Abstract
Biosurfactant-producing bacteria were isolated from various sources in the south of Thailand. Isolates were screened for biosurfactant production using jackfruit seed powder (JSP) as a novel and promising substrate. The highest biosurfactant activity was obtained with a bacterial strain which was identified by 16S rRNA gene sequence analysis as Deinococcus caeni PO5. D. caeni PO5 was able to grow and reduce the surface tension of the culture supernatant from 67.0 to 25.0 mN/m after 87 h of cultivation when 40 g/l of JSP and 1 g/l of commercial monosodium glutamate were used as carbon and nitrogen sources, respectively. The biosurfactant obtained by ethyl acetate extraction showed high surface tension reduction (47.0 mN/m), a small critical micelle concentration value (8 mg/l), thermal and pH stability with respect to surface tension reduction and emulsification activity, and a high level of salt tolerance. Chemical characterization by biochemical testing, Fourier transform infrared spectroscopy, and mass spectra revealed that the obtained biosurfactant was a glycolipid-type biosurfactant. The obtained biosurfactant was capable of forming stable emulsions with various hydrocarbons and had the ability to enhance oil recovery, the solubility of polyaromatic hydrocarbons, heavy metal removal, and antimicrobial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Biosurfactants are a diverse group of surface-active agents with both hydrophilic and hydrophobic moieties and are produced by many living microorganisms, such as bacteria and fungi (Desai and Banat 1997). Biosurfactants have many advantages over synthetic surfactants. These include a lower toxicity and higher biodegradability, which makes them more environmentally friendly, better foaming properties, milder production conditions, lower critical micelle concentration, and greater stability towards temperature and pH (Mulligan 2005). These advantages clearly put the biosurfactants ahead of their synthetic counterparts and have, as a result, elevated their commercial potential (Franzetti et al. 2012). Despite possessing many commercially attractive properties and clear advantages compared with their synthetic counterparts, the production of microbial surfactants on a commercial scale has not been realized because of their high production costs (Muthusamy et al. 2008). However, this cost can be reduced by selecting efficient strains, optimizing medium composition and/or using alternative inexpensive substrates (Saimmai et al. 2012c). The choice of inexpensive raw materials is important to the overall economy of the process as these materials account for the major portion of the final production cost. Synthetic media commonly employed for the production of biosurfactants are not economically attractive, and there is therefore a need to identify inexpensive raw materials (Saimmai et al. 2012c, 2013a, b). One option is to replace expensive traditional carbon sources for surfactant production with cheaply available natural raw materials, by-products of industrial processes, or waste from agro-industry. The selection of waste substrates involves the difficulty of finding a residue with the right balance of nutrients to support optimal growth and production. Agro-industrial wastes with a high content of carbohydrates or lipids meet the requirements for use as promising substrates for biosurfactant production.
Jackfruit (Atrocarpus heterophyllus Lam.) is a monoecious evergreen tree that is popular in several tropical countries, such as Cambodia, Malaysia, Philippines, Thailand, and Vietnam. It is an excellent example of a food prized in some areas of the world and allowed to go to waste in others (Babitha et al. 2006). The largest of all tree-borne fruits, the jackfruit, can vary in length from 8 inches to 3 feet (20–90 cm) and in width from 6 to 20 inches (15–50 cm); its mass generally ranges from 10 to 60 lb (4.5–20 kg) but can reach 110 lb (50 kg) (Morton 1987). There may be 100 or up to 300 seeds in a single fruit. Seeds make up around 10–15 % of the total fruit mass and have high carbohydrate and protein contents. They are normally discarded, but can be steamed and eaten as a snack or used in some local dishes (Babitha et al. 2006). Therefore, we suggest that the jackfruit seed may be a suitable low-cost substrate for the production of biosurfactant in Thailand since it is cheap and renewable, especially in the Southern part of Thailand. To the best of our knowledge, this is the first report on biosurfactant production using jackfruit seed powder as the substrate.
Biosurfactants are produced by a wide variety of microorganisms, secreted either extracellulary or localized on the cell surface (Desai and Banat 1997). However, for cultivation, each species requires specific components in the medium and specific cultivation conditions. The object of this study was to investigate the potential of jackfruit seed powder as a substrate for biosurfactant production. Potential microbes were isolated locally and screened to identify the best isolate that could utilize jackfruit seed powder for biosurfactant production. The optimal medium components were determined to maximize the biosurfactant production from the selected isolates.
Materials and methods
Raw material and isolation of microorganisms
Jackfruit seeds obtained from a local market in Phuket Province, Thailand, were used as the substrate. To obtain jackfruit seed powder (JSP), we first peeled off the white arils of the seeds (seed coats), then sliced the seeds into thin chips, dried the chips at 60 °C for 12 h, and finally ground the chips into JSP. The chemical composition of the JSP was (g/100 g): carbohydrate, 39.05; crude protein, 7.32; fiber, 1.87; ash, 1.68; fat, 0.51.
Biosurfactant-producing bacteria were isolated from various sources in the south of Thailand, such as oil sludge, soil contaminated with palm oil or wastewater from the palm oil industry (Krabi Province, Nakhon Si Thammarat Province, Phang Nga Province, Phuket Province, Satun Province, Songkhla Province, Suatthani Province, and Trang Province), and mangrove sediment in the south of Thailand. The samples were collected in zipper bags and transported to the laboratory for screening and isolation of microorganisms. The method used for screening consisted of making serial dilutions of the samples and plating these on a minimal salt medium [MSM (g/l): K2HPO4, 0.8; KH2PO4, 0.2; CaCl2, 0.05; MgCl2, 0.5; FeCl2, 0.01; (NH4)2SO4, 1.0; NaCl, 5.0; Saimmai et al. 2012a]. MSM agar with 20 g/l JSP as the carbon source was used for the isolation of bacteria. Morphologically distinct colonies were re-isolated by transfer onto fresh JSP-containing agar plates at least three times to obtain pure cultures and subsequently Gram-stained. Pure cultures were stored at −20 °C in MSM mixed with sterile glycerol at a final concentration of 30 %.
Preparation of the seed culture
A loopful of isolated bacterial colony, previously maintained on a nutrient agar plate, was transferred to 50 ml nutrient broth (Difco Laboratories, Detroit, MI). The culture was grown on a rotary incubator shaker for 24 h at 30 °C and 200 rpm. This primary inoculum was grown until the optical density at 600 nm wavelength (OD600) reached 1.50 absorbance units and used to inoculate the production medium (5 % v/v).
Preparation of production medium
The production medium consisted of MSM supplemented with 20 g/l of JSP. The medium was sterilized by autoclaving at 121 °C for 15 min. Fermentation was carried out in 250-ml Erlenmeyer flasks with a 50 ml working volume. For inoculation, the flasks were allowed to cool down to room temperature before 2 % (v/v) primary inocula was transferred into the production medium. The cultures were incubated in a rotary incubator shaker for 72 h at 30 °C and 150 rpm. All experiments were carried out in triplicate.
Screening for potential biosurfactant-producing strains
Each isolated strain was subjected to liquid fermentation in the production medium as described above. The ability of each isolate to produce biosurfactant was measured qualitatively using a drop collapse method and quantitatively based on surface tension and emulsification activity values. The strain which showed the lowest surface tension value was selected for further study. The ability of a strain to grow in the presence of JSP was also one of the criteria used for strain selection.
Identification of selected strain
The selected strain was identified first based on biochemical methods (Kuda et al. 2011), 16S rRNA gene sequencing, and BLAST analysis (DNA Data Bank of Japan: http://www.ncbi.nlm.nih.gov/BLAST/) as reported by Saimmai et al. (2012a). The 16S rRNA gene was amplified with a forward (27 F: AGAGTTTGATCCTGGCTCAG) and reverse (1492R: GGTTACCTTGTTACGACTT) primer set.
Medium optimization
Medium optimization was conducted in a series of experiments during which in each experiment only one variable was changed and the other factors kept fixed at a specific set of conditions. Six factors were chosen for study with the aim of obtaining a higher productivity of the biosurfactant: the nitrogen source (N); carbon:nitrogen (C:N) ratio; inoculum concentration; initial pH; temperature; addition of amino acids. The nitrogen sources used were beef extract, commercial monosodium glutamate (CMSG), NaNO3, (NH4)2SO4, NH4Cl, NH4NO3, and peptone or yeast extract at a concentration of 1 g/l. The C:N ratio (with optimized nitrogen sources) was varied from 0 to 50 while keeping the concentration of the nitrogen source constant at 1 g/l. The inoculum concentration was varied between 2 and 12 % (v/v) of a 24-h culture of Deinococcus caeni PO5 in NB medium. In order to evaluate the effect of pH and temperature on the biosurfactant production, the pH of the medium was adjusted in the range of 5.0 and 9.0 and the temperature was set at 20, 25, 30, 35 40, 45, and 50 °C, respectively. Different amino acids, such as arginine, asparagine, aspartic acid, glutamic acid, isoleucine, leucine, and valine, were filtrated separately and then added to the medium at a final concentration of 10 g/l.
Recovery of biosurfactant
Four solvent systems, namely, a mixture of chloroform:methanol (2:1), cold acetone, dichloromethane, and ethyl acetate, were examined for the biosurfactant extraction (Saimmai et al. 2012c, 2013a, b). The solvent showing the highest biosurfactant activity was used to recover biosurfactant from D. caeni PO5.
Chemical analysis of biosurfactant
The carbohydrate content of the biosurfactant was determined by the phenol–sulfuric acid method (Dubois et al. 1956) using d-glucose as a standard. Protein content was determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard. The lipid content was estimated by following the procedure of Folch et al. (1956). The chemical nature of the biosurfactants obtained was determined by thin layer chromatography as previously described by Saimmai et al. (2012c). Further characterization of the biosurfactant was carried out using Fourier transform infrared spectroscopy (FT-IR) by the KBr pellet method (Saimmai et al. 2013a).
Biosurfactant stability
The effects of NaCl concentration, temperature, and pH on biosurfactant activity were investigated according the methods reported by Saimmai et al. (2012a, b). The effect of salinity was investigated by the dissolution of a specific concentration of NaCl (0–24 % w/v). To determine the effect of pH on biosurfactant activity, the pH of the biosurfactant was adjusted to 2–12. To verify the heat stability, the biosurfactant was maintained at 4–100 °C for 1 h and 110–121 °C for 20 min.
Application of the biosurfactant to remove used motor lubricating oil from contaminated sand
The suitability of the biosurfactant for enhanced oil removal was investigated as previously described by Saimmai et al. (2012c).
Laboratory experiment on biodegradation of used motor lubricating oil with biosurfactant
An experiment was conducted to study the impact of the biosurfactant derived from D. caeni PO5 on the biodegradation of used motor lubricating oil (ULO) in natural seawater as previously described by Saimmai et al. (2013a).
Application of the biosurfactant in removing metal from aqueous solutions
Biosurfactant solutions at different concentrations were incubated for 15 h with various concentrations of lead and cadmium at 30 °C and shaking (200 rpm). The content of unbound metals was quantified by atomic absorption spectroscopy after centrifugation at 10,000 g to separate the metal–biosurfactant co-precipitate from the supernatant. The resulting solutions were treated as metal solutions and measured using appropriate blanks and standards for calibration by using an atomic absorption spectrophotometer (model AA-6300; Shimadzu, Kyoto, Japan). The unbound metal concentration was represented as a function of biosurfactant concentration.
Solubilization assay for polyaromatic hydrocarbons
A solubilization assay for polyaromatic hydrocarbons (PAHs) was conducted as previously described by Saimmai et al. (2013a).
Antimicrobial activity of surface active compound
The extracted compound was tested for antimicrobial activity using an agar well diffusion method and the inhibition zone was measured as previously described by Saimmai et al. (2013b).
Growth
Biomass determination was performed as previously described by Saimmai et al. (2012c).
Drop-collapse test
The drop-collapse test was performed as previously described by Youssef et al. (2004).
Emulsification activity assay
The emulsification activity test was performed as previously described by Plaza et al. (2006).
Surface tension measurement
The surface tension of the culture supernatant was measured using a Model 20 Tensiometer (Fisher Science Instrument Co., Pittsburgh, PA) at 25 °C (Saimmai et al. 2012a).
Statistical analysis
All experiments were carried out in triplicate for the calculation of the mean value. Statistical analysis was performed using the Statistical Package for Social Science (SPSS ver. 10.0 for Windows; SPSS Inc., Chicago, IL).
Results and discussion
Potential biosurfactant-producing strains
Among the 452 bacterial isolates obtained from the soil samples (PO1–PO5) and waste water (PO6–PO12) associated with the palm oil refinery, mangrove sediments from the south of Thailand (MS1–MS18), and soil contaminated with ULO (US1–US18), only 48 bacterial isolates showed positive results with the drop-collapse method. Microbial growth in the production medium was used as a parameter during the primary screening of the biosurfactant-producing bacteria. After centrifugation of the fermentation broth, only a few strains showed a significant amount of biomass in the form of pellets, which indicated their abilities to grow in the production medium. Table 1 shows the Gram staining results, growth, emulsification activity, and surface activity of selected bacterial isolates. Of the selected bacterial isolates, 78 % (37/48) were Gram-negative. It has previously been reported that most bacteria isolated from sites with a history of contamination by hydrocarbon oil or its by-products and other immiscible substrate are Gram-negative due to the presence of outer membranes which act as biosurfactants (Batista et al. 2006; Ruggeri et al. 2009; Saimmai et al. 2012a, c). However, in our study, the highest biosurfactant activity was identified in a Gram-positive bacterium (PO5) isolated from a soil contaminated by a palm oil refinery in Phang Nga Province, with a drop-collapse result of 4.0 mm. Interestingly, this strain did not show high emulsification activity (41.26 %), but it could produce biosurfactants which exhibited the lowest surface tension value (39.5 mN/m) among those tested in our study. One explanation for this result may lay with the type of carbon source used for growth and also the type of hydrocarbon utilized in the emulsification activity test. When the medium is a single phase type (water-soluble carbon source), with no requirement for an emulsifier to make an insoluble substrate more accessible, there is no relation between surface tension reduction and emulsification activity (Saimmai et al. 2012c).
Identification of selected bacterial strain
Isolate PO5 was identified either through cell and colony morphology or biochemical and physiological characteristics (data not shown). However, due to their intrinsic limitations, biochemical and physiological tests can only provide a preliminary identification (Saimmai et al. 2012b). Final identification of the strain was accomplished by combining the alignment results of the 16S rRNA gene sequence analysis with biochemical and physiological characteristics. The results from 16S rRNA gene analysis were in agreement with the colony morphology, cell shape, and the results of Gram staining and biochemical testing (data not shown).
The sequences were assigned in the NCBI database and deposited in the DDBJ/EMBL/GenBank under accession number AB809363. The phylogenetic analysis of strain PO5 using the 16S rRNA gene nucleotide sequences data showed that this strain had the highest homology (100 %) with Deinococcus caeni Ho-08 (DQ017709). The phylogenetic tree based on neighbor joining analysis of the 16S rRNA gene nucleotide sequences is given in Fig. 1. We therefore named this isolate Deinococcus caeni PO5. There have been many reports of the isolation of biosurfactant-producing bacteria in the last few decades (Batista et al. 2006; Abdel-Mawgoud et al. 2010; Janek et al. 2010; Saimmai et al. 2012b, c; Chooklin et al. 2013). However, to date there have been few reports on biosurfactants produced by bacteria isolated from palm oil-contaminated soil and water samples (Saimmai et al. 2012c). It should also be noted that, to the best of our knowledge, this is the first report on the capacity of the genus Deinococcus to produce a biosurfactant.
Phylogenetic tree of Deinococcus caeni strain PO5 and closest NCBI (BLASTn) strains based on 16S rRNA gene sequences (neighbor joining tree method). Scale bar 0.01 nucleotide substitutions per nucleotide position. Numbers at node Bootstrap values obtained with 1,000 resampling analyses. The GenBank accession numbers are reported in parenthesis
Effect of N source
The effect of the nitrogen source on biosurfactant production is shown in Table 2. D. caeni PO5 was able to use nitrogen sources such as ammonia and urea for biosurfactant production. However, in order to obtain high concentrations of biosurfactant it is necessary to have restricted conditions for these macro-nutrients. We determined that commercial CMSG was the best source of nitrogen for growth and biosurfactant synthesis. The maximum biosurfactant yield (0.28 g/l) and emulsifying activity (45.53 %) and the lowest surface tension (35.5 mN/m) were obtained in media containing CMSG. No significant change in pH was observed in this case (data not shown). A similar result was reported in biosurfactant isolated from Selenomonas ruminantium CT2 (Saimmai et al. 2013a).
Effect of C:N ratio on biosurfactant production
Table 2 presents the effect of the C:N ratio on cell growth and biosurfactant production from D. caeni PO5. The results show that the biosurfactant concentration was higher and significantly different for C:N ratios of >40 than for C:N ratios of 5–35. There were no significant differences between C:N ratios of 40–60 in relation to biosurfactant production, but a C:N ratio of 65 presented a significant difference in relation to cell biomass. Several authors consider a nitrogen-limiting condition favorable to biosurfactant production. Saimmai et al. (2012c) commented that nutritional limitations direct cellular metabolism towards product formation. These authors established C:N ratios of 40 for the maximization of biosurfactant production from Oleomonas sagaranensis AT18 (Saimmai et al. 2012c).
Effect of inoculum size on biosurfactant production
To optimize the amount of inoculum for biosurfactant production, we varied the concentration of inoculum from 2.0 to 12.0 % (v/v). As shown in Table 2, with an increase in inoculum size from 2.0 to 8.0 %, growth and biosurfactant production by D. caeni PO5 was enhanced linearly. Although the dry cell weight seemed to increase with an increase in the amount of inoculum (2.0–12.0 %), there was no corresponding increase in the amounts of excreted biosurfactants.
Effect of initial pH
Certain microorganisms have the ability to grow and produce biosurfactants in a wide pH range. This renders possible the microbial production of surfactant for in situ applications, such as bioremediation and enhanced oil recovery (Mulligan 2005). Based on our results, D. caeni PO5 is able to grow under a pH range of 5.0–9.0; in our study, the highest levels of cell growth were recorded at pH values ranging from 6.5 to 8.5 (data not shown). However, the highest levels of biosurfactant production were obtained at pH 7.0. Other pH values were accompanied by decreased biosurfactant production, especially at pH ≥8.5 and ≤ 6.0.
Effect of incubation temperature on growth and biosurfactant production
The cell growth of D. caeni PO5 markedly increased (2.71 to 3.50 g/l) when the incubation temperature increased (20 to 35 °C) (data not shown).). The maximum cell growth was obtained when the incubation temperature was 30–35 °C. Further increases in the incubation temperature resulted in a dramatically decreased cell growth of D. caeni PO5. Incubation temperature had an effect not only on cell growth but also on biosurfactant production by D. caeni PO5. When the temperature was increased from 20 to 35 °C, the surface activity and biosurfactant yield increased as well. Increasing the incubation temperature above 35 °C resulted in a dramatic decrease of surface activity and biosurfactant yield, especially when the incubation temperature was >40 °C. This result is in agreement with that reported by Thavasi et al. (2008) who found that incubation temperature had a large effect on growth and biosurfactant produced by Bacillus megaterium and that the optimum temperature was 38 °C. In addition, the optimal temperature for rhamnolipid production by Pseudomonas aeruginosa J4 and lipopeptide production by Selenomonas ruminantium CT2 has been reported to be 30 °C (Saimmai et al. 2013a). From these results, we selected an incubation temperature of 30 °C for subsequent experiments.
Effect of different amino acids on growth and biosurfactant production
The effect of various amino acids was examined with the aim to optimize the biosurfactant production by D. caeni PO5. Cultivation in MSM supplemented with 10 g/l arginine resulted in a more than twofold increase in biosurfactant production (2.81 g/l) compared with the control cultivated in the absence of amino acid (data not shown). In fact, biosurfactant production by D. caeni PO5 increased as a result of the direct uptake of amino acids as precursors for the surfactant biosynthesis, thus improving biosurfactant yield. Saimmai et al. (2013a) reported that adding amino acid to a medium resulted in a 1.5-fold enhancement of biosurfactant production from Selenomonas ruminantium CT2. The addition of arginine to the medium also influenced the C:N ratio of the medium, with the optimal C:N ratio for biosurfactant production from D. caeni PO5 changing to 40:11.
Time course of growth and biosurfactant production
Figure 2a shows the biosurfactant production kinetics from D. caeni PO5 using the optimal medium and cultivation conditions reported above. Biosurfactant production and surface tension were dependent on the growth of the culture in the fermentation medium. The surface tension dropped rapidly after 18 h of cultivation, reaching its lowest value (25.0 mN/m) after 63 h of cultivation. However, the highest biosurfactant yield (3.12 g/l) was obtained as the cultivation time approached 87 h, which also corresponded to the stationary phase of microbial growth. These results lead to the conclusion that the biosurfactant produced by D. caeni PO5 is a primary metabolite. Growth-associated production of biosurfactant has been reported for Pseudomonas sp. (Obayori et al. 2009), Selenomonas ruminantium CT2 (Saimmai et al. 2013a), Oleomonas sagaranensis AT18 (Saimmai et al. 2012c), and Bacillus spp. (Suwansukho et al. 2008). From the results it can be seen that a cultivation time of 87 h gave the highest biosurfactant activity.
Recovery of biosurfactant
Crude extract of the biosurfactant was recovered from the culture supernatant of D. caeni PO5 by extraction with several organic solvents. Among the four solvent systems, ethyl acetate extraction was the most efficient in terms of biosurfactant recovery from the culture supernatant of D. caeni PO5 (data not shown). A recovery yield of 3.12 g/l was obtained. The use of ethyl acetate resulted in a greater activity of the crude extract when compared with systems based on mixtures of chloroform and methanol, cold acetone, or dichloromethane (data not shown). It has also been reported that the extraction of bioproducts with considerably high polarity by ethyl acetate solvent is quite efficient (Saimmai et al. 2013a). Because the recovery and concentration of biosurfactants from fermentation broth largely determines the production cost, our results indicate that ethyl acetate is a better choice than the highly toxic chloro-organic compounds.
Surface tension and critical micelle concentration
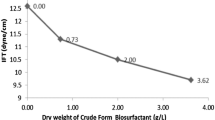
Surfactant molecules in aqueous solutions at concentrations above their critical micelle concentration (CMC) aggregate to form supramolecular structures several times; these aggregations are referred to as micelles. The onset of micellization can be determined by many experimental methods where the physical property of a surfactant solution undergoes an abrupt change at the CMC (Monteiroa et al. 2011). Here we report the determination of the CMC of the obtained biosurfactant by surface tension measurement. The relationship between the surface tension and concentration of the isolated biosurfactant solution was determined using a du Noüy ring tensiometer (Fig. 2b). The biosurfactant produced exhibited excellent surface tension reducing activity. The surface tension of the water was 72 mN/m and decreased to 25.0 mN/m by increasing the solution concentration up to 8.0 mg/l. Further increases in the concentration of the biosurfactant solution did not reduce the surface tension of the water, indicating that the CMC was reached at this concentration. The biosurfactant from D. caeni PO5 showed a lower minimum surface tension and CMC value than that of the biosurfactants from Lactobacillus paracasei (41. mN/m, 2.5 mg/ml) (Gudina et al. 2010), Pseudomonas fluorescens (31.5 mN/m, 72 mg/l) (Janek et al. 2010), and Selenomonas ruminantium CT2 (25.5 mN/m, 8.0 mg/l) (Saimmai et al. 2013a), respectively, and was equal to that of Oleomonas sagaranensis AT18 (25.0 mN/m, 8.0 mg/l) (Saimmai et al. 2012c).
Effect of temperature, pH, and salinity on biosurfactant stability
The applicability of biosurfactants in several fields depends on their stability at different temperatures and pH values and at a wide range of sodium chloride concentrations (Saimmai et al. 2013a). Stability tests on the biosurfactant produced by D. caeni PO5 with respect to temperature, pH, salinity, and pressure were conducted. The biosurfactant produced by D. caeni PO5 was shown to be thermostable (data not shown) and exposing the biosurfactant to 121 °C had only a minor effect on its stability. The surface tension and E24 were quite stable at the temperatures tested (approx. 29 mN/m and approx. 50 %, respectively). This result indicates the usefulness of the biosurfactant in the food processing, pharmaceutical, and cosmetics industries where heating to achieve sterility is of paramount importance (Abdel-Mawgoud et al. 2010). The surface activity of the crude biosurfactant remained relatively stable at pH changes between pH 6 and 10, showing a higher stability at alkaline pH 8 than in acidic conditions (data not shown). At pH 12, the surface tension value was 32.0 mN/m and the E24 showed 47 % activity. However, at pH 2 the surface tension increased up to 61.0 mN/m and the E24 decreased to 31 %. In addition, at pH values of <5, the samples become turbid due to partial precipitation of the biosurfactant. Little changes in biosurfactant activity were observed with increased concentrations of NaCl up to 10 % (w/v) (data not shown). At higher concentrations of NaCl the biosurfactant retained 45 % of its emulsification activity and its surface activity also increased (30.0 mN/m) (data not shown).
Emulsification properties of biosurfactant
To determine the hydrocarbon specificity for emulsification activity at 24 h (E24) and 48 h (E48), we investigated a wide range of pure and mixed substrates. The biosurfactant from D. caeni PO5 showed good E24 and E48 values against several hydrophobic substrates (data not shown). Hexane, kerosene, and toluene were good substrates for E24 and E48 when tested on the crude biosurfactant from D. caeni PO5; there were no significant differences between these three substrates. Olive oil, soybean oil, motor oil, and xylene also formed stable emulsions. Poor emulsification occurred with hexadecane and ULO, probably due to the inability of the biosurfactant to stabilize the microscopic droplets of these compounds. The structure of the ULO consists of a mixture of paraffin, naphthalene, and aromatic hydrocarbon, which is difficult to emulsify by a crude biosurfactant (Muthusamy et al. 2008). The ability of the crude biosurfactant from D. caeni PO5 to emulsify various hydrophobic substrates indicates that it has a good potential for applications in microbial-enhanced oil removal and that it can also be used as an emulsifying agent in the food industry.
Chemical characterization of the biosurfactant
The biochemical composition of the biosurfactant revealed that it is a mixture of carbohydrate (67 %) and lipid (28 %). FT-IR spectral analysis of the biosurfactant showed a band at 3,309 cm−1 which was assigned to the OH stretching band indicating the presence of carboxylic acids and another band at 2,958 cm−1 which was assigned to N–H/C–H bonds of protein. CH2/C-H asymmetric vibrations were found at 2,927 and 2,855 cm−1 which confirmed the presence of alkanes (C–H) (Fig. 3a). CH and CH2 deformation was found at 801, 1,094, and 1,261 cm−1. The presence of a C = O, C = C, and C–O bond was found at 1,711, 1,654, and 1,542 cm−1, respectively. These data confirmed the glycolipid nature of the biosurfactant (Thavasi et al. 2007).
The above structure of biosurfactant obtained was fully supported by the mass spectrometry (MS) analysis. Analysis of the intact molecules using a LCQ Fleet Ion Trap Mass Spectrometer (Thermo Finnigan, Waltham, MA) revealed four molecular ion peaks with molecular masses [M + H]+ of 548 and 576, respectively (Fig. 3b). The spectra clearly indicate the presence of higher and lower homologs of surfactants for the difference between prominent M+, the peaks being around 14, corresponding to a difference in the number of methylene groups (CH2). This finding is in agreement with the results reported by Abdel-Mawgoud et al. (2010) who found that glycolipid has a molecular mass in the range of 302–803 Da.
Removal of ULO from contaminated sand
The capacity of aqueous biosurfactant solutions to remove ULO from contaminated sand was also investigated and compared with that of synthetic surfactants [sodium dodecyl sulfate (SDS) and Tween 80] at several concentrations (Fig. 4a). For SDS, the maximum ULO removal was attained at the CMC, namely, a removal of 69 %. For Tween 80 and biosurfactant, increasing concentrations enhanced the removal of ULO, increasing from about 67 % at the CMC to about 88 and 92 % at 5× CMC and 10× CMC, respectively (Fig. 4a). The maximum percentage of oil removal by the biosurfactant and Tween 80 was around 4.4-fold higher than that of the negative control (distilled water; 21 % oil removal). Two mechanisms are associated with the removal of crude oil from soils: mobilization and solubilization. Mobilization occurs at concentrations below the CMC, and the phenomena associated with this mechanism include the reduction of surface and interfacial tension. Solubilization occurs above the CMC of the surfactants, as the apparent solubility of oil increases dramatically due to aggregation within the surfactants’ micelles (Urum and Pekdemir 2004).
Antimicrobial activity of biosurfactant
The level of antimicrobial activity of the crude biosurfactant produced by D. caeni PO5 depended on the microorganism (Fig. 4b). We found that the biosurfactant obtained exhibited a high antimicrobial activity against Bacillus cereus, Candida albicans, Pseudomonas aeruginosa, and Staphylococcus aureus at the concentrations tested. We also observed that the biosurfactant obtained showed no antimicrobial activity against Enterococcus faecium and only a low activity against Escherichia coli, Listeria monocytogenes, Salmonella sp., Salmonella typhimurium, Vibrio vulnificus, and Vibrio cholerae. Biosurfactants with high surface-active properties generally show certain antimicrobial activities to some extent. Indeed, several lipopeptides and glycolipids have been identified to have potent antibiotic activity and these have been the subject of several studies relating to the discovery of new antibiotics. The list includes surfactin from Bacillus subtilis and rhamnolipid from P. aeruginosa, which is the most powerful biosurfactant known to date (Pornsunthorntawee et al. 2008). These compounds have many pharmacological activities, including antibacterial, antifungal, antiviral, and antimycoplasma properties, inhibition of fibrin clot formation and hemolysis, formation of ion channels in lipid bilayer membranes, antitumor activity against Ehrlich’s ascites carcinoma cells, and inhibition of the cyclic adenosine 3,5-monophosphate phosphodiesterase (Seydlova and Svobodova 2008; Vatsa et al. 2010).
Laboratory experiment on ULO biodegradation by biosurfactant
Maximum ULO biodegradation was found when only the biosurfactant was added to the system (73.5 %), followed by the addition of biosurfactant and fertilizer (71.0 %), and the addition of only fertilizer (60.5 %); in comparison, in the absence of both biosurfactant and fertilizer ULO degradation was 42.0 %. ULOs are highly hydrophobic and require solubilization by surfactants to increase bioavailability to microbial cells (Cameotra and Singh 2008). The use of synthetic surfactants is associated with concerns related to toxicity and environmental pollution, and the microbial production of biosurfactants therefore represents an interesting alternative. Previous studies have shown that, similar to chemical surfactants, biosurfactants can either promote, inhibit, or have no effect on hydrocarbon biodegradation (Urum and Pekdemir 2004; Cameotra and Singh 2008). Moreover, the use of microbial surfactants for the bioremediation of hydrophobic contaminants is considered a very environmental friendly and effective strategy.
Application of the biosurfactant for metal removal from aqueous solutions
Atomic absorption spectroscopy revealed that the metal content in the solution containing biosurfactant was significantly lower than that at the beginning of the experiment. This results indicates that the metal was co-precipitated by the biosurfactant. The metal–biosurfactant co-precipitate could be seen as an off-white precipitate following the addition of biosurfactant to the metal solutions and subsequent appropriate incubation. The percentage removal for the metals tested varied with the different concentrations of metals and the biosurfactant used. Generally, higher concentrations of metals were removed when the biosurfactant was present in greater amounts. The percentage removal at 0.5× CMC was 20.45, 18.16, 13.67, 12.16, and 25.03, 24.87, 20.09, 17.74 for 100, 200, 500, and 1,000 ppm of cadmium and lead, respectively. The percentage removal increased to 79.76, 73.05, 59.54, 55.17 and 94.75, 90.85, 74.65, and 68.14 for cadmium and lead, respectively, at 10× CMC of biosurfactant (Fig. 5a, b). These observations are in agreement with the report of Mulligan et al. (2001) who found that biosurfactants present at levels below the CMC were less efficient in metal remediation. At higher concentrations of biosurfactant (10× CMC) almost complete removal of the metals was obtained.
Effect of biosurfactant on solubilization of PAHs
The effect of biosurfactant on the apparent aqueous solubility of PAHs was determined by test tube solubilization assays in the presence of increasing concentrations of biosurfactant (0–50 mg/l) (Table 3). In general, the biosurfactant obtained enhanced the apparent solubility of PAHs in a dose-dependent manner. However, the solubilization of fluorene, naphthalene, or phenanthrene by the biosurfactant produced by D. caeni PO5 (at the highest tested concentration the apparent solubility was about three- to fourfold higher that that of the control) was significantly lower (p < 0.05) than the solubilization of anthracene, fluoranthene, or pyrene (at the highest tested concentration it was about 26- to 40-fold higher than the control). Biosurfactants have potential applications for the remediation of PAHs contaminations, such as in situ soil flushing and ex situ soil washing for the remediation of unsaturated zone and pump, and treated for aquifer remediation (Franzetti et al. 2012), as well as in bioremediation technologies to improve the biodegradation rate of organic compounds (Mulligan 2005). Pump-and-treat processes are too slow to clean up PAHs within reasonable periods of time (Cameotra and Singh 2008; Franzetti et al. 2012). Biosurfactant flushing has been used for removing those compounds with a low water solubility, since surfactants can enhance the cleanup of soils and groundwater contaminated with PAHs by mobilizing and partitioning them into the hydrophobic cores of surfactant micelles.
Conclusions
In this study, we isolated and tested Deinococcus caeni PO5, a novel biosurfactant-producing bacterial strain isolated from soil contaminated by a palm oil refinery. The production of the biosurfactant from D. caeni PO5 in a system using JSP as a novel and promising substrate is reported. The growth characteristics were obtained, and the results of studies on the properties of the biosurfactant relating to a low-cost fermention medium indicate that this strain has a potential for industrial applications. Chemical characterization of the biosurfactant by biochemical analysis, the FT-IR and electrospray ionization-MS revealed the presence of glycolipids in the sample. The potential of this biosurfactant for industrial uses was shown by studying its physical properties, namely, the surface tension, CMC, and emulsification activity, and its stability in relation to environmental stresses, such as salinity, pH, and temperature. The surface tension of an aqueous solution of this biosurfactant at a CMC value of 8 mg/l reached 25.0 mN/m. These values are very low compared with those of synthetic surfactants. The properties of the biosurfactant obtained suggest its potential applications, especially for microbial-enhanced oil recovery and/or bioremediation of environmental contamination by hydrophobic compounds.
References
Abdel-Mawgoud AM, Lepine F, Deziel E (2010) Rhamnolipids: Diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Babitha S, Soccol CR, Pandey S (2006) Jackfruit seed for production of monascus pigments. Food Technol Biotechnol 44:465–471
Batista SB, Mounteer AH, Amorim FR, Totola MR (2006) Isolation and characterization of biosurfactant/bioemulsifier producing bacteria from petroleum contaminated sites. Bioresource Technol 97:868–875
Cameotra SS, Singh P (2008) Bioremediation of oil sludge using crude biosurfactants. Int Biodeter Biodegr 62:274–280
Chooklin CC, Phertmean S, Cheirsilp B, Maneerat S, Saimmai A (2013) Utilization of palm oil mill effluent as a novel substrate for biosurfactant production by Nevskia ramosa NA3. Songklanakarin J Sci Technol 35:167–176
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Folch JM, Lees M, Stanly HS (1956) A simple method for the isolation and quantification of total lipids from animal tissues. J Biol Chem 226:497–509
Franzetti A, Tamburini E, Banat IM (2012) Applications of biological surface active compounds in remediation technologies. Adv Exp Med Biol 672:121–134
Gudina EJ, Teixeira JA, Rodrigues LR (2010) Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloid Surf B 76:298–304
Janek T, Lukaszewicz M, Rezanka T, Krasowska A (2010) Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the arctic archipelago of Svalbard. Bioresource Technol 101:6118–6123
Kuda T, Kyoi D, Takahashi H, Obama K, Kimura B (2011) Detection and isolation of p-nitrophenol-lowering bacteria from intestine of marine fishes caught in Japanese waters. Marine Pollut Bull 62:1622–1627
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Monteiroa AS, Mirandaa TT, Lulab I, Denadaic AML, Sinisterra RD, Santoroe MM, Santosa VL (2011) Inhibition of Candida albicans CC biofilms formation in polystyrene plate surfaces by biosurfactant produced by Trichosporon montevideense CLOA72. Colloid Surface B 84:467–476
Morton J (1987) Jackfruit. In: Morton JF (ed) Fruits of warm climates. Florida Flair Books, Miami, pp 58–64
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollut 133:183–198
Mulligan CN, Yong CN, Gibbs BF (2001) Heavy metal removal from sediments by biosurfactants. J Haz Mat 85:111–125
Muthusamy K, Gopalakrishnan S, Ravi TK, Sivachidambaram P (2008) Biosurfactants: Properties, commercial production and application. Curr Microbiol 94:736–747
Obayori O, Ilori M, Adebusoye S, Oyetibo G, Omotayo A, Amund O (2009) Degradation of hydrocarbons and biosurfactant production by Pseudomonas sp. strain LP1. World J Microbiol Biotechnol 25:1615–1623
Plaza GA, Zjawiony I, Banat IM (2006) Use of different methods for detection of thermophilic biosurfactant-producing bacteria from hydrocarbon contaminated and bioremediated soils. J Pet Sci Eng 50:71–77
Pornsunthorntawee O, Arttaweeporn N, Paisanjit S, Somboonthanate P, Abe M, Rujiravanit R, Chavadej S (2008) Isolation and comparison of biosurfactants produced by Bacillus subtilis PT2 and Pseudomonas aeruginosa SP4 for microbial surfactant-enhanced oil recovery. Biochem Eng J 42:172–179
Ruggeri C, Franzetti A, Bestetti G, Caredda P, La Colla P, Pintus M, Sergi S, Tamburini E (2009) Isolation and characterization of surface active compound-producing bacteria from hydrocarbon-contaminated environments. Int Biodeter Biodegr 63:936–942
Saimmai A, Kaewrueng J, Maneerat S (2012a) Used lubricating oil degradation and biosurfactant production by SC-9 consortia obtained from oil contaminated soil. Ann Microbiol 62:1757–1767
Saimmai A, Rukadee O, Onlamool T, Sobhon V, Maneerat S (2012b) Characterization and phylogenetic analysis of microbial surface active compounds-producing bacteria. Appl Biochem Biotechnol 168:1003–1018
Saimmai A, Rukadee O, Onlamool T, Sobhon V, Maneerat S (2012c) Isolation and functional characterization of a biosurfactant produced by a new and promising strain of Oleomonas sagaranensis AT18. World J Microbiol Biotechnol 28:2973–2986
Saimmai A, Rukadee O, Onlamool T, Sobhon V, Maneerat S (2013a) An efficient biosurfactant-producing bacterium Selenomonas ruminantium CT2, isolated from mangrove sediment in south of Thailand. World J Microbiol Biotechnol 29:87–102
Saimmai A, Rukadee O, Onlamool T, Sobhon V, Maneerat S (2013b) Production and characterization of biosurfactant from marine bacterium of Inquilinus limosus KB3 grown on low-cost raw materials. Ann Microbiol 29(1):87–102
Seydlova G, Svobodova J (2008) Review of surfactin chemical properties and the potential biomedical applications. Cent Eur J Med 3:123–133
Suwansukho P, Rukachisirikul V, Kawai F, Kittikun AH (2008) Production and applications of biosurfactant from Bacillus subtilis MUV4. Songklanakarin J Sci Technol 30:87–93
Thavasi R, Jayalakshmi S, Balasubramanian T, Banat IM (2007) Biosurfactant production by Corynebacterium kutscheri from waste motor lubricant oil and peanut oil cake. Lett Appl Microbiol 45:686–691
Thavasi R, Jayalakshmi S, Balasubramaniam T, Banat IM (2008) Production and characterization of a glycolipid biosurfactant from Bacillus megaterium using economically cheaper sources. World J Microbiol Biotechnol 24:917–925
Urum K, Pekdemir T (2004) Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 57:1139–1150
Vatsa P, Sanchez L, Clement C, Baillieul F, Dorey S (2010) Rhamnolipid biosurfac-tants as new players in animal and plant defense against microbes. Int J Mol Sci 11:5095–5108
Youssef NH, Dunacn KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ (2004) Comparison of methods to detect biosurfactant production by diverse microorganism. J Microbiol Methods 56:339–347
Acknowledgments
We are grateful to Phuket Rajabhat University for providing a scholarship to Saimmai A. This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission and International Foundation for Science (Sweden) No. F/5204-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chooklin, C.S., Petmeaun, S., Maneerat, S. et al. Isolation and characterization of a biosurfactant from Deinococcus caeni PO5 using jackfruit seed powder as a substrate. Ann Microbiol 64, 1007–1020 (2014). https://doi.org/10.1007/s13213-013-0738-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-013-0738-2