Abstract
The role of plant growth-promoting rhizobacteria along with nanochitosan on maize productivity remains unexplored. In the present study we report the effect of nanochitosan and PGPR on growth, productivity and mechanism(s) involved in defence response in Zea mays under field conditions. Application of nanochitosan (50 mg L−1) along with plant growth-promoting rhizobacteria enhanced seed germination, plant height, root length, leaf area, fresh and dry weight of shoot and root, chlorophyll, carotenoids, total sugar and protein content upto 1.5–2 fold over control in maize after 60 days of the field experiment. Treated maize plants also showed enhanced level of defence-related biomolecules like phenolic compounds (103%), catalase (60.09%), peroxidase (81.57%) and superoxide dismutase (76.50%) over control. Level of phenols and sugar content in maize plants enhanced which was analysed by GC–MS (Gas chromatography–mass spectrometry). Significant increase in cob length, cob weight/plot, grain yield/plot and 100 grain weight was observed in treated maize plants over control. As per the results, the combination of nanochitosan and plant growth-promoting rhizobacteria was reported to improve the health and yield of maize. The interaction can be further studied in field trials for improvement in agriculture production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Corn (Zea mays L), an important cereal crop grown worldwide is known as the queen of cereal crops. It has an adequate short growing period and produces a high yield. It ranks third in terms of production following rice and wheat globally (Raji, 2003). Complete understanding of microbial ecology and diversity linked with maize rhizosphere can be exploited to improve plant health. This practice may reduce our dependence on chemical fertilizers and support to develop a sustainable approach for enhanced crop yield (Filion et al. 2004).
Plant growth-promoting rhizobacteria (PGPR), a discursive group of bacteria found in the rhizosphere at the rhizoplane or in association with roots, enhance plant growth directly or indirectly (Ahmad et al. 2008). Different bacterial genera like Arthrobacter, Azoarcus, Azotobacter, Agrobacterium, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Enterobacter, Erwinia, Flavobacterium, Klebsiella, Micrococcous, Rhizobium, Pantoea, Pseudomonas and Serratia with numerous plant growth-promoting characteristics have been reported by a number of authors. These PGPR are used as biocontrol and/or plant growth promontory agents in different crops as they protect plants from fungal attack or facilitate the availability of essential nutrients to the plants for enhanced growth (Ahemad and Kibret 2014; Bruto et al. 2014; Chaudhary and Sharma 2019).
Nanotechnology and nano-enabled products have received much attention in recent times due to their remarkable properties. Nanocompounds have observed numerous applications in different sectors including agriculture. The improved and unique properties of nanocompounds are related to their high surface-to-volume ratio, size-dependent qualities and unique optical properties (Saharan et al. 2015). Chitosan-based nanoparticles are being used worldwide for various purposes due to their low cost, biodegradability, solubility, high absorptive nature and non-toxicity to humans (Bueter et al. 2013; Manikandan and Sathiyabama 2015). Application of NPs, planned for sustainable crop production decreases nutrient loss, prevents disease development and improves yield. Chitosan is used in agriculture for seed treatment and as a biopesticide which helps plants to fight against fungal infections. Uptake efficiency and effect of nanocompound(s) on the growth and metabolic functions may vary among different plants. Concentration of nanocompounds affects seed germination and plant growth (Zheng et al. 2005; Li et al. 2016). The chelating property of chitosan towards various organic and inorganic compounds makes it a suitable biopolymer for improved stability, solubility and biocidal activity in agriculture practices (Shukla et al. 2013). Chandra et al. (2015) reported that the application of nanochitosan on Camellia sinensis leaves showed enhanced defence response and accumulation of defence enzymes. Chitosan has been recognized as one of the most important elicitors of plant defence (Li et al. 2009). It affects different physiological responses like membrane permeability, production of antioxidant enzymes (superoxide dismutase, catalase and peroxidase), biosynthesis of jasmonic acid, lignification and ion flux, etc. in plants. Chitosan elicits plant defence response against a wide range of phytopathogens, including plant viruses (Terry and Joyce 2004). Oligochitosan is reported to induce accumulation of H2O2 in plants which takes part in oxidative burst and in the induction of ROS scavenging system. Enzymatic systems consisting of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) are responsible for scavenging of ROS in plants (Agrawal 2002; Lin et al. 2005; Ahmad et al. 2013). According to Chaudhary et al. (2021b), metagenomic analysis of soil under maize cultivation revealed that the application of nanochitosan and Bacillus sp. improved the population of beneficial microbes.
A preliminary pot trial by the same group under the same treatment (Bacillus spp. and nanochitosan) in maize was significantly effective in terms of plant health and microbial diversity of maize rhizosphere. Experimental trial of nanocompounds in combination with plant growth-promoting rhizobacteria has not been conducted under field conditions on maize hence present study aims to investigate the combined effect of PGPR and nanochitosan on plant health parameters, defence response and yield of maize plants under field condition.
Materials and methods
A field experiment on maize was conducted in year 2017 (June–September) at Crop Research Center (CRC) of G.B. Pant University of Agriculture & Technology, Pantanagar (location of 29 °N latitude and 79.3 °E longitude). The experimental site lies in Tarai plains which is about 30 km Southward of foothills of “Shivalik” range of the Himalayas and at 243 m above sea level. In this zone, summers are warm and hot. Generally, South West monsoon commences in the second or third week of June and continues upto September.
The experimental soil was classified under the subgroup Aquic hapludoll in order Mollisols. The soil belongs to silt clay type and has pH 7.4 and has Electrical Conductivity- 0.206 (dS/m), Organic carbon (%) 0.78, available nitrogen (215.79 kg/ha), available phosphorus (27 kg/ha) and available potassium (136 kg/ha) (Chaudhary et al. 2021a). The experiment was carried out using a randomized block design (RBD) with three replications. In each replication, seeds were sowed at 5 cm depth and the area of each plot was 14.70 m2, where distance between row to row was 60 cm and plant to plant was 20 cm. Each plot had six rows. Total six treatments comprised of uninoculated control, PS2 (Bacillus sp.), PS10 (Bacillus sp.), nanochitosan (Nch), PS2 with nanochitosan (PS2 + Nch) and PS10 with nanochitosan (PS10 + Nch) were used in this experiment.
Biological material and chemicals used
Based on 16S rDNA sequencing, bacterial cultures (PS2-KX650178 and PS10- KX650179) used in field experiment were characterized as Bacillus spp. (Khati et al. 2019a). These bacterial isolates with potential plant growth-promoting activities enhanced plant and soil health parameters in the presence of nanochitosan (50 mg L−1) in our earlier studies on maize (Khati et al. 2017). Nanochitosan used in the present study was purchased from Intelligent Material Pvt. Ltd India. The size of nanochitosan was < 80 nm, pH- 7–9, refractive index- 1.47 and purity > 99%. Chemicals for enzyme assay and other experiments were purchased from SRL and Hi media Laboratories Pvt. Ltd. India. Seeds of maize variety ‘DH296’ were kindly provided by the Department of Plant Breeding and Genetics of the University.
Plant material and growth conditions
Maize seeds were washed thoroughly with tap water. Floating seeds were discarded and healthy seeds were surface sterilized for 2 min in 0.1% mercuric chloride (HgCl2) solution and then rinsed three times with sterilized distilled water to eliminate the residual traces of HgCl2.
Seed bacterization For seed bacterization, 1% carboxy methyl cellulose (CMC) was added in overnight grown bacterial culture(s) having 0.6 O.D (optical density) at 600 nm. Maize seeds soaked in bacterial culture (PS2 or PS10 or the treatments consisted of bacteria and nanochitosan (50 mg L−1) had bacterial counts in the range of 3 × 108 cfu seed−1. Control did not have either culture or nanocompound. Treated seeds were incubated at 25 °C on a rotary shaker at 70 rpm for 15 min in the flasks to allow proper adherence of bacterial cells onto the seeds. After incubation, seeds were dried and then sown in the field.
Measurement of plant health parameters
Percent seed germination from different plots was evaluated by using following formula:
Germination percentage of seeds
Plant parameters
To check agronomical and biochemical analysis of plant parameters, four maize plants from each plot (total 12 plants) were gently removed after 20, 40 and 60 days after sowing (DAS) and used after washing with running water. Data were recorded for total leaf number, leaf area calculated by using the formula (0.75 × length × breadth of leaf), root and shoot fresh weight. Fresh weights of shoots and roots were determined directly after removing the plants from the plot. To measure dry weight, plant material was dried in an oven at 80 °C until a constant weight was obtained.
Total chlorophyll
Chlorophyll content of maize leaves after 20, 40 and 60DAS was estimated according to the method of Hiscox and Israelstam (1979). To extract chlorophyll, healthy maize leaves were washed with deionized water to remove surface contamination. Then 50 mg of leaves were cut into small pieces and placed in test tubes containing 10 ml dimethyl sulfoxide (DMSO). Tubes were kept in water-bath for 3 h at 60 °C till leaves became colourless. After filtration, the extract was maintained at room temperature. The absorbance of the extract was taken at 663 and 645 nm using a visible spectrophotometer (Labtronics Model LT-39). DMSO was used as a blank, and the amount of total chlorophyll in the extract was calculated in mg g−1 of leaves using the following formula:
Carotenoid content
Same leaf extract was used to estimate carotenoid content. Absorbance of the extract was taken at 470 nm using a spectrophotometer (Kirk and Allen 1965). Carotenoid content was calculated using following formula.
Total sugar content
To estimate total sugar, fresh leaves were dried in a hot air oven at 800C for 48 h and then 0.1 g dried leaves were powdered with the help of mortar and pestle. Powdered leaves were added to 3 ml of 80% ethyl alcohol, boiled in water bath and then centrifuged for 15 min at 1000 rpm. The supernatant was taken in a test tube and the final volume was made 6 ml with 80% ethyl alcohol. To 1 ml ethanolic leaf extract, 4 ml ice-cold Anthrone reagent was added. The mixture was shaken properly and boiled in the water bath for 10 min. After cooling, absorbance was recorded at 620 nm. The amount of total sugar was estimated using a standard graph prepared by taking glucose in the range of 10–100 µg ml−1 (Dubois et al. 1956).
Protein estimation
For protein content, thoroughly washed fresh maize leaves were transferred to a clean mortar and pestle. After adding 5 ml of 0.2 M Tris–Cl (pH-8), leaves were crushed gently for 20 min to get fine slurry. The obtained slurry was centrifuged at 10,000 rpm at 4 °C for 20 min. The supernatant was transferred to a fresh tube and stored at 40C for further use. Protein was estimated according to Bradford (1976). To estimate protein content, 20 µl supernatant was mixed with extraction buffer (280 µl) to which 3 ml Coomassie brilliant blue (CBB) G-250 was added. The mixture was kept at 37 °C for 5 min and absorbance was read at 595 nm in a spectrophotometer against a reagent blank. The amount of protein was calculated using a standard curve prepared with different concentrations of BSA (10-100 µg ml−1).
Estimation of total phenolic content (TPC)
Total phenolic content of maize leaves was estimated according to the method of Ainsworth and Gillespie (2007). Plant leaves (200 mg) were homogenized in 800 µl ice cold 95% methanol in a cold mortar and pestle. Homogenized leaves were incubated for 48 h in dark at room temperature and then centrifuged at 10,000 rpm for 5 min. Obtained supernatant was used to determine total phenolic content by the Folin-Ciocalteu method. Gallic acid (10–100 µg ml−1) was used as a standard, and total phenolic contents were expressed as mg g−1 leaves.
Analysis of antioxidant enzymes
Fresh maize leaves (1 g) from all the treatments were homogenized separately in 3 ml of 50 mM sodium phosphate buffer (pH-7) in an ice cold mortar and pestle. The obtained slurry was centrifuged at 10,000 rpm at 4 °C for 20 min and supernatant was kept under the refrigerated condition to estimate enzyme activities.
Catalase activity (CAT)
Catalase activity was determined according to the method described by Chandlee and Scandalios (1984). The reaction was initiated by adding 100 μl of enzyme extract to 3 ml reaction mixture containing 100 mM sodium phosphate buffer (pH-7) and 0.1 ml of 10 mM H2O2. A decrease in optical density was monitored at 230 nm for 3 min in a spectrophotometer. Assay mixture without enzyme extract served as control. CAT activity was calculated by using an extinction coefficient of 39.4 mM −1 cm−1 and enzyme activity was expressed as decomposition of 1 mM of H2O2 min−1.
Peroxidase activity (POD)
Peroxidase activity was determined according to the method of Mali et al. (1989). For enzyme assay, 3 ml reaction mixture containing 0.4 ml of pyragallol in phosphate buffer, 0.1 ml enzyme extract and 0.5 ml H2O2 was added in a cuvette and change in absorbance at 420 nm was noted at an interval of 15 s for a period of 3 min. Reaction mixture without enzyme extract served as control. POD activity was calculated by using extinction coefficient of 26.6 mM−1 cm−1.
Superoxide dismutase activity (SOD)
The method of Giannopolitis and Ries (1977) was used to estimate SOD activity. For enzyme activity, reaction mixture was prepared by using 100 mM phosphate buffer (pH-7.5), EDTA (3 mM), methionine (200 mM), riboflavin (75 mM) and enzyme extract (100 µl). SOD enzyme activity was expressed as units of enzyme g-1 FW.
Gas chromatography–mass spectroscopy (GC–MS)
For GC–MS analysis, maize leaves from the treated and control samples were shade dried for 20 days and crushed in methanol for extraction. After centrifugation at 10,000 rpm extract was used for GC-MS analysis. GC–MS (Shimadzu GC–MS QP Ver. 2010) was performed using silica column (31 m 90.25 mm at 60 °C) at a flow rate of 1 ml min−1 and injection temperature was raised to 270 °C. The organic compounds present in different samples were identified by comparing the mass spectrum matched with the inbuilt library (Wiley 8).
Crop yield
Observations on cob length, cob weight/plot, grain yield/plot and 100 grain weight were recorded at the time of harvesting after 85 days of sowing.
Statistical analysis
Data were analysed using two-way analysis of variance (ANOVA) by Statistical Package for the Social Sciences (SPSS) software 16.0. The difference between means was evaluated for significance by putting Duncan’s multiple comparison tests (P < 0.05). Data represented in the tables and figures are expressed as means of three replicates ± standard deviation (SD).
Results
Effect of PGPR and nanochitosan on seed germination
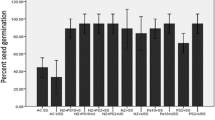
It is evident from Table 1 that application of nanochitosan and PGPR stimulated percent seed germination in maize. Maximum percent germination of 94.52 and 95.23% was recorded in the combined treatments consisted of bacterial culture(s) and nanochitosan respectively. Percent seed germination was significantly higher in the combined treatment than the control and other treatments.
Analysis of agronomical parameters
Maximum plant height was observed in the combined treatment of PGPR and nanochitosan. Percent increase in plant height was in the range of 25.21 in PS10 + Nch, 23.90 in PS2 + Nch, 14.41 in Nch, 12.63 in PS10 and 11.62 in PS2 treated soil over control after 20 days of the experiment. An increase in plant height was more in all the treated plants till the end of the experiment as compared to control (Table 1).
In the present study, root length was significantly better in treated plants as compared to control. It is evident from the observations that root parameters like root length and root biomass at 20, 40 and 60 DAS were significantly higher in maize with the application of PGPR along with nanochitosan than control. An increase of 29.95, 46.67, 49.25, 77.95 and 90% in root length was observed in PS2, PS10, Nch, PS2 + Nch and PS10 + Nch treatments respectively as compared to control after 60 days (Table 1).
Number of leaves per plant was high in all the treated plants. The combined treatment of PGPR and nanochitosan showed a maximum increase in leaf number. An increase in leaf area was also observed in all the treated plants in comparison to control. The highest leaf area (10.28% and 10.98%) over control was observed in PS2 and PS10 treatments respectively when applied with nanochitosan. Order of percent increase in root fresh weight in all the treatments over control was: Nch (29.91%), PS2 + Nch (15.54%) and PS10 + Nch (16.41%). Application of individual bacterial cultures PS2 and PS10 showed 8.31% and 9.99% increase in root fresh weight than control. Percent fresh and dry weight of shoot/root was significantly higher in all the treated plants than control (Tables 2, 3).
Analysis of biochemical parameters
All the treated plants showed a significant increase in chlorophyll content as compared to control (Table 4). An increase of 2.22 and 2.26 fold over control was observed in PS2 + Nch and PS10 + Nch treatments respectively. Other treatments like PS2, PS10 and nanochitosan showed 1.75, 1.80 and 1.85 fold increase in chlorophyll as compared to control. An increase in carotenoid content was also observed in treated plants as compared to control. PS2, PS10, Nch, PS2 + Nch and PS10 + Nch showed 1.50, 1.51, 1.54, 1.67 and 1.70 fold increase in carotenoid content respectively as compared to control (Table 4).
Total sugar content was highest (1.82 and 1.84 fold as compared to control) in the maize plants under the combined treatment of nanochitosan with PS2, PS10. On the other hand individual treatment(s) of PS2, PS10 and nanochitosan showed 1.49, 1.56 and 1.43 fold increases in total sugar respectively over control in maize (Table 4). Protein content was also high in all the treated plants. PS2, PS10, Nch, PS2 + Nch and PS10 + Nch treatments showed 1.43, 1.46, 1.48, 1.72 and 1.75 fold increase respectively in protein level as compared to control after 60 days of the experiment (Table 5). Total phenolic content in maize plants was also highest in the combined treatment of nanochitosan and PGPR. The increase was 1.88 and 1.89 fold in PS2 + Nch and PS10 + Nch treatments respectively, 1.61 fold in Nch and 1.57 and 1.54 fold in PS2 and PS10 respectively as compared to control (Table 5).
Analysis of antioxidant enzymes
Application of nanochitosan and PGPR showed a significant increase in innate immune response in maize plants by inducing catalase, peroxidase and SOD activities throughout the experimental period. Observations on enzyme activities (CAT, POD and SOD), taken after 20, 40 and 60DAS were significantly high in all the treated plants as compared to control and the order was SOD > POD > CAT. PS2, PS10, Nch, PS2 + Nch and PS10 + Nch treatments showed 1.17, 1.19, 1.19, 1.52 and 1.54 fold increase in CAT activity respectively as compared to control after 60 days. Similarly, PS2, PS10, Nch, PS2 + Nch and PS10 + Nch treatments showed 1.30, 1.34, 1.38, 1.74 and 1.82 fold increase respectively in POD activity as compared to control. A linear increase in SOD activity was also observed under the treatments of PS2, PS10, Nch, PS2 + Nch and PS10 + Nch and the increase was 1.20, 1.21, 1.26, 1.70 and 1.75 fold respectively over control (Table 6).
GC–MS analysis of plant metabolites
Results of GC–MS analysis revealed an increased level of phenols, acid esters, ketones and sugar in treated plant samples over control (Fig. 1, SM1). These chemicals were found responsible for stress tolerance in plants caused by environmental factors or by exposure to any biotic/abiotic stress.
Crop yield
Cob length, cob weight/plot, grain yield/plot and 100 grain weight were high in all the treated maize plants (Fig. 2). Maximum cob length was observed in PS2 + Nch and PS10 + Nch treatments. 100 grain weight was also significantly influenced by the combination of nanochitosan along with PGPR.
Discussion
The positive effect of nanocompounds on seed germination can be explained on the basis of the role of nanocompounds in regulating aquaporins, the water channels, which regulate water permeability and play important role in seed germination and plant growth (Heinen et al. 2009; Khodakovskaya et al. 2011). Ma et al. (2014) reported that oligochitosan promoted wheat growth in terms of germination, root length, seedling height and increase in root activity. Studies revealed that the application of Cu-chitosan NPs enhanced the growth of maize seedlings by mobilizing reserve food through the enhanced activities of α-amylase and protease enzymes (Saharan et al. 2016).
Plant growth-promotory activities of Bacillus spp. used in the present study supported plant vigour by providing minerals and nutrition to maize plants through enhanced solubilization (Agri et al. 2021). An increase in plant height may be related to increased level of gibberellic acid which is responsible for shoot elongation (Stepanova et al. 2007). Song et al. (2012) reported that an increase in root and shoot length and biomass was high when Brassica juncea plants were treated with 500, 1000, 1500 mg L−1 of TiO2. Increased root length and biomass could be related to the application of nanochitosan which supported the growth of Bacillus spp. around the root zone and helped in cell elongation and multiplication due to enhanced nutrient uptake. Khati et al. (2019b) reported that presence of nanozeolite supported beneficial bacterial spp. in the rhizospheric soil of maize. Application of PGPRs and titania nanoparticles in wheat crop showed enhanced seedling health and plant biomass under the condition of drought and salt stress (Timmusk et al. 2018). Similar findings (soil and plant health) were reported by Sillen et al. (2015) on applying AgNPs in maize.
Gulnaz et al. (2017) observed higher root length, root biomass, root volume at 30, 60 and 90 DAS with the application of PGPRs along with phosphorus fertilizer. Application of Cu-chitosan NPs (250 mg Kg−1) showed increased plant height, stem diameter, root length, root number and chlorophyll content in Zea mays (Choudhary et al. 2017). Leaf area and leaf number of the plants are regulated by a complex interaction of various genes under the influence of ethylene, an important growth hormone (Gonzalez et al. (2010). Similar findings were observed by Hediat (2012), when maize plants were treated with 60 ppm silver nanoparticles. Percent fresh and dry weight was significantly higher in the treated plants. According to Ghafariyan et al. (2013), exposure of supermagnetic iron oxide nanoparticles also improved chlorophyll contents in soybean.
Photosynthesis is an important metabolic process and can be directly related to the growth and productivity of green plants. Chlorophyll is present in the membrane of chloroplast and is considered as the major light-harvesting complex of green plants by taking part in the electron transport mechanism. Carotenoids act as “accessory” pigment and play important role in photosynthetic energy transduction and protect chlorophyll by preventing the formation of singlet species. Chlorophyll and carotenoid content were high in all the treated plants as compared to control. This possibly could be due to the enhancement in the level of photosynthates (Urbonaviciute et al. 2006). Application of 0.01 and 0.12% of Cu-nanochitosan in maize showed an increase of 10.58–16.22 mg g−1 in chlorophyll content respectively (Choudhary et al. 2017). Similarly, Tarafdar et al. (2013) reported 276.2% enhancement in chlorophyll content in cluster bean as compared to control when provided 10 ppm ZnO. Our results are comparable to the findings of Javad et al. (2014) who reported a significant increase in carotenoids in maize when treated with SiO2 nanoparticles (400 mg L1).
Total sugar, phenolic and protein content of the maize plants, treated with nanochitosan and PGPRs were high as compared to control. This might be due to the defensive mechanism of nanochitosan and Bacillus sp.. Similar results were observed by Hediat (2012) when maize plants were treated with 60 ppm silver nanoparticles. Application of low concentration of nanocalcium carbonate enhanced soluble sugar in peanut (Lin et al. 2005). Hediat (2012) tested the effect of silver nanoparticles at different concentrations (20, 40 and 60 ppm) on Zea mays and Phaseolus vulgaris and found a 30% increase in protein content of common bean and 24% increase in maize at 60 ppm. Kukreti et al. (2020) also observed an increase in protein content in maize plants, when treated with nanosilicon dioxide and PGPRs at 10 ppm concentration after 15 and 30 days respectively. Same pattern of phenols was observed by Chandra et al. (2015) when tea plants were treated with nanochitosan and chitosan. They reported 24 and 20% increase in phenolic compound as compared to control under the treatment of nanochitosan and chitosan respectively.
Combined treatment of nanochitosan and PGPR in maize plants showed highest activity of CAT, POD and SOD after 60 days of the experiment. Our findings can be correlated with the results reported by Siddaiah et al. (2018), who observed that application of nanochitosan (250 mg Kg−1) protected pearl millet from downy mildew by upregulation of activities of CAT, POD and SOD by enhancing 2.59, 3.29 and 3.09 fold activities respectively as compared to control. Ortega-Ortíz et al. (2007) showed that the application of chitosan and salicylic acid can induce CAT activity in tomato fruits. Increased expression of SOD and CAT due to chitosan or Chitosan NP treatment might provide protection to the plants from the oxidative stress associated with pathogenic invasion. It is reported that Pseudomonas fluorescens supported catalase and peroxidase activities in the leaves, stem and roots of Catharanthus roseus (Jaleel et al. 2010). Yin et al. (2008) reported upregulation of PAL, PPO, POX, CAT and SOD activities in tobacco and Brassica napus under the treatment of oligochitosan. Our results are comparable with the findings of various authors who have suggested that PGPR and nanochitosan perform better in combination as compared to individual treatment(s) and showed a significant effect on antioxidant enzymes as compared to control.
GC–MS results revealed an increased level of phenols, acid esters, ketones and sugar in treated maize plants over control. These compounds have been found responsible for stress reduction under harsh environments or by exposure to any biotic/abiotic stress. This observation can be correlated to better stress adaptation by maize plants in the presence of nanochitosan with PGPR (Suriyaprabha et al. 2012; Kumari et al. 2020).
Cob length, cob weight/plot, grain yield/plot and 100 grain weight were also high in all the treated plants. 100 grain weight was significantly influenced by the combined treatment of nanochitosan and PGPR. This could be related to an enhanced level of biochemical parameters of maize plants which resulted in higher yield. Siddaiah et al. (2018) also reported enhanced values of cob length, cob weight/plot, grain yield/plot and 100 grain weight of maize plants when treated with Cu-chitosan.
Conclusion
We report the effect of combined application of nanochitosan and Bacillus spp. on plant vigour, antioxidant enzymes and yield of maize plants under field conditions. Results showed that combined application of PGPR and nanochitosan in maize plants significantly improved plant health parameters like biomass, photosynthetic pigments, sugar, protein, phenolic content and also enhanced the activities of antioxidant enzymes which provided an innate immune response to the plants and helped in higher productivity as compared to control. Nanochitosan and PGPR have been proved to be a promising agent for plant protection and sustainable growth. Our observations on the biochemical and physiological aspects provide useful information for the safe application of nanochitosan along with PGPR in agriculture practices.
Abbreviations
- PGPR:
-
Plant growth-promoting rhizobacteria
- GC–MS:
-
Gas chromatography–mass spectrometry
- NPs:
-
Nanoparticles
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
- ROS:
-
Reactive oxygen species
- DMSO:
-
Dimethyl sulfoxide
- CBB:
-
Coomassie brilliant blue
- PAL:
-
Phenylalanine ammonia lyase
- PPO:
-
Polyphenol oxidase
- SiO2 :
-
Silicon dioxide
- ZnO:
-
Zinc oxide
- TiO2 :
-
Titanium oxide
- HgCl2 :
-
Mercuric chloride
References
Agrawal GK, Rakwal R, Tamogami S, Yonekura M, Kubo A, Saji H (2002) Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol Biochem 40:1061–1069. https://doi.org/10.1016/S0981-9428(02)01471-7
Agri U, Chaudhary P, Sharma A (2021) In vitro compatibility evaluation of agriusable nanochitosan on beneficial plant growth-promoting rhizobacteria and maize plant. Natl Acad Sci Lett. https://doi.org/10.1007/s40009-021-01047-w
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud University Sci 26(1):1–20. https://doi.org/10.1016/j.jksus.2013.05.001
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163(2):173–181. https://doi.org/10.1016/j.micres.2006.04.001
Ahmad I, Basra SMA, Afzal I, Farooq M, Wahid A (2013) Growth improvement in spring maize through exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide. Int J Agric Biol 15:95–100
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bruto M, Prigent-Combaret C, Muller D, Moënne-Loccoz Y (2014) Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci Rep 4:6261. https://doi.org/10.1038/srep06261
Bueter CL, Specht CA, Levitz SM (2013) Innate sensing of chitin and chitosan. PLoS Pathog 9:e1003080. https://doi.org/10.1371/journal.ppat.1003080
Chandlee JM, Scandalios JG (1984) Analysis of variants affecting the catalase developmental program in maize scutellum. Theoret Appl Genetics 69:71–77. https://doi.org/10.1007/BF00262543
Chandra S, Chakraborty N, Dasgupta A, Sarkar J, Panda K, Acharya K (2015) Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep 5:15195. https://doi.org/10.1038/srep15195
Chaudhary P, Sharma A (2019) Response of nanogypsum on the performance of Plant Growth Promotory Bacteria recovered from nanocompound infested agriculture field. Environ Ecol 37(1B):363–372
Chaudhary P, Khati P, Chaudhary A, Gangola S, Kumar R, Sharma A (2021a) Bioinoculation using indigenous Bacillus spp. improves growth and yield of Zea mays under the influence of nanozeolite. 3Biotech. 11:11. https://doi.org/10.1007/s13205-020-02561-2
Chaudhary P, Sharma A, Chaudhary A, Sharma P, Gangola S, Maithani D (2021b) Illumina based high throughput analysis of microbial diversity of rhizospheric soil of maize infested with nanocompounds and Bacillus sp. Appl Soil Ecol 159:103836. https://doi.org/10.1016/j.apsoil.2020.103836
Choudhary RC, Kumaraswamy VR, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V (2017) Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci Rep 7:9754. https://doi.org/10.1038/s41598-017-08571-0
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Filion M, Hamelin RC, Bernier L, St-Arnaud M (2004) Molecular profiling of rhizosphere microbial communities associated with healthy and diseased black spruce (Picea mariana) seedlings grown in a nursery. Appl Environ Microbiol 70(6):3541–3551. https://doi.org/10.1128/AEM.70.6.3541-3551.2004
Ghafariyan HM, Malakouti JM, Dadpour M, Stroeve P, Mahmoudi M (2013) Effects of magnetite nanoparticles on soybean chlorophyll. Environ Sci Technol. https://doi.org/10.1021/es402249b
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gonzalez P, Neilson RP, Lenihan JM, Drapek RJ (2010) Global patterns in the vulnerability of ecosystems to vegetation shifts due to climate change. Global Ecol Biogeograph 10:1–14. https://doi.org/10.1111/j.1466-8238.2010.00558.x
Gulnaz Y, Fathima PS, Denesh GR, Kulmitra KA, Kumar HS (2017) Effect of plant growth promoting rhizobacteria (PGPR) and PSB on root parameters, nutrient uptake and nutrient use efficiency of irrigated maize under varying levels of phosphorus. J Entomol Zool Studies 5(6):166–169
Hediat MHS (2012) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res J Biotechnol. 3(10):190–197
Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. J Exp Bot. 11:2971–2985. https://doi.org/10.1093/jxb/erp171
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf and tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
Jaleel CA, Salem MA, Hasanuzzaman M, Nahar K (2010) Plant growth regulator interactions results enhancement of antioxidant enzymes in Catharanthus roseus. J Plant Interactions. https://doi.org/10.1080/17429140903377456
Javad SR, Javed K, Mohsenzadeh S, Majid SR, Javad M (2014) Evaluating SiO2 nanoparticles effects on developmental characteristic and photosynthetic pigment contents of Zea maysL. Bull Environ, Pharmacol Life Sci. 3:6
Khati P, Chaudhary P, Gangola S, Bhatt P, Sharma A (2017) Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3Biotech. 7:81. https://doi.org/10.1007/s13205-017-0668-y
Khati P, Parul, Bhatt P, Nisha, Kumar R, Sharma A (2018) Effect of nanozeolite and plant growth promoting rhizobacteria on maize. 3Biotech. 8:141. https://doi.org/10.1007/s13205-018-1142-1
Khati P, Chaudhary P, Gangola S, Sharma A (2019a) Influence of nanozeolite on plant growth promotory bacterial isolates recovered from nanocompound infested agriculture field. Environ Eco 37(2):521–527
Khati P, Sharma A, Chaudhary P, Singh AK, Gangola S, Kumar R (2019b) High- throughput sequencing approach to access the impact of nanozeolite treatment on species richness and evens of soil metagenome. Biocatalysis Agric Biotechnol 20:101249. https://doi.org/10.1016/j.bcab.2019.101249
Khodakovskaya MV, Silva K, Nedosekin DA, Dervishi E, Biris AS, Shashkov EV, Galanzha EI, Zharov VP (2011) Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc Natl Acad Sci U S A 108(3):1028–1033. https://doi.org/10.1073/pnas.1008856108
Khodakovskaya MV, de Silva K, Biris AS, Dervishi E, Villagarcia H (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano. https://doi.org/10.1021/nn204643g
Kirk JOT, Allen RL (1965) Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun 21(6):523–530. https://doi.org/10.1016/0006-291x(65)90516-4
Kukreti B, Sharma A, Chaudhary P, Agri U, Maithani D (2020) Influence of nanosilicon dioxide along with bioinoculants on Zea mays and its rhizospheric soil. 3Biotech. 10:345. https://doi.org/10.1007/s13205-020-02329-8
Kumari S, Sharma A, Chaudhary P, Khati P (2020) Management of plant vigor and soil health using two agriusable nanocompounds and plant growth promontory rhizobacteria in Fenugreek. 3Biotech. 10:461. https://doi.org/10.1007/s13205-020-02448-2
Li Y, Yin H, Wang Q, Zhao X, Du Y, Li F (2009) Oligochitosan induced Brassica napus L. production of NO and H2O2 and their physiological function. Carbohyd Polym 75:612–617. https://doi.org/10.1016/j.carbpol.2008.09.005
Li J, Hu J, Ma C, Wang Y, Wu C, Huang J, Xing B (2016) Uptake, translocation and physiological effects of magnetic iron oxide (g-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 10(05):083. https://doi.org/10.1016/j.chemosphere.2016.05.083
Lin W, Hu X, Zhang W, Rogers WJ, Cai W (2005) Hydrogen peroxide mediates defense responses induced by chitosan of different molecular weights in rice. J Plant Physiol 162:937–944. https://doi.org/10.1016/j.jplph.2004.10.003
Ma LJ, Li YY, Yu CM, Wang Y, Li XM, Li N (2014) Germination and physiological response of wheat (Triticum aestivum) to pre-soaking with oligochitosan. Int J Agric Biol 16:766–770
Mali PC, Vyas SP, Satish LL (1989) Biochemical components of clusterbean genotypes in relation to bacterial blight. Indian Phytopathol 42:559–561
Manikandan A, Sathiyabama M (2015) Green synthesis of copper chitosan nanoparticles and study of its antibacterial activity. Nanomed Nanotech 6:1–5. https://doi.org/10.4172/2157-7439.1000251
Ortega-Ortíz H, Benavides-Mendoza A, Mendoza-Villarreal R, Ramírez-Rodríguez H, Alba-Romenus K (2007) Enzymatic activity in tomato fruits as a response to chemical elicitors. J Mexican Chem Soc 51:141–144
Raji JA (2003) Intercropping soybean and maize in a derived savanna echology. African J Biotechnol 6(16):1885–1887
Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A (2015) Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol 75:346–353. https://doi.org/10.1016/j.ijbiomac.2015.01.027
Saharan V, Kumaraswamy RV, Choudhary RC, Kumari S, Pal A, Raliya R, Biswas P (2016) Cu-Chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J Agric Food Chem 64:6148–6155
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: a state of the art review. Int J Biol Macromol 59:46–58. https://doi.org/10.1016/j.ijbiomac.2013.04.043
Siddaiah CN, Prasanth KVH, Satyanarayana NR, Mudili V, Gupta VK, Kalagatur NK, Satyavati T, Dai X-F, Chen J-Y, Mocan A, Singh BP, Srivastava RK (2018) Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci Rep 8:2485. https://doi.org/10.1038/s41598-017-19016-z
Sillen WM, Thijs S, Abbamondi GR, Janssen J, Weyens N, White JC, Vangronsveld J (2015) Effects of silver nanoparticles on soil microorganisms and maize biomass are linked in the rhizosphere. Soil Biol Biochem 91:14–22. https://doi.org/10.1016/j.soilbio.2015.08.019
Song G, Gao Y, Wu H, Hou W, Zhang C, Ma H (2012) Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ Toxicol Chem 31:2147–2152. https://doi.org/10.1002/etc.1933
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19:2169–2185. https://doi.org/10.1105/tpc.107.052068
Suriyaprabha R, Karunakaran G, Yuvakkumar R, Prabu P, Rajendran V, Kannan N (2012) Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J Nanoparticle Res 14:1294–1296. https://doi.org/10.1007/s11051-012-1294-6
Tarafdar JC, Sharma S, Raliya R (2013) Nanotechnology: interdisciplinary science of applications. African J Biotechnol 12(3):219–226. https://doi.org/10.5897/AJB12.2481
Terry LA, Joyce DC (2004) Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Post Harvest Biol Technol 32:1–13. https://doi.org/10.1016/j.postharvbio.2003.09.016
Timmusk S, Seisenbaeva G, Behers L (2018) Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci Rep 8:617. https://doi.org/10.1038/s41598-017-18939-x
Urbonaviciute A, Samuoliene G, Sakalauskaite J, Duchovskis P, Brazaityte A, Siksnianiene JB, Ulinskaite R, Sabajeviene G, Baranauskis K (2006) The effect of elevated CO2 concentrations on leaf carbohydrate, chlorophyll contents and photosynthesis in radish. Polish J Environ Studies 15:921–925
Yin H, Bai X, Du YG (2008) The primary study of oligochitosan inducing resistance to Sclerotinia sclerotiorum on Brassica napus. J Biotechnol 136:S600–S660. https://doi.org/10.1016/j.jbiotec.2008.07.1217
Acknowledgements
The authors gratefully acknowledge the UCB, Haldi Uttarakhand and facilities provided by the Department of Microbiology, G. B. Pant University of Agriculture & Technology, Pantnagar.
Author information
Authors and Affiliations
Contributions
PC: developed the concept, participated in all the experiments, and created the manuscript. PK: isolated and characterized the bacterial cultures used in the experiment, SG and AK: participated in analysis agronomical parameters of maize crop, RK: provided the field facilities and nanochitosan used in the experiment, AS: provided the laboratory facilities and critical checking of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaudhary, P., Khati, P., Gangola, S. et al. Impact of nanochitosan and Bacillus spp. on health, productivity and defence response in Zea mays under field condition. 3 Biotech 11, 237 (2021). https://doi.org/10.1007/s13205-021-02790-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02790-z