Abstract
The cancer cells in brain tumors interact with their microenvironment, which includes stromal cells, the extracellular matrix (ECM), and the physical properties of tissues. The reciprocal interaction between cancer cells and the surrounding microenvironment regulates the biological behavior of cancer cells. To improve our understanding of the progression of brain tumors, it is useful to construct physiologically relevant brain tumor models. Consequently, versatile in vitro tumor models ranging from simplistic two-dimensional (2D) cultures to three-dimensional (3D) cultures have been developed to mimic the microenvironments of the brain. This review covers the recent progress in the in vitro reconstruction of brain tumor microenvironments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A brain tumor is a growth of abnormal cells in the brain tissues that multiply in an uncontrolled manner. Clinically, the World Health Organization (WHO) classifies brain tumors into four grades according to histological and molecular parameters.1 Grades I and II are benign tumors that grow slowly and are the least aggressive. Malignant, high-grade (grades III and IV) brain tumors grow rapidly and consist of abnormal-appearing cells that infiltrate the surrounding tissues and have a poor prognosis.2 The standard treatments for brain tumors are surgical resection, radiotherapy, and chemotherapy with alkylating agents.3 However, these therapies focus on inhibition of the neoplasm or proliferating cells, and not on the cells infiltrating the brain.4,5 Therefore, these conventional therapies lack efficacy in most high-grade brain tumors and the patients have poor outcomes or develop recurrent tumors.
To enhance treatment effectiveness and predict the prognosis, it is important to understand the characteristics of brain tumors, including their growth and invasion. The brain tumor microenvironment, including blood vessels, immune cells, inflammatory cells, signaling molecules, and the extracellular matrix (ECM), can regulate tumor progression by interacting directly with cancer cells.6–8 Brain tissues have unique microenvironments that distinguish them from other tissues with low stiffness and loosely connected cellular network,9 including the composition of the ECM, anatomical structures, and specialized cell types, such as neurons, astrocytes, and microglia. The ECM component of brain tissue contains high amounts of hyaluronic acid (HA), glycosaminoglycans (GAGs), and proteoglycans, but lacks fibrous materials such as collagen, fibronectin, etc.9,10 The interaction between cancer cells and the unique extracellular microenvironment of the brain can affect the progression of aggressive tumors. Clinical and experimental data have demonstrated that diffusive invasion of cancer cells is regulated by several independent mechanisms including different anatomic and molecular pathways.11–13 Therefore, an understanding of these complex interactions is essential for developing new therapeutic strategies.
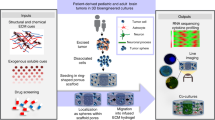
Recently, versatile in vitro tumor models have been developed to mimic the brain tumor microenvironment, reflecting the unique features of the brain stroma, including its structure and the ECM composition. In this review, we introduce the latest in vitro brain tumor models (Figure 1) used to reconstruct complex brain microenvironments. Then, future perspectives for recapitulating the brain microenvironments are suggested.
Two-dimensional (2D) Models
Over several decades, various in vitro tumor models have been developed to study cancer biology and drug screening. In the simplest approach, 2D tumor models were used for a wide variety of fundamental cancer research. However, 2D culture models are too simple to reflect the complexity of the in vivo environment.14,15
To replicate the microenvironment features of the brain in the 2D model, researchers have been culturing cancer cells on substrates coated with ECM bio-molecules or materials that exhibit the characteristics of native brain tissues (Table 1). This enables cell–ECM interactions within brain tumor microenvironments. In the brain, the ECM contains few fibrous proteins and large amounts of PG, GAG, and glycoproteins. The greatest volume of brain parenchyma is filled with HA, a negatively charged, unbranched GAG, which is the main organizer of the ECM, interacting with proteins and PG. By contrast, fibrous proteins such as collagen, fibronectin, and laminin are expressed only in the brain vasculature.10,12,16 Therefore, a substrate coated with an HA-based hydrogel17–19 is preferred to elucidate the effects of the brain-specific ECM microenvironment on cell behaviors. On HA-coated substrate, the Caucasian glioblastoma (GBM)–astrocytoma cell lines U87MG and U373MG showed increased migration speed.17 Moreover, to elucidate the effects of substrate stiffness on cell behavior, many studies have cultured cells on 2D substrates with different mechanical properties, such as polyacrylamide,11,20 silicon rubber,21 and HA.17–19 A rigid substrate tends to increase the motility, actin formation, adhesion, and proliferation of brain tumor cells.22
2.5D Models
In addition to the brain-specific ECM composition, the brain has unique anatomical structures, including the grey and white matter. The grey matter is composed of neurons and the white matter is formed from bundles of aligned axons; both are fibrous structures with submicron-sized fibers.23 Specific anatomical structures called Scherer structures may significantly increase the speed of invasion and distance traveled by cancer cells through the brain parenchyma, such as along white matter tracts and capillaries.24–26 These topological features of the brain can be reproduced using micro-engineered fabrication techniques, including micro-patterned substrates,20,27 and aligned nanofibers.28–30 On these substrates, cancer cells display increased polarity and migration speed compared to on a flat substrate. For instance, in micro-tracts smaller than 3 µm, cancer cells exhibited the native characteristics and behavior induced by topographic cues, allowing saltatory migration.26
Three-dimensional (3D) Culture Models
Although 2D culture models have been used in basic research to provide various types of information, the absence of the complexity of the in vivo microenvironment, such as cell-cell, results in the high failure rate of drug screening studies.14,15 Recent studies have shown that 3D culture allows a systemically designed microenvironment that includes cell type, dimensionality, ECM, and the enrichment of soluble factors (i.e., growth factors). In addition, 3D culture models exhibit several in vivo-like features, including cell-cell interactions,31 hypoxia,32 oxygen/medium penetration,33 and drug resistance.14,15 Therefore, these culture models have been used increasingly for a wide variety of basic cancer and pre-clinical research.34–36 Here, we present an overview of the latest 3D in vitro brain tumor models (Table 1) used to mimic brain microenvironments.
Multicellular Tumor Spheroid (MCTS)
Since Sutherland and coworkers introduced MCTS, one of the simplest 3D culture methods, in the early 1970s,37,38 they have provided important insights into tumor biology because of their in vivo-like features (cell-cell interactions, proliferation, and nutrient/oxygen gradients). There are versatile culture models for MCTS formation, including the culture of cells on non-adherent plates, spinner flasks, or rotary cell culture systems.39–41 To enhance production efficiency and size uniformity, various methods have been developed, including hanging drops,42,43 microwells,44 and microfluidic devices.45
Indeed, cells within tissues or organs interact with the surrounding microenvironment, such as resident cells, the ECM, soluble factors, and nutrient/oxygen gradients. These interactions establish a communication network that regulates tissue function and homeostasis.46 MCTS re-establish such cell-cell interactions and nutrient/oxygen gradients, which mimic in vivo-like features better than 2D cultures. MCTS can be used with matrix-free/matrix-based culture models for basic cancer research and pre-clinical research on brain cancer. Most scaffold-free culture models can be used to characterize MCTS39,47, or for drug screening.41,45 For instance, using a microfluidic device, uniformly sized MCTS were produced in mass and could be used for examining multiple-simultaneous drug treatment and testing drug responses.45 In addition, GBM spheroids were genetically more representative of the parental tumor profile than 2D cultures.47 Nevertheless, it is hard to replicate cell–ECM interactions or interactions with other cells (immune cells, fibroblast, etc.) within the MCTS. Therefore, matrix-based culture models have been developed to examine cell–ECM interactions and are introduced in the next section.
Matrix-based Culture Models
As mentioned above, the ECM of the brain has a composition distinct from that of peripheral tissues, with few fibrous proteins and large amounts of PG, GAG, and glycoproteins.12,16 Most of the brain parenchyma is composed of HA, which interacts with CD44 and RHAMM receptors, promoting the proliferation, invasion, and drug resistance of brain tumors.18,48,49 Moreover, there is accumulating evidence that specific ECM components such as HA, vitronectin, and tenascin-C are dysregulated in brain tumors, which may alter cellular invasiveness.10,26 Indeed, dysregulation of ECM remodeling is common in cancer and fibrosis.50 Therefore, it is important to examine the brain ECM features of tumors to understand brain tumor biology, including biophysical and biochemical characteristics.
To study the effects of biophysical cues on tumor cell behavior, it is important to control the mechanophysical properties of the 3D matrix, including stiffness, degradability, and pore size. Alginate,51 chitosan-alginate hydrogels,52 collagen-agarose hydrogels,53 matrix metalloproteinase (MMP)-degradable poly (ethylene glycol) (PEG) gels,54,55 gelatin methacrylate (GelMA),56 and HA-based hydrogels19,57 are used to investigate biophysical impacts on cancer cell behaviors in 3D brain tumor models. Use of these materials showed that biophysical cues play an important role in regulating brain tumor progression, including proliferation, gene expression, and invasion. For example, the increased MMP-degradable sites of PEG promote the invasion of GBM.55 U87R and U118 showed reduced migration distance on increasing the stiffness of HA-based hydrogels.57
HA-based hydrogels have been widely utilized in in-vitro 3D culture models to mimic the ECM components of the brain because HA is the most abundant component of the ECM of the brain. However, HA cannot form cross-links alone and must be mixed with chemically modified HA, such as thiolated57 or methacrylated18,19,58 HA, or with other materials, such as interpenetrating polymer networks.59,60 Within these HA-based hydrogels, brain tumor cells were not only highly invasive and proliferated via CD44-mediated adhesion,60 but also increased oncogenic markers.61
Advanced strategies to mimic the brain ECM have used decellularized matrix obtained from brain tissues to reconstruct in vitro models. Decellularization removes the cellular components from tissues or organs, leading to the production of cell- or tissue- derived ECM that preserve the complex mixture of in vivo ECM components and structure without antigenicity.62 In brain research, the decellularized porcine brain is widely used to reconstruct the brain ECM.63,64 Recently, an in vitro model that utilized patient-derived brain tissues and GBM was introduced.65 Within the decellularized ECM, GBM cells displayed heterogeneous invasion strategies and upregulated brain ECM-specific component-related genes, such as HA.
Conclusion
This review provided an overview of the latest in vitro brain tumor models used in biomedical research. The use of biomimetic in vitro brain tumor models enables the investigation and evaluation of the characteristics of brain tumors, such as invasion and proliferation, and could be applied to drug screening. For further progress, in vitro brain tumor models should incorporate other environmental factors, such as intratumoral heterogeneity, growth factors, and interactions with surrounding cells, to improve our understanding of the characteristics of brain tumors. In the future, we expect that the integration of various environmental components of the brain will enhance our understanding of brain tumor biology and inform the choice of ECM-targeted therapeutic options for patients.
References
Preusser, M. & Marosi, C. Neuro-oncology in 2016: Advances in brain tumour classification and therapy. Nat. Rev. Neurol. 13, 71–72 (2017).
Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820 (2016).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Bredel, M. Anticancer drug resistance in primary human brain tumors. Brain Res. Rev. 35, 161–204 (2001).
Yip, S. et al. MSH6 Mutations Arise in Glioblastomas during Temozolomide Therapy and Mediate Temozolomide Resistance. Clin. Cancer Res. 15, 4622–4629 (2009).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Sciences (N. Y.) 348 (2015).
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
Nelson, C. M. & Bissell, M. J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287–309 (2006).
Ruoslahti, E. Brain extracellular matrix. Glycobiology 6, 489–492 (1996).
Wiranowska, M. & Rojiani, M. V. in Glioma–Exploring Its Biology and Practical Relevance Glioma–Exploring Its Biology and Practical Relevance (ed Anirban Ghosh) Ch. 12, (InTech, 2009).
Ulrich, T. A., de Juan Pardo, E. M. & Kumar, S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167–4174 (2009).
Bellail, A. C., Hunter, S. B., Brat, D. J., Tan, C. & Van Meir, E. G. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int. J. Biochem. Cell Biol. 36, 1046–1069 (2004).
Quail, D. F. & Joyce, J. A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 31, 326–341 (2017).
Hickman, J. A. et al. Three-dimensional models of cancer for pharmacology and cancer cell biology: Capturing tumor complexity in vitro/ex vivo. Biotechnol. J. 9, 1115–1128 (2014).
Rich, J. N. & Bigner, D. D. Development of novel targeted therapies in the treatment of malignant glioma. Nat. Rev. Drug Discovery 3, 430–446 (2004).
Zamecnik, J. The extracellular space and matrix of gliomas. Acta Neuropathol. 110, 435–442 (2005).
Rape, A. D., Zibinsky, M., Murthy, N. & Kumar, S. A synthetic hydrogel for the high-throughput study of cell-ECM interactions. Nat. Commun. 6 (2015).
Kim, Y. & Kumar, S. CD44-Mediated Adhesion to Hyaluronic Acid Contributes to Mechanosensing and Invasive Motility. Mol. Cancer. Res. 12, 1416–1429, doi:10.1158/1541–7786.Mcr–13–0629 (2014).
Ananthanarayanan, B., Kim, Y. & Kumar, S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials 32, 7913–7923 (2011).
Pathak, A. & Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. U. S. A. 109, 10334–10339 (2012).
Thomas, T. W. & DiMilla, P. A. Spreading and motility of human glioblastoma cells on sheets of silicone rubber depend on substratum compliance. Med. Biol. Eng. Comput. 38, 360–370 (2000).
Grundy, T. J. et al. Differential response of patientderived primary glioblastoma cells to environmental stiffness. Sci. Rep. 6 (2016).
Payan, Y. & Ohayon, J. Biomechanics of living organs: hyperelastic constitutive laws for finite element modeling. pp. 127–146 (Academic Press, an imprint of Elsevier, 2017).
Giese, A. & Westphal, M. Glioma invasion in the central nervous system. Neurosurgery 39, 235–250 (1996).
Holland, E. C. Glioblastoma multiforme: The terminator. Proc. Natl. Acad. Sci. U. S. A. 97, 6242–6244 (2000).
Giese, A. et al. Migration of human glioma cells on myelin. Neurosurgery 38, 755–764 (1996).
Cha, J. et al. Tapered Microtract Array Platform for Antimigratory Drug Screening of Human Glioblastoma Multiforme. Adv. Healthcare Mater. 4 (2015).
Johnson, J. et al. Quantitative Analysis of Complex Glioma Cell Migration on Electrospun Polycaprolactone Using Time-Lapse Microscopy. Tissue Eng., Part C Methods. 15, 531–540 (2009).
Beliveau, A., Thomas, G., Gong, J. X., Wen, Q. & Jain, A. Aligned Nanotopography Promotes a Migratory State in Glioblastoma Multiforme Tumor Cells. Sci. Rep. 6 (2016).
Rao, S. S. et al. Mimicking white matter tract topography using core-shell electrospun nanofibers to examine migration of malignant brain tumors. Biomaterials 34, 5181–5190 (2013).
Baker, B. M. & Chen, C. S. Deconstructing the third dimension -how 3D culture microenvironments alter cellular cues. J. Cell Sci. 125, 3015–3024 (2012).
Mehta, G., Hsiao, A. Y., Ingram, M., Luker, G. D. & Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Controlled Release 164, 192–204 (2012).
Kim, J. B. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 15, 365–377 (2005).
Tibbitt, M. W. & Anseth, K. S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 103, 655–663 (2009).
Fischbach, C. et al. Engineering tumors with 3D scaffolds. Nat. Methods 4, 855–860 (2007).
Inch, W. R. Growth of Nodular Carcinomas in Rodents Compared with Multi-Cell Spheroids in Tissue Culture. Growth 34, 271–& (1970).
Sutherland, R. M., Inch, W. R., Mccredie, J. A. & Kruuv, J. A multi-component radiation survival curve using an in-vitro tumour model. Int. J. Radiat. Biol. Relat. Stud. Phys., Chem. Med. 18, 491–495 (1970).
Khaitan, D., Chandna, S., Arya, M. B. & Dwarakanath, B. S. Establishment and characterization of multicellular spheroids from a human glioma cell line; Implications for tumor therapy. J. Transl. Med. 4, 12 (2006).
Lin, R. Z. & Chang, H. Y. Recent advances in threedimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 3, 1172–1184 (2008).
Vinci, M. et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 10 (2012).
Tung, Y. C. et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 136, 473–478 (2011).
Del Duca, D., Werbowetski, T. & Del Maestro, R. F. Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J. Neuro-Oncol. 67, 295–303 (2004).
Wong, S. F. et al. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials 32, 8087–8096 (2011).
Fan, Y. T., Nguyen, D. T., Akay, Y., Xu, F. & Akay, M. Engineering a Brain Cancer Chip for High-throughput Drug Screening. Sci. Rep. 6 (2016).
Pampaloni, F., Reynaud, E. G. & Stelzer, E. H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845 (2007).
Hamer, P. C. D. W. et al. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene 27, 2091–2096 (2008).
Koochekpour, S., Pilkington, G. J. & Merzak, A. Hyaluronic acid/CD44H interaction induces cell detachment and stimulates migration and invasion of human glioma cells in vitro. Int. J. Cancer 63, 450–454 (1995).
Wiranowska, M., Tresser, N. & Saporta, S. The effect of interferon and anti-CD44 antibody on mouse glioma invasiveness in vitro. Anticancer Res. 18, 3331–3338 (1998).
Cox, T. R. & Erler, J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Models Mech. 4, 165–178 (2011).
Zustiak, S. P. et al. Three-Dimensional Matrix Stiffness and Adhesive Ligands Affect Cancer Cell Response to Toxins. Biotechnol. Bioeng. 113, 443–452 (2016).
Kievit, F. M. et al. Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials 31, 5903–5910 (2010).
Ulrich, T. A., Jain, A., Tanner, K., MacKay, J. L. & Kumar, S. Probing cellular mechanobiology in threedimensional culture with collagen-agarose matrices. Biomaterials 31, 1875–1884 (2010).
Wang, C., Tong, X. & Yang, F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEGbased hydrogels. Mol. Pharmaceutics 11, 2115–2125 (2014).
Wang, C., Tong, X., Jiang, X. & Yang, F. Effect of matrix metalloproteinase-mediated matrix degradation on glioblastoma cell behavior in 3D PEG-based hydrogels. J. Biomed. Mater. Res., Part A 105, 770–778 (2017).
Pedron, S. & Harley, B. A. Impact of the biophysical features of a 3D gelatin microenvironment on glioblastoma malignancy. J. Biomed. Mater. Res., Part A 101, 3404–3415 (2013).
Heffernan, J. M., Overstreet, D. J., Le, L. D., Vernon, B. L. & Sirianni, R. W. Bioengineered Scaffolds for 3D Analysis of Glioblastoma Proliferation and Invasion. Ann. Biomed. Eng. 43, 1965–1977 (2015).
Pedron, S., Becka, E. & Harley, B. A. Spatially Gradated Hydrogel Platform as a 3D Engineered Tumor Microenvironment. Adv. Mater. 27, 1567–+ (2015).
Rao, S. S. et al. Glioblastoma Behaviors in Three-Dimensional Collagen-Hyaluronan Composite Hydrogels. ACS Appl. Mater. Interfaces 5, 9276–9284 (2013).
Cha, J., Kang, S. G. & Kim, P. Strategies of Mesenchymal Invasion of Patient-derived Brain Tumors: Microenvironmental Adaptation. Sci. Rep. 6, 24912 (2016).
Pedron, S., Becka, E. & Harley, B. A. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials 34, 7408–7417 (2013).
Gilbert, T. W., Sellaro, T. L. & Badylak, S. F. Decellularization of tissues and organs. Biomaterials 27, 3675–3683 (2006).
Crapo, P. M. et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33, 3539–3547 (2012).
DeQuach, J. A., Yuan, S. H., Goldstein, L. S. & Christman, K. L. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng., Part A 17, 2583–2592, (2011).
Koh, I. et al. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci. Rep. 8 (2018).
Acknowledgments
This research was supported from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant number: HI14C1324).
Author information
Authors and Affiliations
Corresponding author
Conflict of Interests
Conflict of Interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Koh, I., Kim, P. In Vitro Reconstruction of Brain Tumor Microenvironment. BioChip J 13, 1–7 (2019). https://doi.org/10.1007/s13206-018-3102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-018-3102-6