Abstract
Biosurfactant-producing bacteria, isolate CT2, was isolated from mangrove sediment in the south of Thailand. The sequence of the 16S rRNA gene from isolate CT2 showed 100 % similarity with Selenomonas ruminantium. The highest biosurfactant production (5.02 g/l) was obtained when the cells were grown on minimal salt medium containing 15 g/l molasses and 1 g/l commercial monosodium glutamate supplemented with 1 g/l NaCl, 0.1 g/l leucine, 5 % (v/v) inoculum size at 30 °C and 150 rpm after 54 h of cultivation. The biosurfactant obtained by extraction with ethyl acetate showed high surface tension reduction (25.5 mN/m), a small CMC value (8 mg/l), thermal and pH stability with respect to surface tension reduction and emulsification activity and a high level of salt tolerance. The biosurfactant obtained was confirmed as a lipopeptide by using a biochemical test, FT-IR, MNR and mass spectrometry. The crude biosurfactant showed a broad spectrum of antimicrobial activity and also had the ability to emulsify oil and enhance PAHs solubility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biosurfactants are a structurally diverse group of surface-active molecules synthesized by microorganisms (Nitschke and Coast 2007). These amphiphilic compounds contain a hydrophobic and a hydrophilic moiety, and have the ability to reduce interfacial tension between different fluid phases. Their uses and potential commercial applications have been reported in several fields, and include microbial enhanced oil recovery, bioremediation of pollutants, health care and food processing (Nitschke and Pastore 2006). Biosurfactants have gained attention because they exhibit some advantages when compared with chemical surfactants, such as biodegradability, low toxicity, ecological acceptability and ability to be produced from renewable cheaper substrates, the possibility of their production through fermentation and specific activity at extreme temperature, pH level and salinity (Kim et al. 2006). Microbial surfactants exhibit high specificity and are consequently suited to new applications as evidenced by numerous reports published during the last decade on the application of biosurfactants in various industrial sectors (Banat et al. 2000; Singh and Cameotra 2004; Mulligan 2005, 2009; Brown 2010; Fathabad 2011) and in environment protection (Banat 1995; Das and Mukherjee 2007).
Mangrove ecosystems, which are important inter-tidal estuarine wetlands along the coastlines of tropical and subtropical regions, have been considered as significant sinks for pollutions from freshwater discharges as well as from contaminated tidal water (Holt et al. 1994; Bernard et al. 1996; Reda and El-Nagar 2009). Diverse groups of indigenous microorganisms capable of utilizing and degrading contaminants such as hydrocarbons and PAHs might have been present in the contaminated sediment (Ke et al. 2003). The biodegradation is maximized when the water-insoluble substrate is dissolved or emulsified. In these ways, biosurfactants are capable of increasing the bioavailability of poorly soluble hydrocarbons. Biosurfactants also increase water insoluble uptake of microorganisms as well as increasing efficiency of bioremediation. There have been many reports made on biosurfactants in the last few decades (Gudiña et al. 2010; Janek et al. 2010; Burgos-Diaz et al. 2011). However, the reports on biosurfactants produced by mangrove sediment microorganisms have been limited to date (Saimmai et al. 2012a, 2012b, 2012d). The objective of the present study was to isolate and characterize biosurfactants produced by bacteria isolated from mangrove sediments and their evaluation for prospective applications including antimicrobial activity, microbial enhanced PAH solubility and oil removal.
Materials and methods
Microorganism isolation
One hundred gram of mangrove sediment from five different sites (each site yielded 10 samples) in southern of Thailand were collected at a depth of 0–5 cm. The enrichment and isolation of the biosurfactant producing bacterial consortium was done by using used lubricating oil (ULO) as the sole carbon and energy source. Initially, the bacterial consortium was enriched by adding 1 g of soil sample to 50 ml of minimal salt medium (MSM) in a 250 ml Erlenmeyer flask. The composition of MSM was (g/l): K2HPO4, 0.8; KH2PO4, 0.2; CaCl2, 0.05; MgCl2, 0.5; FeCl2, 0.01; (NH4)2SO4, 1.0; NaCl, 5.0; ULO, 10 (Saimmai et al. 2012d). The flasks were incubated at 150 rpm and 30 °C for 5 days or until oil emulsion was observed. Thereafter, 1 ml aliquot of the culture broth was transferred to a fresh 50 ml MSM in a 250 ml flask and incubated under the same conditions as described above. This procedure was repeated five times.
Sediment enriched cultures were diluted in a sterile 0.85 % saline solution and plated on MSM agar using ULO (1 %, w/v) as the carbon source for the isolation of microorganisms. Morphologically distinct colonies were re-isolated by transfer to ULO containing agar plates at least three times to obtain pure cultures and were subsequently Gram-stained. Pure cultures were stored at −20 °C in MSM mixed with sterile glycerol at a final concentration of 30 %.
Screening of potential biosurfactant-producing strains
One hundred and sixty five isolates were streaked on MSM agar containing 1 % (w/v) of ULO and incubated for 5 days at 30 °C. Subsequently one loop full of each isolate was transferred to test tubes containing 5 ml of Nutrient broth (NB, Difco, MI, USA) and incubated at 150 rpm and room temperature for 24 h. One hundred microlitters of cell culture were transfer to 5 ml of MSM medium supplemented with 1 % (w/v) of ULO and incubated in a rotary shaker (Vision Scientific Co., Daejon, Korea) at room temperature and 150 rpm for 5 days. The screening of the biosurfactant-producing isolates was performed by using the qualitative drop-collapsing test and measuring the emulsification activity and the surface tension of culture supernatant after oil removal by hexane extraction and centrifugation at 8,500 rpm 4 °C for 15 min.
16S rRNA gene sequence analysis
Selected isolates were incubated for 48 h at 30 °C on MSM agar supplemented with 1 % (w/v) of suitable carbon source. For 16S rRNA gene amplification, selected bacterial chromosomal DNA was isolated using the MagNA Pure LC DNA isolation kit (Roche Applied Science, Mannheim, Germany) in accord with the manufacture’s instructions. The 16S rRNA gene was amplified using the PCR method with 1U of Taq DNA polymerase (Bio-Lab Ltd., Auckland, New Zealand) and universal bacterial primers UFUL (GCCTAACACATGCAAGTCGA) and URUL (CGTATTACCG CGGCTGCTGG) (Nilsson and Strom 2002). These two primers were target at highly conserved regions of the prokaryotic 16S rRNA gene (Phalakornkule and Tanasupawat 2006) and gave a PCR product of about 1,500 bps. The 16S rRNA gene was sequenced by using the ABI Prism BigDye terminator kit (Perkin-Elmer Applied Biosystems, Massachusetts, USA), according to the manufacturer’s protocol, with UFUL as the primer. The 1,500 bps 16S rRNA gene sequences obtained were compared with the sequences of type strains obtained from the GenBank database by using the ClustalW program (Thompson et al. 1997). Sequence homologies were examined using BLAST version 2.2.12 of the National Center for Biotechnology Information and a consensus neighbor-joining tree was constructed using Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0 (Tamura et al. 2007).
Optimization of biosurfactant production by Selenomonas ruminantium CT2
The medium optimization was conducted in a series of experiments changing one variable at a time, keeping the other factors fixed at a specific set of conditions. Five factors were chosen aiming at obtaining higher productivity of the biosurfactant: carbon sources (C); nitrogen source (N); carbon/nitrogen (C/N) ratio; NaCl concentration and addition of amino acids. The carbon sources used were glucose, glycerol, molasses, oleic acid, palm oil, soybean oil, stearic acid, ULO or used vegetable oil (UVO) at a concentration of 1 g/l. A medium with no carbon source was used as the control assay. The nitrogen sources used were beef extract, commercial monosodium glutamate (CMSG), NaNO3, (NH4)2SO4, NH4Cl, NH4NO3, peptone or yeast extract at a concentration of 1 g/l. A medium with no nitrogen source was used as control assay. The C/N ratio (with optimized nitrogen sources) was varied from 1 to 40 by keeping constant the nitrogen source concentration (1 g/l). To examine the effect of increased medium osmolarity on biosurfactant production, sodium chloride was added to the medium at final concentrations of 0–3 % (w/v). Different amino acids such as arginine, asparagine, aspartic acid, glutamic acid, isoleucine, leucine, valine were filtrated separately and then added to medium at final concentrations of 0.1 % (w/v).
Recovery of biosurfactant
Four solvent systems were examined for biosurfactant extraction (Saimmai et al. 2012a, 2012b, 2012c). These were a mixture of chloroform:methanol (2:1), cold acetone, dichloromethane and ethyl acetate were examined for biosurfactant extraction. The method showing the highest biosurfactant activity was used to recover biosurfactant from S. ruminantium CT2.
Stability of biosurfactant
Biosurfactant solutions at the CMC concentration in distilled water were prepared. To investigate the effects of pH, sodium chloride (NaCl) concentrations and temperature on biosurfactant activity, the biosurfactant solution was adjusted with 1.0 N HCl or NaOH to obtain the pHs of 2.0–12.0. NaCl was added to the sample to obtain the final concentrations of 1.0–11.0 % (w/v). For the thermal stability study, the biosurfactant solution was incubated at 4–80 °C for 24 h, 100 °C for 1 h and at 121 °C for 15 min and cooled to 25 °C. The remaining activity was then determined.
Chemical analysis of biosurfactant
Fourier transform infrared spectroscopy (FT-IR) of the obtained biosurfactant was done on a Nexus-870 FT-IR spectrometer (Thermo Electron Co., Yokohama, Japan) by the KBr pellet method. The dried biosurfactant samples (0.3–0.5 mg) were ground in about 80 mg of spectral grade KBr (Merck, Darmstadt, Germany) and pressed into pellets under about 6 t/cm2 pressure with the help of a hydraulic press (Specac, Orpington, Kent, UK). A 1H- and 13C- nuclear magnetic resonance (NMR) spectrum was recorded at 298 K on an AMX 300 NMR spectrometer (Bruker, 300 MHz). This was equipped with an Aspect 3,000 computer (Bruker) locked to the deuterium resonance of solvent, CDCl3, without spinning. Data were recorded at 32 K (the number of data points per parts per million of the plot). The electrospray ionization mass spectrometry (ESI–MS) analysis of the biosurfactant was carried out in an LCQ™ quadrupole ion-trap mass spectrometer (Finnigan MAT, San Jose, CA, USA). This utilizes ESI. The electrospray source was operated at ionization source temperature 80 °C, electrolyte voltage 200 V, and spray inlet temperature 120 °C. The equipment was run in a positive ion mode.
Application of the biosurfactant in used motor lubricating oil (ULO) removal from contaminated sand
Biosurfactant suitability for enhanced oil removal was investigated using 800.0 g of acid washed sand impregnated with 50.0 ml of ULO. Fractions of 20.0 g of the contaminated sand were transferred to 250 ml flasks which were submitted to the following treatments: addition of 60.0 ml of distilled water (control); addition of 60.0 ml of aqueous solution of SDS; Triton X-100; and biosurfactant at the CMC level of each compound. The samples were incubated on a rotary shaker at 200 rpm for 24 h at 30 °C and centrifuged at 5,000 rpm for 20 min for separation of the laundering solution and the sand. The amount of oil residing in the sand after the treatment with the biosurfactant was gravimetrically determined as the amount of material extracted from the sand by hexane (Sobrinho et al. 2008).
PAHs solubilization asssy
A polyaromatic hydrocarbons (PAHs) solubilization assay was done as described by Barkay et al. (1999). Briefly, 0.6 μg of PAHs (anthracene, fluoranthene, fluorine, naphthalene, phenanthrene or pyrene (from 0.6 mg/ml stock in acetone) were distributed into glass test tubes (10 mm × 170 mm) and kept open inside an operating chemical fume hood to remove the solvent. Subsequently, 3.0 ml of assay buffer (20 mM Tris–HCl, pH 7.0) and the biosurfactant at increasing concentrations (0–50 mg/ml) obtained from the bacterial strain were used in this study. Assay buffer containing the biosurfactant, but no PAH, was used as a control. Tubes were capped with plastic closures and incubated overnight at 30 °C with shaking (200 rpm) taking place in the dark. Samples were filtered through 1.2 μm filters (Whatman, Springfield Mill, UK) and 2.0 ml of this filtrate was extracted with equal volume of hexane. This emulsion was centrifuged at 8,500 rpm for 10 min to separate the aqueous and hexane phases. The concentration of PAH was measured spectrophotometrically (Libra S22; Biochrom, Cambridge, UK) at specific wave length of each compound (Barkay et al. 1999). From a calibration curve of individual PAH (in hexane), the concentration of each PAH was determined. Assay buffer with biosurfactant but without PAH was extracted with hexane identically and served as a blank.
Antimicrobial activity of surfactive compound
The extracted compound was tested for antimicrobial activity using the agar well diffusion method and the area of the inhibition zone was measured (Candan et al. 2003). The active compound extracted was tested against pathogenic bacteria including Bacillus cereus, Candida albicans, Enterococcus faecium, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella sp., Salmonella typhimurium, Staphylococcus aureus, Vibrio cholerae and Vibrio vulnificus. All strains were obtained from Songklanagarind Hospital, Prince of Songkla University, Thailand. The extract was weighed and dissolved in distilled water at a concentration of 10 mg/ml and filter-sterilized using a 0.2 μm membrane filter. Each tested microorganism was grown in Brain Heart Infusion (BHI; Hi-Media Laboratories, Mumbai, India) and diluted to a concentration of 106 CFU/ml. Microbial suspensions were overlaid onto the surface of BHI agar plates, which were dried for 20 min at room temperature. The wells were cut from the agar plates and 50 μl of extract solution were added. The plates were incubated at 37 °C for 24 h. After incubation, the clear zone was measured.
Analytical methods
Biomass was determined as dry cell weight. At different times of fermentation, samples were mixed in pre-weighted tubes with chilled distilled water and centrifuged at 8,500 rpm for 30 min. The biomass obtained was dried overnight at 105 °C and weighed.
Emulsification activity was performed according to Wu et al. (2008). Thus 4 ml of hydrocarbon or oil was added to 4 ml of aqueous solution of culture supernatant in a screw cap tube, and vortexed at high speed for 2 min. The emulsion stability was determined after 24 h [E24 (%)] and 48 h [E48 (%)], and the E24 and E48 was calculated by dividing the measured height of emulsion layer by the mixtures’s total height and multiplying by 100.
The surface tension was measured using a Model 20 Tensiometer (Fisher Science Instrument Co., PA, USA) at 25 °C. Critical micelle concentration (CMC) was determined by plotting the surface tension versus concentration of biosurfactant in the solution.
All experiments were carried out at least in triplicate. Two well-defined synthetic surfactants, Triton X-100 and SDS were used as positive controls, while distilled water and MSM medium were used as negative controls. Statistical analysis was performed using the Statistical Package for Social Science (SPSS 10.0, for Windows Inc., Chicago, IL).
Results and discussion
Isolation and identification of selected microorganisms
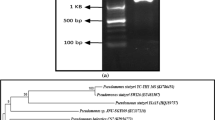
The isolation of a new biosurfactant-producing bacterium was carried out from mangrove samples from southern Thailand. From 45 samples, 165 bacterial isolates (94 Gram-negative bacilli, 63 Gram positive bacilli, five Gram-positive cocci and three yeasts) were selected for study. Among the isolated bacteria, strain CT2 was eventually chosen for further work on the basis of its ability to grow in a mineral medium and as it showed the highest reduction of surface tension (17.6 mN/m). The bacterial identification was performed based on the 16S rRNA gene sequence analysis. Examination with two sets of primers for almost fully and partial 16S rRNA sequence alignment revealed that strain CT2 was closely related to the species in genus Selenomonas, exhibiting the highest similarity (99 %) to S. ruminantium GA129 (M62702). We thus tentatively classified strain CT2 as S. ruminantium CT2 (Fig. 1). The 16S rRNA gene sequence of strain CT2 was submitted to GenBank with an accession number AB685262.
Phylogenetic tree of the strain CT 2 and closest NCBI (BLASTn) strains based on the 16S rRNA gene sequences (neighbor joining tree method). The scale bar indicates 0.01 nucleotide substitutions per nucleotide position. The numbers at node show the bootstrap values obtained with 1,000 resampling analyses. The GenBank accession numbers are reported in parenthesis
Effect of carbon source on growth and biosurfactant production
Literature revealed that the type and concentration of carbon and nitrogen substrates markedly affected the production yield of biosurfactant (Wu et al. 2008). In light of this, this study started with the investigation of carbon and nitrogen sources on biosurfactant production. S. ruminantium CT2 was grown on each of 9 types of carbon sources. As seen in Table 1, the type of carbon source affected both the biosurfactant concentration and E24. Molasses differed from the others in relation to the biosurfactant concentration and E24, and was the most appropriate carbon source. Surface tension reduction reached 30.08 mN/m with a biosurfactant concentration of 0.52 g/l and E24 (%) about 50 % for both kerosene and xylene. Table 1 also shows that there seems to be clear trend between biomass and biosurfactant yields and strongly dependent on the carbon source used. Although vegetable oils or glucose have been frequently used as the carbon substrates for biosurfactant production (Banat et al. 2010), S. ruminantium CT2 attained a lower biosurfactant yield from palm oil, soybean oil and glucose than that from molasses and glycerol (Table 1). Direct use of fatty acids (i.e., oleic acid and stearic acid) as the carbon source did not improve biosurfactant production, suggesting that hydrolysis of the oils was not a bottle-neck in the process. Moreover, ULO and UVO were also inefficient in cell growth and biosurfactant production, resulting in a low biosurfactant yield of only 0.20 g/l and 0.25 g/l, respectively probably due to its poor biodegradability (Chayabutra et al. 2001) or toxic to bacterial cell (Li and Chen 2009).
Effect of nitrogen source on growth and biosurfactant production
After examining the most commonly used organic and inorganic nitrogen sources reported in the literature (Wei et al. 2005), it was found that the type of nitrogen source affected growth and biosurfactant production of S. ruminantium CT2 (Table 2). The highest biomass was obtained when yeast extract was used. However, commercial monosodium glutamate (CMSG) exhibited the highest surface tension reduction and biosurfactant yield (33.49 mN/m and 1.60 g/l, respectively). This yield was nearly threefolds when compared with using (NH4)2SO4 as the nitrogen source. Moreover, using CMSG as the organic nitrogen source not only increased in biosurfactant yield but also improved in biomass and E24 toward xylene as 5.02 g/l and 54.80 %, respectively.
Effect of C:N ratio on growth and biosurfactant production
The C:N ratio was also known as a vital factor influencing the performance of biosurfactant production (Santos et al. 2002). As indicated in Table 3, the best biosurfactant activity in surface tension reduction and yield (45.3 mN/m and 2.46 g/l) occurred at a C:N ratio of 15. This yield was about 1.5 folds of that obtained from control (C:N ratio at 1:1). The productivity of biosurfactant tended to decrease as the C:N ratio increased from 20 to 40, especially for C:N ratio >25. Some reports mentioned that biosurfactant production is more efficient under nitrogen-limiting conditions (Benincasa et al. 2002). The results show that a possible inhibitory effect on the bacterial metabolism may occur due to a likely deficiency in nutrient transport. That is, nitrate first undergoes dissimilatory nitrate reduction to ammonium and then is assimilated by glutamine-glutamate metabolism. It is likely that assimilation of nitrate as nitrogen source is so low, leading to a simulated nitrogen-limiting condition (Barber and Stuckey 2000).
Effect of NaCl concentration on growth and biosurfactant production
The kinetics of biosurfactant included the biosurfactant yield, E24 and surface tension reduction decreases by S. ruminantium CT2 was performed at various NaCl concentrations (Fig. 2a). There was no production in the absence of added NaCl. The production increased with salt concentration up to 2.0 %, and then decreased again at 2.5 % NaCl. Thus, at 2.0 % NaCl S. ruminantium CT2 exhibited the highest surface tension reduction and bisurfactant yield of 45.2 mN/m and 3.19 g/l, respectively. This result clearly indicates that production of biosurfactants is salt dependent.
Effect of NaCl concentrations (a) and addition of amino acids (b) on growth and biosurfactant production by S. ruminantium CT2, which were cultivated in 250 ml flask containing 50 ml MSM in a shaking incubator at 150 rpm and 30 °C for 48 h. Bars indicate that standard deviation from triplicate determinations
Effect of different amino acids on growth and biosurfactant production
The effect of various amino acids was examined to optimize the biosurfactant production by S. ruminantium CT2. MSM supplemented with 0.1 % (w/v) leucine showed a considerable increase in the biosurfactant production by more than one and half folds (4.8 g/l) compared with the control without amino acid (Fig. 2b). In fact, the increased production of biosurfactant by S. ruminantium CT2 may take place as a result of the direct uptake of amino acid as precursors for the surfactant biosynthesis, thus improving the biosurfactant yield. In a previous report, Lotfabad et al. (2009) and Abdel-Mawgoud et al. (2008) showed that some of amino acids are excellent substrates as nitrogen sources for the production of glycolipid and surfactin by P. aeruginosa MR01 and Bacillus subtilis BS5, respectively.
Time course of growth and biosurfactant production
The results in Fig. 3a showed that biosurfactant production started early in the exponential phase and the production kinetics paralleled the biomass kinetics up to 2 days of incubation. On the basis of these facts, it could be concluded that biosurfactant production is growth associated. It was found that the maximum level of cell biomass was obtained after 48 h of incubation. However, maximum biosurfactant concentration was obtained 6 h later (5.02 g/l), that is after 54 h of incubation. After those periods, a sharp reduction in either biomass or biosurfactant production levels was observed. Growth-associated production of biosurfactant has been reported for Aeromonas sp. (Ilori et al. 2005), B. subtilis (Abdel-Mawgoud et al. 2008) and Pseudomonas sp. (Obayori et al. 2009). Tabatabaee et al. (2005) also documented that a biosurfactant synthesized by a strain of Bacillus sp. was a primary metabolite produced during cellular biomass formation. From the result obtained, it can be seen that a cultivation time of 54 h gave the highest biosurfactant yield.
Recovery of biosurfactant
The ability of various solvent systems to recover surface-active components from the culture supernatant of S. ruminantium CT2 after 54 h of cultivation was examined. The use of ethyl acetate resulted in a greater activity of crude extract when compared with systems based on mixtures of chloroform and methanol, cold acetone or dichloromethane (data not shown). It was also reported previously that the extraction of bioproducts with considerably high polarity by ethyl acetate solvent is rather efficient (Chen and Juang 2008). Because the recovery and concentration of biosurfactants from fermentation broth largely determines the production cost, ethyl acetate is a better choice than the highly toxic chloro-organic compounds.
Chemical characterization of the biosurfactant
The FT-IR spectrum of biosurfactant from S. ruminantium CT2 showed strong absorption bands, indicating presence of peptides at 3,310 cm−1 resulting from N–H stretching mode (Fig. 3b). At 1,650 cm−1, the stretching mode of a CO–N bond was observed, and at 1,540 cm−1 the deformation mode of the NH bond combined with C–N stretching mode occurred. The presence of an aliphatic chain was indicated by the C–H stretching modes at 2,970–2,854 cm−1 and 1,450–1,380 cm−1. These results strongly indicate that the biosurfactant contains aliphatic and peptide-like moieties. The band at 1,730 cm−1 was due to lactone carbonyl absorption.
To further confirm the results of this study, an NMR analysis was performed (Fig. 4a, b). The result obtained from 1H-NMR clearly indicated that the molecule being studied is a lipopeptide. The spectrum confirms the presence of a long aliphatic chain (CH2 at 1.55–1.25 ppm) and a peptide backbone (NH at 8.00–7.24 ppm and CH at 4.8–4.2 ppm) (Fig. 4a). A resonance at 4.7 ppm indicates the presence of an ester group which may be a part of lactone ring. Most of the seven backbone-amine (-NH) groups are in the region from 8.3 to 7.3 ppm downfield from tetramethylsilane. α-Hs (alpha hydrogens) of the amino acids come into resonance from 5.3 to 4.3 ppm. 1H-NMR analysis of the compound showed a doublet at δ = 0.859 ppm for the (CH3)2-CH group, indicating terminal branching in the fatty acid component. Owing to the presence of CH at 1.2 ppm, the ratio of the methylene and terminal groups could not be resolved (Fig. 4a). The 13C-NMR spectrum was strongly signals at 14.0, 22.98–37.68, 171.4 and 174.2 ppm from methyl, methlene, ester and carboxyl group, respectively (Fig. 4b). The present of NMR spectrograms of purified biosurfactant are similar to the surfactin, a member of lipopeptide type biosurfactant (Tang et al. 2007).
Analysis of the intact molecules with LCQ-MS revealed four molecular ion peaks with molecular masses [M+ H] of 990, 1,004, 1,018 and 1,032, respectively (Fig. 5a). The spectra clearly indicate the presence of higher and lower homologus of surfactants for the difference between prominent M+, peaks being around 14, corresponding to a difference in the number of methylene groups (CH2). The overall chemical determination of biosurfactant from S. ruminantium CT2 was very similar with cyclic lipopeptides produced by bacilli like surfactin (produced by B. subtilis), the most effective biosurfactant discovered so far (Roongsawang et al. 2002; Joshi et al. 2008). To our best knowledge, this is the first report of the production of a lipopeptide biosurfactant by S. ruminantium.
Hydrocarbon specificity for emulsification activity and emulsification index
To determine hydrocarbon specificity for emulsification activity (E24) and emulsification index (E48), a wide range of pure and mixed substrates were investigated. According to Fig. 5b, emulsions can be formed and stabled with a wide range of hydrophobic compounds, an important property for applications of biosurfactant. Olive oil, soybean oil and toluene were good substrates for E24 and E48 by the crude biosurfactant from S. ruminantium CT2, showing no significant differences. Hexane, kerosene and motor oil also formed stable emulsions. Hexadecane, ULO and xylene resulted in poor emulsification, probably due to the inability of the biosurfactant to stabilize the microscopic droplets of these compounds. The structure of ULO is consisted of the mixture of paraffin, naphthalene and aromatic hydrocarbon. Thus, it was difficult to emulsify by crude biosurfactat (Muthusamy et al. 2008). The ability of crude biosurfactant from S. ruminantium CT2 to emulsify various hydrophobic substrates indicates that it has a good potential for applications in microbial enhanced oil removal, as cleaning and emulsifying agent in food industry and also for bioremediation.
Surface tension and critical micelle concentration (CMC)
The relationship between surface tension and concentration of the isolated biosurfactant solution was determined by a De Noüy ring tensiometer (Fig. 6a). The biosurfactant produced exhibited excellent surface tension reducing activity. The surface tension of water of 72 mN/m decreased to 25.5 mN/m by increasing the solution concentration up to 8 mg/l. Further increase in the concentration of the biosurfactant solution did not reduce the surface tension of water, indicating that the CMC was reached at this concentration. The biosurfactant from S. ruminantium CT2 showed a lower minimum surface tension and CMC value than that of the biosurfactant from B. subtilis (26.7 mN/m, 10 mg/l) (Ghojavand et al. 2008), from Lactobacillus paracasei (41.8 mN/m, 2.5 mg/ml) (Gudiña et al. 2010) and from P. aeruginosa Bs20 (29.5 mN/m, 13.4 mg/l) (Abdel-Mawgoud et al. 2009).
Effect of temperature, pH and salinity on biosurfactant stability
The results obtained from thermal stability analysis of biosurfactant over a wide range of temperature (4–121 °C) revealed that the biosurfactant from S. ruminantium CT2 was thermostable (Fig. 6b). Temperature up to 100 °C (or its autoclaving at 121 °C) caused no effect on the biosurfactant performance and its emulsification capacity. Such extreme Brevibacterium aureum MSA13, Bacillus spp. and B. subtilis EG1, respectively. It was found that only 5 % of E24 becomes lost, when the biosurfactant was stored at 4 °C. The surface tension reduction and E24 were relatively stable at the temperatures used (ST = 25.5 mN/m, E24 ≈ 65 and 69 against kerosene and xylene, respectively). This suggests that the biosurfactant isolated may be used in microbial enhanced oil removal processes where high temperatures prevail.
As can be seen in Fig. 6c, the activity of biosurfactant and its emulsification ability was also affected by the pH. When the pH was acidic and set to 2, 3 and 4, the biosurfactant activities were 65, 63 and 50 mN/m, respectively. Correspondingly, the emulsification ability was limited to the acidic to neutral pH and emulsification index up to 45, 47 and 50 %, respectively were obtained. The optimum pH for both parameters namely biosurfactant activity (ST = 25.5 mN/m) and emulsification capacity (E24 = 65 and 69 against kerosene and xylene, respectively) was determined to be 7. However, the emulsification activity was relatively stable between pH 8 and pH 12. Similar result had been reported for biosurfactants produced by Bacillus spp. and B. subtilis EG1, which remained stable in the range of pH 5.0–10.0 and 5.0–11.0, respectively (Gudiña et al. 2012; Vaz et al. 2012). The crude biosurfactants produced by B. subtilis TD4 and P. aeruginosa SU7 were also stable in a wide range of pH (4.0–11.0) (Saimmai et al. 2012c).
Figure 6d demonstrates the effect of addition of NaCl on the surface tension and E24 of the biosurfactant obtained. As is shown, negligible changes were occurred in the biosurfactant activity with an increase in the NaCl concentration up to 14 %. Likewise, an increase in NaCl concentration up to 18 % did not have a significant effect on E24. However, at the highest level of NaCl (24 %), E24 dropped severely to 43.6 % and surface activity was increased as well (48 mN/m). NaCl activated biosurfactant activity of many strains isolated from several environmental sources had been observed including seawater (Maneerat and Phetrong 2007), mangrove sediment (Saimmai et al. 2012a, 2012b, 2012d), palm oil contaminated soil (Saimmai et al. 2012c) or petroleum reservoirs (Lotfabad et al. 2009). Nevertheless, biosurfactant extracted from Bacillus spp. exhibited zero saline tolerance (Gudiña et al. 2012), whereas the biosurfactant from Pseudomonas sp. 2B tolerated 8 % NaCl and lost 15 % of its activity in 20 % NaCl (Aparna et al. 2012). More recently, biosurfactant produced by P. aeruginosa MA01 has shown good stability of surface tension and emulsion in the presence of NaCl up to 300 g/l (Abbasi et al. 2012).
Our findings indicate that the biosurfactant has potential application over a wide range of temperature, pH values and saline environment. This is applicable in situations where extreme conditions of temperature, pH and salinity prevail such as bioremediation of contaminated environments such as marine, sediment or soil. It can also be used to enhance oil removal processes.
Application of the biosurfactant in motor oil removal from contaminated sand
Petroleum hydrocarbon compounds bind to soil components and are difficult to remove and degrade (Sobrinho et al. 2008). Biosurfactants can emulsify hydrocarbons enhancing their water solubility, decreasing surface tension and increase the displacement of oil substances from soil particles (Banat et al. 2010). The ability of biosurfactant from S. ruminantium CT2 to enhance motor oil removal from contaminated sand was examined in comparison with those of synthetic surfactants, this is a nonionic surfactant Triton X-100 and anionic surfactants SDS (Fig. 7). Biosurfactant from S. ruminantium CT2 and Triton X-100 recovered 25–30 % of motor oil from contaminated sand at 25 °C, 50 % at room temperature (30 ± 2 °C), 65 % at 45 °C and 85 % at 60 °C. The synthetic surfactant SDS was found to be less efficient. In the case of control (distilled water), and very little recovery (5–18 %) could be obtained in the temperature range. These results have implications for the potential use of a biosurfactant produced by S. ruminantium CT2 to enhance sorbed motor oil from environment. However, it is important not to rule out that efficiency of the removal could be vary depending on the characteristic of the contaminants and site characteristics.
Effect of biosurfactant on PAH solubilization
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental polluants. Excessive inputs from anthropogenic activities have caused serious contamination and adversely affect the health of aquatic and human life through bioaccumulation. PAHs are hydrophobic and readily adsorbed onto particulate matter; therefore, coastal and marine sediments become the ultimate sinks and elevated concentrations have been recorded (Aniszewski et al. 2010). Solubilization of PAHs depends on the type and dose of the surfactant, the hydrophobicity, the surfactant-soil interactions and the time that a contaminant has been in contact with the soil (Zhou and Rhue 2000). The effect of biosurfactant on the apparent aqueous solubility of PAHs was determined by test tube solubilization assays in the presence of increasing concentrations of biosurfactant (0 to 30 mg/l) is depicted in Table 4. In general, the obtained biosurfactant enhanced the apparent solubility of PAHs in a dose dependent manner. However, solubilization of fluorene, naphthalene or phenantrene by biosurfactant from this strain (about 3–5 time higher apparent solubility compared to control) was significantly lower (p < 0.05) when compared with anthracene, fluoranthene or pyrene affect by biosurfactant (15–20 times higher compared to control). In the present study, the crude biosurfactant showed ability to solubilize PAHs in aqueous phase indicating its possible role in increasing the bioavailability of non-soluble organic compounds for bacterial metabolism.
Antibacterial activity of biosurfactant
The biosurfactant obtained of mangrove S. ruminantium CT2 was found to be an antimicrobial agent, depending on the microorganism (Fig. 7b). It was found that the biosurfactant exhibited a high antimicrobial activity against B. cereus, C. albicans, P. aeruginosa and S. aureus at tested concentrations. In addition, it was observed that the biosurfactant obtained showed no antimicrobial activity against V. vulnificus and V. cholerae and low activity against E. faecium, E. coli, L. monocytogenes, Salmonella sp. and S. typhimurium. Low antimicrobial activity correlating with higher resistance were often seen when Gram-negative microorganisms were tested (Das and Mukherjee 2007). Higher resistance of Gram-negative bacteria to external substances had been reported (Negi et al. 2005). It is attributed to the presence of lipopolysaccarides, making them naturally resistant to certain antibacterial agents, in their outer membrane (Elving et al. 2000; Luna et al. 2011). On the other hand, Gram-positive tested organisms showed higher sensitivity against the biosurfactant than the Gram-negative bacteria. The reason could be attributed to the differences between their cell wall compositions (Scherrer and Gerhardt 1971).
Conclusion
In the present study, biosurfactant production by S. ruminantium CT2 which was isolated from mangrove sediment has been reported. The growth characteristics were obtained and studies on the properties of the biosurfactant indicate the possibility of its industrial application. The spectra obtained from FT-IR spectroscopy and LC–MS confirmed the presence of lipopeptide in the sample. The potential of this biosurfactant for industrial use was shown by studying its physical properties, such as the surface tension, critical micelle concentration and emulsification activity, and its stability to environmental stresses such as salinity, pH and temperature. The surface tension of an aqueous solution of this biosurfactant at a CMC value of 8 mg/l, reached 25.5 mN/m. The properties of the biosurfactant obtained have potential applications especially for microbial enhanced oil removal and/or reducing the intensity of environmental contamination.
References
Abbasi H, Hamedi MM, Lotfabad TB, Zahiri HS, Sharafi H, Masoomi F, Moosavi-Movahedi AA, Ortiz A, Amanlou M, Noghabi KA (2012) Biosurfactant-producing bacterium, Pseudomonas aeruginosa MA01 isolated from spoiled apples: physicochemical and structural characteristics of isolated biosurfactant. J Biosci Bioeng 113:211–219
Abdel-Mawgoud AM, Aboulwafa AM, Hassouna NAH (2008) Optimization of surfactin production by Bacillus subtilis isolate BS5. Appl Biochem Biotechnol 150:305–325
Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NAH (2009) Characterization of rhamnolipid produced by Pseudomonas aeruginosa isolate Bs20. Appl Biochem Biotechnol 157:329–345
Aniszewski E, Peixoto SR, Mota FF, Leite SGF, Rosado SA (2010) Bioemulsifier production by Microbacterium sp. strains isolated from mangrove and their application to remove cadmium and zinc from hazardous industrial residue. Braz J Microbiol 41:235–245
Aparna A, Srinikethan G, Smitha H (2012) Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloid Surf B 95:23–29
Banat IM (1995) Biosurfactants production and possible uses microbial enhanced oil recovery and oil pollution remediation: a review. Bioresour Technol 51:1–12
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444
Barber WP, Stuckey DC (2000) Nitrogen removal in a modified anaerobic baffled reactor (ABR): 1, denitrification. Water Res 10:2413–2422
Barkay T, Navon-Venezia S, Ron EZ, Rosenberg E (1999) Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl Environ Microbiol 65:2697–2702
Benincasa M, Contiero J, Manresa MA, Moraes IO (2002) Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J Food Eng 54:283–288
Bernard D, Pascaline H, Jeremie JJ (1996) Distribution and origin of hydrocarbons in sediments from lagoons with fringing mangrove communities. Mar Pollut Bull 32:734–739
Brown LR (2010) Microbial enhanced oil recovery (MEOR). Curr Opin Microbiol 13:316–320
Burgos-Diaz C, Pons R, Espuny MJ, Aranda FJ, Teruel JA, Manresa A, Ortiz A, Marques AM (2011) Isolation and partial characterization of a biosurfactant mixture produced by Sphingobacterium sp. isolated from soil. J Colloid Interf Sci 361:195–204
Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sokmen A, Akpulat HA (2003) Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol 87:215–220
Chayabutra C, Wu J, Ju LK (2001) Rhamnolipid production by Pseudomonas aeruginosa under denitrification: effects of limiting nutrients and carbon substrates. Biotechnol Bioeng 72:25–33
Chen HL, Juang RS (2008) Recovery and separation of surfactin from pretreated fermentation broths by physical and chemical extraction. Biochem Eng J 38:39–46
Das K, Mukherjee AK (2007) Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon source: some industrial applications of biosurfactants. Process Biochem 42:1191–1199
Elving GJ, van der Mei HC, Busscher HJ, Amerogen EC, van Weissenbruch R, Albers FW (2000) Antimicrobial activity of synthetic salivary peptides against voice prosthetic microorganisms. Laryngoscope 110:321–324
Fathabad EG (2011) Biosurfactants in pharmaceutical industry. Am J Drug Discov Dev 1:58–69
Ghojavand H, Vahabzadeh F, Roayaei E, Khodabandeh A (2008) Production and properties of a biosurfactant obtained from a member of the Bacillus subtilis group (PTCC 1696). J Colloid Interf Sci 324:172–176
Gudiña EJ, Teixeira JA, Rodrigues LR (2010) Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloid Surf B 76:298–304
Gudiña EJ, Pereira JFB, Rodrigues LR, Coutinho JAP, Teixeira JA (2012) Isolation and study of microorganisms from oil samples for application in microbial enhanced oil recovery. Int Biodeterior Biodegradtion 68:56–64
Holt JG, Kreig NR, Sneath PHA, Stanley JT, William ST (1994) Bergey’s manual determinative bacteriology. William and Wilkins, Baltimore
Ilori MO, Amobi CJ, Odocha AC (2005) Factors affecting biosurfactant production by oil degrading Aeromonas sp., isolated from a tropical environment. Chemosphere 61:985–992
Janek T, Lukaszewicz M, Rezanka T, Krasowska A (2010) Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the arctic archipelago of Svalbard. Bioresour Technol 101:6118–6123
Joshi S, Bharucha C, Jha S, Yadav S, Nerurkar A, Desai AJ (2008) Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour Technol 99:195–199
Ke L, Wang WQ, Wong TW, Wong YS, Tam NF (2003) Removal of pyrene from contaminated sediments by mangrove microcosms. Chemosphere 52:1581–1591
Kim HS, Jeon JW, Kim BH, Ahn CY, Oh HM, Yoon BD (2006) Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Appl Microbiol Biotechnol 70:391–396
Li JL, Chen BH (2009) Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials 2:76–94
Lotfabad TB, Shourian M, Roostaazad R, Najafabadi AR, Adelzadeh MR, Noghabi KA (2009) An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloid Surf B 69:183–193
Luna JM, Rufino RD, Sarubbo LA, Rodrigues LR, Teixeira JA, de Campos-Takaki GM (2011) Evaluation antimicrobial and antiadhesive properties of the biosurfactant lunasan produced by Candida sphaerica UCP 0995. Curr Microbiol 62:1527–1534
Maneerat S, Phetrong K (2007) Isolation of biosurfactant-producing marine bacteria and characteristics of selected biosurfactant. Songklanakarin J Sci Technol 29:781–791
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollut 133:183–198
Mulligan CN (2009) Recent advances in the environmental applications of biosurfactants. Curr Opin Colloid Interface Sci 14:372–378
Muthusamy K, Gopalakrishnan S, Ravi TK, Sivachidambaram P (2008) Biosurfactants: properties, commercial production and application. Curr Microbiol 94:736–747
Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS (2005) Antioxidant and antimicrobial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem 92:119–124
Nilsson WB, Strom MS (2002) Detection and identification of bacterial pathogens of fish in kidney tissue using terminal restriction length polymorphism (T-RFLP) analysis of 16S rRNA genes. Dis Aquat Org 48:175–185
Nitschke M, Coast SG (2007) Biosurfactants in food industry. Trends Food Sci Technol 18:252–259
Nitschke M, Pastore G (2006) Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour Technol 97:336–341
Obayori O, Ilori M, Adebusoye S, Oyetibo G, Omotayo A, Amund O (2009) Degradation of hydrocarbons and biosurfactant production by Pseudomonas sp. strain LP1. World J Microbiol Biotechnol 25:1615–1623
Phalakornkule C, Tanasupawat S (2006) Characterization of lactic acid bacteria from traditional Thai sausages. J Cult Collect 5:46–57
Reda AB, El-Nagar AY (2009) Safe control methods of petroleum crude oil pollution in the mangrove forests of the Egyptian red sea coast. J Appl Sci Res 5:2435–2447
Roongsawang N, Thaniyavarn J, Thaniyavarn S, Kameyama T, Haruki M, Imanaka T, Morikawa M, Kanaya S (2002) Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: bacillomycin L, plipastatin, and surfactin. Extremophiles 6:499–506
Saimmai A, Rukadee O, Onlamool T, Sobhon V, Maneerat S (2012a) Isolation and functional characterization of a biosurfactant produced by a new and promising strain of Oleomonas sagaranensis AT18. World J Microbiol Biotechnol. doi:10.1007/s11274-012-1108-0
Saimmai A, Sobhon V, Maneerat S (2012b) Production of biosurfactant from a new and promising strain of Leucobacter komagatae 183. Ann Microbiol 62:391–402
Saimmai A, Sobhon V, Rukadee O, Maneerat S (2012c) Comparison of biosurfactants produced by Bacillus subtilis TD4 and Pseudomonas aeruginosa SU7 for microbial surfactant-enhanced oil recovery. J Sci Ind Res 71:396–406
Saimmai A, Tani A, Sobhon V, Maneerat S (2012d) Mangrove sediment, a new source of potential biosurfactant producing bacteria. Ann Microbiol. doi:10.1007/s13213-012-0424-9
Santos AS, Sampaio AP, Vasquez GS, Anna LMS, Pereira N Jr, Freire DM (2002) Evaluation of different carbon and nitrogen sources in the production of rhamnolipids by a strain of Pseudomonas. Appl Biochem Biotechnol 98:1025–1035
Scherrer R, Gerhardt P (1971) Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol 107:718–735
Singh P, Cameotra SS (2004) Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol 22:142–146
Sobrinho HBS, Rufino RD, Luna JM, Salgueiro AA, Campos-Takaki GM, Leite LFC, Sarubbo LA (2008) Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem 43:912–917
Tabatabaee A, Assadi MM, Noohi AA, Sajadian VA (2005) Isolation of biosurfactant producing bacteria from oil reservoirs. J Environ Health Sci Eng 2:6–12
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tang JS, Gao H, Hong K, Yu Y, Jiang MM, Lin HP, Ye WC, Yao XS (2007) Complete assignments of 1H- and 13C-NMR spectral data of nine surfactin isomers. Magn Reson Chem 45:792–796
Thompson JD, Gibbons TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vaz DA, Gudiña EJ, Alameda EJ, Teixeira JA, Rodrigues LR (2012) Performance of a biosurfactant produced by a Bacillus subtilis strain isolated from crude oil samples as compared to commercial chemical surfactants. Colloid Surf B 89:167–174
Wei YH, Chou CL, Chang JS (2005) Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. J Biochem Eng 27:146–154
Wu JY, Yeh KL, Lu WB, Lin CL, Chang JS (2008) Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour Technol 99:1157–1164
Zhou M, Rhue RD (2000) Screening commercial surfactants suitable for remediating DNAPL source zones by solubility. Environ Sci Technol 34:1985–1990
Acknowledgments
We are grateful to Phuket Rajabhat University for providing a scholarship to AS. This research was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saimmai, A., Onlamool, T., Sobhon, V. et al. An efficient biosurfactant-producing bacterium Selenomonas ruminantium CT2, isolated from mangrove sediment in south of Thailand. World J Microbiol Biotechnol 29, 87–102 (2013). https://doi.org/10.1007/s11274-012-1161-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1161-8