Abstract
Pseudomonas nitroreducens MILB-8054A isolated from petroleum-contaminated soil, immobilized on calcium alginate beads, and under resting cell condition, produced biosurfactants. Immobilized cells gave a best yield of 5.6 g rhamnolipid l−1 using sucrose as carbon source. Time course study using resting cells showed that 2 % v/v of palm oil (preculture carbon source) and 10 % diesel (carbon source) gave the best rhamnolipid yield of 5.1 g l−1 at pH 8 and temperature of 30 °C. Carbon utilization by resting cells was compared with that of growing cells. The best biosurfactant recovery procedure was acetone extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
A variety of microorganisms, such as bacteria, yeasts, and fungi, are able to produce biosurfactants (Desai and Banat 1997). Compared to synthetic surfactants, biosurfactants have higher surface activity, lower toxicity, higher biodegradability, and better environmental compatibility (Banat et al. 2000). Biosurfactants can be categorized in five groups in terms of their chemical composition, being glycolipids, lipopeptides, phospholipids, fatty acids, and polymeric biosurfactants (Wei et al. 2007). Among them, rhamnolipid, a glycolipid-type biosurfactant, primarily produced by Pseudomonas sp., can be applied in the petrochemical industry for enhanced oil recovery, hydrocarbon remediation, removal of heavy metals from soils, and decontamination of oil from soil (Mulligan 2005). Although rhamnolipid is an effective biosurfactant and is well suited for applications in bioremediation of oil pollutants (Wei et al. 2008), its production could be limited by several factors such as limited yield, recovery, and purification difficulties, as well as a limited productivity of batch process or occurrence of substrate inhibition (Abouseoud et al. 2008b). Therefore, there is an urgent demand to develop an efficient biosurfactant producer and a cost-effective bioprocess for the production of rhamnolipid. A possible strategy for enhanced rhamnolipid production is immobilization of bacterial cells on hydrogel beads and keeping the microbial cell under resting condition. The major problem associated with use of hydrogel beads is to find the right one that will not be harmful to the microbial cells.
For the continuous production of rhamnolipids, immobilization methods have been used (Jeong et al. 2004; Abouseoud et al. 2008b). The successful application of an immobilization technique requires that the carriers have a strong consistency, durability, and high cell viability as well as low cost (Hashimoto and Furukawa 1987). Immobilizing microbial cells in a high density not only improves the productivity of a bioreactor but also provides many benefits over free cells. The microbial cells immobilized in a hydrogel matrix can be protected from harsh environmental conditions such as pH, temperature, organic solvent, and poison. Immobilized microbial cells can also be handled more easily and recovered from the solution without difficulty. Continuous processes can be operated in a high cell density without loss of microbial cells even at high dilution rates, which results in a higher bioreactor volumetric productivity. An entrapment method has been commonly used for the preparation of immobilized microbial cells (Park and Chang 2000). There are two categories of hydrogel material for cell immobilization, natural and synthetic. Of the former, agar, agarose, polyacrylamides, κ-carrageenan, and alginate are common examples. Examples of synthetic gels include poly(carbamoyl)sulphonate (PCS), polyhydroxyethylmethacrylate (polyHEMA), polyacrylamide, and polyvinyl alcohol (PVA) (Cunningham et al. 2004). However, conditions under which these hydrogels form may be inhibitory to microbial growth, e.g., cross-linking with toxic chemicals or UV light (Webb and Dervakos 1996). The encapsulation of microbial cells in biodegradable matrices such as alginate do not harm the cells, while the resulting carriers are biodegradable and non-toxic, and may provide a means for slow release of microorganisms over time, initially protecting encapsulated cells while the beads are degraded (Cassidy et al. 1995).
In order to improve rhamnolipid production by Pseudomonas sp., the use of resting microbial cells has also been reported by several authors (Reiling et al. 1986; Ramana and Karanth 1989). Production by resting cells is a mode of biosurfactant production in which there is no cell multiplication. The cells nevertheless continue to utilize the carbon source for the synthesis of biosurfactant. Biosurfactant production by resting cells is important for the reduction of cost of product recovery, as the growth and the product formation phases can be separated (Desai and Banat 1997).
Though there have been reports on biosurfactant production by various species of Pseudomonas, no report has been made on biosurfactant production by Pseudomonas nitroreducens at the time of this report. Alginate has been widely studied and utilized due to the milder conditions (e.g., ambient temperature) of the encapsulation process (Cassidy et al. 1995). To this end, this work was aimed at immobilization of Pseudomonas nitroreducens on Ca2+ alginate beads for enhanced production of rhamnolipid biosurfactants. Also, we report the production of biosurfactants by this organism under resting cells condition.
Materials and methods
Microorganism and inocula
Pseudomonas nitroreducens MILB-8054A with accession number (AY297786) used in this study was previously isolated from petroleum-contaminated soil in Awka, Nigeria (Onwosi and Odibo 2011).
Growth medium
A mineral salts medium (MSM), according to Pruthi and Cameotra (1997) with a few modifications, containing the following components (g l−1) was used for the growth of the isolate: KH2PO4, 2.0; K2HPO4, 5.0; (NH4)2SO4, 3.0; NaNO3, 2.0; NaCl, 0.10; MgSO .4 7H2O, 0.2; FeSO .4 7H2O, 0.01; CaCl2, 0.01. It also contained trace elements solution having the following components (mg l−1) ZnSO4 .7H2O, 5.25; MnSO4 .4H2O, 200; CuSO4 .5H2O, 70.5; NH4MoO4 .2H2O; 15; CoCl .2 6H2O, 200; H3BO3, 15. The pH of the medium was adjusted to 6.8 using 1 M NaOH and sterilized by autoclaving at 121 °C for 15 min. The inoculum was prepared by transferring a loopful of bacteria from the slant to Erlenmeyer flask (250 ml) containing 50 ml of mineral salt medium and 2 % glucose as carbon source and incubated at 180 rpm for 36 h at 30 °C in an orbital shaker.
Rhamnolipid purification and recovery
The collected fermentation broth was first centrifuged at 9,000 g for 15 min to remove bacterial cells. The pH of the resulting supernatant was adjusted to pH 2.0 with 1 M HCl to precipitate rhamnolipid. The precipitate was harvested by centrifugation (9,000 g, 20 min). In the second approach, the culture broth was centrifuged (10,000 g for 15 min) to remove the cells. The clear supernatant served as the source of crude biosurfactant. The biosurfactant was recovered from the cell-free culture supernatant by cold acetone precipitation as described by Pruthi and Cameotra (1997). Three volumes of chilled acetone were added and allowed to stand for 10 h at 4 °C. Other approaches involved the precipitation of produced biosurfactant using hexane and ethanol.
Rhamnolipid quantification
The orcinol assay (Chandrasekaran and Bemiller 1980) was used for direct assessment of the amount of glycolipids in the samples. Extracellular glycolipids concentration was evaluated in triplicate by measuring the concentration of rhamnose: 0.33 ml of the culture supernatant was extracted twice with 1 ml diethyl ether. The ether fractions were evaporated to dryness and 0.5 ml of H2O was added. To 1 ml of each sample, 9 ml of a solution containing 0.19 % orcinol (in 53 % H2SO4) was added. After heating for 30 min at 80 °C the samples were cooled at room temperature and the OD421 was measured. The rhamnolipid concentrations were calculated from a standard curve prepared with l-rhamnose and expressed as rhamnose equivalents (RE) (mg ml−1).
Biosurfactant production by immobilized cells of Pseudomonas nitroreducens
The culture broth was centrifuged at 10,000 g for 15 min, and the pellets were used for immobilization study. The alginate encapsulation protocol of Abouseoud et al. (2008b) was modified in the following manner. Briefly, 1.4 g sodium alginate (final concentration 1.4 % w/v) was thoroughly mixed with hot distilled water in a 250-ml Erlenmeyer flask. The solution was heated to 70 °C and mixed for 30 min at 180 rpm by magnetic stirring. It was autoclaved for 10 min at 121 °C and the mixture cooled to about 35 °C and then added to a solution of about 2.0 g wet cell weights per 100 ml of alginate solution for 15 min. The resulting mixture was extruded through a sterile 5-ml gauge needle into a sterile solution of 0.05 M CaCl2 maintained at 10 °C and mixed by magnetic stirring at 50 rpm. The resulting beads were held stationary in the CaCl2 solution for 2 h at 10 °C in order to harden. The beads were finally washed with sterile distilled water. Experiments were carried out using mineral salts medium (MSM), according to Pruthi and Cameotra (1997) with a few modifications. Various carbon sources (2 %) were aseptically added to the flasks containing MSM. Laboratory scale biosurfactant production was carried out in 250-ml Erlenmeyer flasks (containing 50 ml of medium inoculated with 5 ml of immobilized cells) incubated for 7 days in an orbital shaker (180 rpm, 30 °C). This experiment was performed in triplicate and the average result taken.
Preparation of resting cells and biosurfactant production by resting cells
The method of Kitamoto et al. (1992) was used in the preparation of the resting cells and evaluating the effects of carbon sources on rhamnolipid production from resting cells. Briefly, the bacterial cells were incubated in an orbital shaker (180 rpm, 30 °C) for 2 days using Nutrient broth as the growth medium. Thereafter, the cells were harvested by centrifugation at 9,500 g for 20 min and washed twice with distilled water under sterile conditions. The washed cells were transferred into Erlenmeyer flasks (250 ml) containing 50 ml of the reaction medium (10 % carbon source and distilled water) and incubated at 30 °C for 6 days. The carbon sources used were groundnut oil, palm oil, diesel, glucose, and glycerol. To examine the effects of pre-culture carbon sources on rhamnolipid production by resting cells, different carbon sources (glucose, sorbitol, maltose, lactose, sucrose, raffinose, glycerol, diesel, palm oil, and groundnut oil) were used for inoculum build-up using MSM as growth medium. The cells were harvested and used for rhamnolipid production. Mineral salts medium (MSM), according to Pruthi and Cameotra (1997) with a few modifications was used for growing cells for rhamnolipid production. Then, 2 % of various carbon sources were aseptically added to the flasks containing MSM. Laboratory-scale biosurfactant production was carried out in 250-ml Erlenmeyer flasks (containing 50 ml of medium inoculated with 3 ml of inoculum) incubated for 7 days in an orbital shaker (180 rpm, 30 °C). The effect of pH on rhamnolipid production from resting cells was examined using different buffers (acetate (pH 4 and 5), citrate-phosphate (pH 6 and 7), phosphate buffer (pH 7), and Tris (pH 9)) instead of distilled water in the reaction medium. The effect of temperature (30–50 °C) on rhamnolipid production by resting and growing cells of Pseudomonas nitroreducens was also evaluated. All the experiments described in this section were performed in triplicates.
Statistical analysis
Statistical analysis was performed by GraphPad Prism 5® software program (Trial version) (GraphPad Software, CA, USA). The data were analyzed by analysis of variance (ANOVA). All values are given as the mean of three replicates ± standard deviation (SD).
Results and discussion
Biosurfactant recovery
During the rhamnolipid recovery, acetone or ethanol was the preferred system. Abouseoud et al. (2008a) also reported the use of acetone in the recovery of biosurfactants. The cold acetone precipitation method used for biosurfactant recovery appeared easy and reliable, and no loss of biosurfactant activity was noted as reported in the available literature (Haba et al. 2000).
Effect of cell immobilization on biosurfactant production
Varying the concentration of immobilized cells did not have outstanding differences in biosurfactant yield in this study. However, the cell loading of 10 % gave the best rhamnolipid yield (data not shown). The medium to bead volume ratio is an important consideration in determining the bioreactor performance (Abouseoud et al. 2008b). The biosurfactant yields from different carbon sources are shown in Table 1. The best carbon source was sucrose. Production of biosurfactant by free and alginate-entrapped cells of Pseudomonas fluorescens Migula 1895-DSMZ was investigated using olive oil as the sole carbon and energy source (Abouseoud et al. 2008b). Jeong et al. (2004) reported that a marine bacterium, Pseudomonas aeruginosa BYK-2, was immobilized by entrapment in polyvinyl alcohol beads and optimized for the continuous production of rhamnolipid. Interestingly, Heyd et al. (2011) also described the continuous rhamnolipid production using Pseudomonas aeruginosa DSM 2874 immobilized in magnetic alginate beads using glycerol as carbon source.
Biosurfactant production by resting cells of Pseudomonas nitroreducens
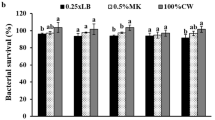
The best pre-culture carbon source was from palm oil with yield of 3.7 g rhamnolipid l−1 (Table 2). Table 3 shows the effects of carbon sources in the reaction medium on the resting and growing cells of Pseudomonas nitroreducens. Glucose and diesel were the best carbon sources for growing and resting cells, respectively. Syldatk et al. (1985) produced four interfacial active rhamnolipids using n-alkanes or glycerol as carbon sources by resting cells of Pseudomonas species DSM 2874. The pH 8 gave the best rhamnolipid yield for both growing and resting cells (Fig. 1a). The optimum temperature for biosurfactant yield was under mesophilic condition (35 °C) for both resting and growing cells (Fig. 1b). In the time course study, optimal conditions of the reaction medium are: pre-culture carbon source (palm oil) = 2 % v/v, carbon source (diesel) = 10 % v/v, pH = 8, temperature = 35 °C) (Fig. 2). There was a linear increase in the concentration of rhamnolipid biosurfactant without a lag phase and it reached 5.1 g rhamnolipid l−1 after 144 h fermentation. According to Syldatk and co-workers, optimal rhamnolipid production was achieved at pH range of 6.0–7.2 and temperature of 30 °C (Syldatk et al. 1985).
Conclusions
The newly isolated Pseudomonas nitroreducens produced rhamnolipid-type biosurfactant. The present study revealed that Pseudomonas nitroreducens immobilized in Ca2+ alginate beads and under resting cells condition produced rhamnolipid. These methods are apparently advantageous in the continuous production and recovery of the biosurfactant.
References
Abouseoud M, Maachi R, Amrane A, Boudergua S, Nabi A (2008a) Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 223:143–151
Abouseoud M, Yataghene A, Amrane A, Maachi R (2008b) Biosurfactant production by free and alginate entrapped cells of Pseudomonas fluorescens. J Ind Microbiol Biotechnol 35:1303–1308
Banat IM, Makkar SS, Cameotra SS (2000) Potential commercial application of microbial surfactants. Appl Microb Biotechnol 53:495–508
Cassidy MB, Leung KT, Lee H, Trevors JT (1995) Survival of lac-lux marked Pseudomonas aeruginosa UG2Lr cells encapsulated in κ-carrageenan and alginate. J Microbiol Methods 23:281–290
Chandrasekaran EV, Bemiller JN (1980) Constituent analyses of glycosaminoglycans. In: Whistler RL (ed) Methods in carbohydrate chemistry. Academic, New York, pp 89–96
Cunningham CJ, Ivshina IB, Lozinsky VI, Kuyukina MS, Philp JC (2004) Bioremediation of diesel-contaminated soil by microorganisms immobilized in polyvinyl alcohol. Int Biodeterior Biodegrad 54:167–174
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Haba E, Espuny MJ, Busquets M, Manresa A (2000) Screening and production of rhamnolipids by Pseudomonas aeruginosa 47 T2 NCIB 40044 from waste frying oils. J Appl Microbiol 88:379–387
Hashimoto S, Furukawa K (1987) Immobilization of activated sludge by PVA-boric acid method. Biotech Bioeng 30:52–59
Heyd M, Franzreb M, Berensmeier S (2011) Continuous rhamnolipid production with integrated product removal by foam fractionation and magnetic separation of immobilized Pseudomonas aeruginosa. Biotechnol Prog 27:706–716. doi:10.1002/btpr.607
Jeong HS, Lim DJ, Hwang SH, Ha SD, Kong JY (2004) Rhamnolipid production by Pseudomonas aeruginosa immobilized in polyvinyl alcohol beads. Biotechnol Lett 26:35–39
Kitamoto D, Fuzishiro T, Yanagishita H, Nakane T, Nakahara T (1992) Production of mannosylerythritol lipids as biosurfactants by resting cells of Candida antarctica. Biotechnol Lett 14:305–310
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollution 133:183–198
Onwosi CO, Odibo FJC (2011) Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J Microbiol Biotechnol. doi:10.1007/s11274-011-0891-3
Park JK, Chang HN (2000) Microencapsulation of microbial cells. Biotechnol Adv 18:303–319
Pruthi V, Cameotra SS (1997) Rapid identification of biosurfactant – producing bacterial strains using a cell surface hydrophobicity technique. Biotechnol Tech 11:229–236
Ramana KV, Karanth NG (1989) Production of biosurfactants by the resting cells of Pseudomonas aeruginosa CFTR-6. Biotechnol Lett 11:437–442
Reiling HE, Wyass UT, Guerra-Santos LH, Hirt R, Kappeli O, Fiechter A (1986) Pilot plant production of rhamnolipid biosurfactant by Pseudomonas aeruginosa. Appl Environ Microbiol 51:985–989
Syldatk C, Lang S, Matulovic U, Wagner F (1985) Production of four interfacial active rhamnolipids from n-alkanes or glycerol by resting cells of Pseudomonas species DSM 2874. Z Naturforsch C 40:60–67
Webb C, Dervakos GA (1996) Studies in viable cell immobilization. Academic, London
Wei YH, Lai CC, Chang JS (2007) Using Taguchi experimental design methods to optimize trace element composition for enhanced surfactin production by Bacillus subtilis ATCC 21332. Process Biochem 42:40–45
Wei YH, Cheng CL, Chien CC, Wan HM (2008) Enhanced di-rhamnolipid production with an indigenous isolate Pseudomonas aeruginosa J16. Process Biochem 43:769–774
Acknowledgement
The authors are grateful to Biotechnology Research Centre, Nnamdi Azikiwe University, Awka, Nigeria for providing some of the facilities used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onwosi, C.O., Odibo, F.J.C. Rhamnolipid biosurfactant production by Pseudomonas nitroreducens immobilized on Ca2+ alginate beads and under resting cell condition. Ann Microbiol 63, 161–165 (2013). https://doi.org/10.1007/s13213-012-0456-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-012-0456-1