters (FAME). Wong et al. (2000) reported that the PHA accumulation by Staphylococcus epidermidis was 2.5 g/L and cell density of 2.68 % was achieved when grown on sesame oil.Obruca et al. (2010) proved that efficient production of co-polymers was found from waste oil in comparison to pure oil and also to glucose. Verlinden et al. (2011) also reported that Cupriavidus necator produced 1.2 g/L of PHB homopolymer from waste and heated frying oil which is similar when glucose is used as the carbon source. They stated that the unsaturated fatty acids, residual carbohydrates, proteins and fats from foods, available nitrogen compounds, peroxides and heat-degradation products in the waste or frying oil could also be metabolized and may have contributed to increased PHB production by bacteria. This indicated that glycerol by-product may contribute towards higher PHA accumulation by the bacterial strain P. aeruginosa JQ866912 when compared to other carbon sources.
IR spectra of pure PHA of P. aeruginosa BP C2 and standard PHB are shown in Fig. 2. It shows mainly two intense absorption bands at 1,740 cm-1 and 1220 cm-1 corresponding to ester carbonyl C = O and C-O stretching grou,ps respectively (Misra et al. 2000). The peaks at wave numbers 3,340, 2,922, represent the presence of O-H bonding and bands of C-H stretching, respectively. Weak peaks at 1,670 cm-1 and 3,085 cm-1 are indicative of the presence of –C = C and alkene C–H stretching in the polymer chain.
The GC pattern of the total ion chromatogram of the polymer from P. aeruginosa JQ866912 (Fig. 3) possessed three methyl ester peaks with the retention time RT = 11.56 min, 18.46 and an additionally large peak with a retention time RT = 19.66 and some minor peaks at RT = 12.59 and 14.61. In Fig. 4(a), the mass spectra of the methyl ester of the saturated 3-hydroxy valeric acid were being dominated by m/z = 103 caused by an α–cleavage between C3 and C4 and M/Z = 143 probably caused by expulsion of methanol. Similarly, in Fig. 4(b), the mass spectra of the decanoic acid methyl esters pattern showed the m/z peak at 90.1, which originated from the carbonyl end of the molecule. This was due to the cleavage between C2 and C3 carbon atoms following McLafferty rearrangement. The fragmentation pattern of the methyl esters of octadecanoic acid methyl esters [Fig. 4(c)] showed the m/z peak at 74.1. This peak originated from the carbonyl end of the molecule due to cleavage between C3 and C4 carbon atoms following McLafferty rearrangement (Verlinden et al. 2011). The peak at m/z = 103.1 represented the hydroxyl end of the molecule which occurred due to the cleavage at bonds between C3 and C4, the alkyl end of this cleavage resulted in the peak at m/z = 71.1. Theref,ore it was concluded that a copolymer of poly-hydroxyvalerate-co-hydroxydecenoate-co-octadecanoate was produced by P. aeruginosa JQ866912.
The number average molecular mass (Mn) of the PHA polymer of P. aeruginosa JQ866912 strain was found to be 3.8 × 104 Da. The weight average molecular mass (Mw) of the polymer was 4.1 × 104 Da with a polydispersity index (PDI) of 1.08. Ouyang et al. (2007) reported that P. putida KT2442 has produced mcl-PHA containing hydroxydodecanoate fractions with the number average molecular mass (Mn) of 8.0 × 104 Da. But the polydispersity index of this polymer is slightly higher (1.25) than the polymer isolated from P. aeruginosa JQ866912 strain. Allen et al. (2010) also reported that P. oleovorans (ATCC 29347) grown in medium supplemented with saponified Jatropha seed oil produced PHA copolymer having molecular mass (Mp) of 1.79 × 105 with polydispersity (Mw/Mn) of 1.3 which is almost similar in range with the polymer from P. aeruginosa JQ866912. They also suggested that the lower polydispersity index of the polymer has uniform chain length, i.e., only one length of polymer is present in the PHA.
From the TGA graph (Fig. 5) it is clear that the commercial standard polymer had a shorter thermal degradation profile in comparison to the polymer from P. aeruginosa JQ866912. The polymer from P. aeruginosa JQ866912 was found to be thermally stable up to the range of 220–229 °C. The initial weight loss step in the TG analysis curves was at around 80–120 °C, which corresponds to the loss of moisture. The second step where major weight loss occurred (about 80 %) in the TGA curves between 350–360 °C was due to degradation of polymers. About 10 % of residue of the all the polymers was finally left when heated to 600 °C. Liu and Chen (2007) reported that mcl-PHA was more thermostable and PHB and P (3HB–12 mol % 3HHx) degraded at 226 and 239 °C, respectively. The high thermal degradability temperature and melting temperature of the isolated PHA from these strains suggests the potential applicability of mcl-PHAs in different industrial applications. Li et al. (2007) reported that for mcl–PHAs consisting of 30 mol % 3HV, the degradation temperature lies between 296 °C and 307 °C. Bengtsson et al. (2010) also reported that decomposition temperatures of the PHAs produced from fermented molasses and synthetic feeds containing single volatile fatty acids (VFAs) by an open mixed culture were 277.2 °C and 294.9 °C, respectively.
Conclusion
The use of a simple, inexpensive, and renewable carbon source for PHA synthesis by microorganisms is crucial from the economic and environmental point of view. From the study, it can be concluded that glycerol by-product generated via the production of biodiesel from KCDL is a better carbon source for PHA synthesis by P. aeruginosa JQ866912 in contrast to glycerol (commercial), sugarcane molasses and glucose. Since glycerol from KCDL is a by-product produced during biodiesel production, therefore, using this glycerol by-product fraction as a carbon source for PHA production can be both cost-effective and environmentally benign. Moreover, the coupling of biodiesel production with PHA production can be a viable means for reducing the economics of biodiesel feedstock utility.
References
Akiyama M, Tsuge T, Doi Y (2003) Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable carbon resources by bacterial fermentation. Polym Degrad Stab 80:183–194
Allen AD, Anderson WA, Ayorinde FO, Eribo BE (2010) Biosynthesis and characterization of copolymer poly (3HB-co-3HV) from saponified Jatropha curcas oil by Pseudomonas oleovorans. J Ind Microbiol Biotechnol 37:849–856
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial use of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Ashby RD, Solaiman DKY, Foglia TA (2002a) Poly (ethylene glycol)-mediated molar mass control of short-chain- and medium-chain-length poly (hydroxyalkanoates) from Pseudomonas oleovorans. Appl Microbiol Biotechnol 60:154–159
Ashby RD, Solaiman DKY, Foglia TA (2002b) The synthesis of short- and medium- chain-length poly (hydroxyalkanoate) mixture from glucose- or alkanoic acid-grown Pseudomonas oleovorans. J Ind Microbiol Biotechnol 28:147–153
Ashby RD, Solaiman DKY, Foglia TA (2004) Bacterial poly (hydroxyalkanoate) polymer production from the biodiesel co-product stream. J Polym Environ 12:105–112
Bengtsson S, Pisco AR, Johansson P, Lemos PC, Reis MAM (2010) Molecular weight and thermal properties of polyhydroxyalkanoates produced from fermented sugar molasses by open mixed cultures. J Biotechnol 147:172–179
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly (β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Castilho LR, Mitchell DA, Freire DMG (2009) Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour Technol 100:5996–6009
Doi Y (1990) Microbial polyesters. VCH publishers, New York
Fernández D, Rodriguéz E, Bassas M, Viñas M, Solanas AM, Llorens J, Marqués AM, Manres A (2005) Agro-industrial oily wastes as substrates for PHA production by the new strain Pseudomonas aeruginosa NCIB40045: Effect of culture conditions. Biochem Eng J 26:159–167
Huijberts GN, de Rijk TC, de Waard P, Eggink G (1994) 13C nuclear magnetic resonance studies of Pseudomonas putida fatty acid metabolic routes involved in poly(3-Hydroxyalkanoate) synthesis. J Bacteriol 176:1661–1666
Jiun-Yee C, Yifen T, Mohd-Razip S, Kumar S (2010) Isolation and characterization of a Burkholderia sp. USM (JCM15050) capable of producing polyhydroxyalkanoate (PHA) from triglycerides, fatty acids and glycerols. J Polym Environ 18:584–592
Kahar P, Tsuge T, Taguchi K, Doi Y (2004) High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym Degrad Stab 83:79–86
Khardenavis AA, Vaidya AN, Kumar MS, Chakrabarti T (2009) Utilization of molasses spent wash for production of bioplastics by waste activated sludge. Waste Manag 29:2558–2565
Lemos PC, Serafim LS, Reis MAM (2006) Synthesis of polyhydroxyalkanoates from different short-chain fatty acids by mixed cultures submitted to aerobic dynamic feeding. J Biotechnol 122:226–238
Li ZG, Lin H, Ishii N, Chen GQ, Inoue Y (2007) Study of enzymatic degradation of microbial copolyesters consisting of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates. Polym Degrad Stab 92:1708–1714
Liu WK, Chen GQ (2007) Production and characterization of mcl-PHA with high 3-hydroxy tetradecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Appl Microbiol Biotechnol 76:1153–1159
Matsumoto K, Nakae S, Taguchi K, Matsusaki H, Seki M, Doi Y (2001) Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) copolymer from sugars by recombinant Ralstonia eutropha harboring the phaC1Ps and the phaGPs Genes of Pseudomonas sp. 61-3. Biomacromolecules 2:934–939
Misra AK, Thakur MS, Srinivas P, Karanth NG (2000) Screening of poly-β-hydroxybutyrate-producing microorganisms using Fourier transform infrared spectroscopy. Biotechnol Lett 22:1217–1219
Mumtaz T, Yahaya NA, Abd-Aziz S, Rahman NA, Yee PL, Shirai Y, Hassan MA (2010) Turning waste to wealth-biodegradable plastics polyhydroxyalkanoates from palm oil mill effluent – a Malaysian perspective. J Clean Prod 18:1393–1402
Obruca S, Marova I, Snajdar O, Svoboda Z (2010) Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rape seed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol Lett 32:1925–1932
Ouyang SP, Luo RC, Chen SS, Liu Q, Chung A, Wu Q, Chen GQ (2007) Production of polyhydroxyalkanoates with high 3-hydroxydodecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Biomacromolecules 8:2504–2511
Page RD (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Philip S, Keshavarz T, Roy I (2006) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol 82:233–247
Phukan MM, Singh SP, Phukon P, Borah T, Konwar BK, Dutta N (2013) Production and statistical optimization of biodiesel from kitchen chimney dump lard. Sustainable Chem Proc 1:12. doi:10.1186/2043-7129-1-12
Qiu YZ, Han J, Guo JJ, Chen GQ (2005) Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol Lett 27:1381–1386
Reddy CS, Ghai R, Rashmi KVC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87:137–146
Rehman S, Jamil N, Husnain S (2007) Screening of different contaminated environments for polyhydroxyalkanoates-producing bacterial strains. Biol Bratislava 62:650–656
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Solaiman DKY, Ashby RD, Foglia TA (2002) Physiological characterization and genetic engineering of Pseudomonas corrugata for medium-chain-length polyhydroxyalkanoates synthesis from triacylglycerols. Curr Microbiol 44:189–195
Taguchi S, Matsusaki H, Matsumoto K, Takase K, Taguchi K, Doi Y (2002) Biosynthesis of biodegradable polyesters from renewable carbon sources by recombinant bacteria. Polym Int 51:899–906
Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Piotrowska-Seget Z, Radecka IK (2011) Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express 1:1–8
Wong AL, Chua H, Fu Yu PH (2000) Microbial production of polyhydroxyalkanoates by bacteria isolated from oil wastes. Appl Biochem Biotechnol 84–86:843–857
Acknowledgments
The first author (PP) gratefully acknowledges the ONGCL, India for providing a research grant for the work. The second author (MMP) expresses his sincere sense of gratitude to the Department of Science & Technology, Government of India for providing a research grant in the form of a DST-INSPIRE fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phukon, P., Phukan, M.M., Phukan, S. et al. Polyhydroxyalkanoate production by indigenously isolated Pseudomonas aeruginosa using glycerol by-product of KCDL biodiesel as an inexpensive carbon source. Ann Microbiol 64, 1567–1574 (2014). https://doi.org/10.1007/s13213-014-0800-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-0800-8