Abstract
Plant growth promoting rhizobacteria are key to soil and plant health maintenance. In the present study, two PGPR strains which were identified as Bacillus spp. (accession number KX650178 and KX650179) with nanozeolite (50 ppm) were applied to the seeds in different combinations and tested on growth profile of maize crop. Various growth related parameters, including plant height, leaf area, number of leaves chlorophyll and total protein were positively increased up to twofold by the nanocompound treatment. GC–MS results reveal increase in total phenolic and acid ester compounds after the treatment of nanozeolite and PGPR, which are responsible for stress tolerance mechanism. Soil physicochemical parameters (organic carbon, phosphorous, potassium, ammoniacal nitrogen and nitrate nitrogen) were assessed qualitatively and a shift towards higher amount was observed. Various biochemical parameters of soil health like dehydrogenase, fluorescein diacetate hydrolysis and alkaline phosphatase activity were significantly enhanced up to threefold with the application of different treatments. The results, for the first time, demonstrate successful use of nanozeolite in enhancing growth of Zea mays, under controlled conditions and present a viable alternative to GM crop for ensuring food security.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize is one of the most important cereal crop of world and third most important crop after rice and wheat in India. It requires good agronomic practices for improvement in yield as it is very sensitive to nutrient status of soil (Suriyaprabha et al. 2014). Nanoparticles are the particles with one or more dimension in nanosize (0.2–100 nm). Nanotechnology in agriculture have several applications but the major concern of the present study is the application of nanocompounds for the crop improvement.
Several studied regarding impact of nanoparticles on plant growth have already been done (Khan and Bano 2016). The majority of reported studies point to positive impact with a few isolated studies pertaining to negative effect. Some studies demonstrate that TiO2 nanoparticles enhance photosynthesis and nitrogen metabolism and thus improve growth of spinach at a very low concentration, i.e., 20 mg/L (Yang et al. 2006). Lin and Xing (2008) investigated toxicity of nanoparticles (carbon nanotubes, aluminium, zinc, zinc oxide) on root growth and seed germination of six plant species (radish, rape, rye grass, lettuce, corn and cucumber). Nanotechnology has begun to make efficient use of different nanoparticles but on the other hand increasing quantities in which nanoparticles are produced and applied, as well as unique characteristics resulting from nanoscale size, has led to the concern over toxicological implication of exposure for nontarget.
Zeolites are complicated silicate minerals with pores and channels within its crystal structure. It has unique higher Cation exchange capacity (CEC) due to which it has high affinity towards cations like Na+, K+, Ca2+ (Navrotsky et al. 1995). Zeolites are responsible for selective retention of NH4+ and K+ ions in soil system (Petrovic 1993). Very few studies have investigated the effect of nanozeolite on maize growth. Nanozeolite are the least worked out nanoparticles on plant growth promotion and soil health, but can give extensive advantage with least side effect due to its biodegradability. Aminiyan et al. (2015) studied the effect of some natural products, zeolite and nanozeolite on aggregation stability and organic carbon status of soil and observed that the nanozeolite gave best results by enhancing the organic carbon of the soil and stabilizing the micro and macroaggregates followed by zeolite.
Plant growth promontory rhizobacteria are already known to support plant growth promotion. (Kloepper et al. 1980). They are also among the most complex and important assemblages in the biosphere found in the vicinity of plant rhizosphere (Khan 2005). PGPR like P. aeruginosa, P. putida, P. fluorescens, B. subtilis and soil nitrogen cycle bacteria (nitrifying bacteria and denitrifying bacteria) have shown varying degree of inhibition when exposed to nanoparticles in pure culture or in aqueous suspensions (Mishra and Kumar 2009). Iron and copper-based nanoparticles are presumed to react with peroxides present in the environment and generate free radicals that are highly toxic to microorganisms like P. aeruginosa. A sublethal dose of CuO nanoparticle impaired pyoverdine (PVD) function in a Gram-negative bacterium. Although most of the reports point out negative effect of nanoparticles on PGPRs but the effect of nanozeolite on PGPRs was never worked out before and nanoparticles like nanozeolite are least toxic and biodegradable, hence are supposed to support the growth of PGPR due to enhanced nutrient use efficiency.
The present study was planned to evaluate the tremendous advantage of nanozeolite in combination of PGPR isolates on maize growth promotion and to look after the possible side effects in parallel. The plant growth parameters (plant height, leave area, number of leaves, chlorophyll content and total protein content) were investigated to observe the direct effect of nanozeolite on plant growth viability but on the other side soil health parameters (pH, Organic carbon, nitrogen, available phosphorous, potassium, FDA hydrolysis activity and enzyme assays) were also investigated to see the effect of nanoparticles on soil health.
Materials and methods
Chemicals used
The nanozeolite was purchased from Intelligent material Pvt. Ltd India and physicochemical properties as supplied are given in Supplementary material 1. Other chemical were purchased from SRL and Hi media Laboratories Pvt. Ltd. India.
Soil collection
The soil was taken from the previous field experiment where the nanoparticles were applied by spray method at a rate of 0.03 g each in 1.5 L. The experimental site was at Norman E. Borlogue Crop Research Centre of G.B. Pant University of Agriculture and Technology, Pantnagar, Dist.Udham Singh Nagar (Uttarakhand), India. This center is situated at an altitude of 243.84 above mean sea level, 29°N latitude and 79.3°E longitudes. The soil without nanoparticle was considered as control.
Soil characteristics
The soil of the experimental plot was classified under order—Mollisol, sub order—udoll, great group—Hapludoll (Deshpande et al. 1971). The composite soil sample was prepared by mixing the soils from different locations in the field. Soil was dug up to a depth of 15 cm and analyzed for different physicochemical properties. The soil was silt clay loam in texture, medium in organic carbon, low in available nitrogen and medium in available phosphorus, and potassium, and near neutral in reaction (Khati et al. 2017b).
Seed variety
Pant sankarmakka 1, a high yielding variety of maize was obtained from Crop Research Centre, Pantnagar. The duration of the crop is 90–120 days (Khati et al. 2017a).
Culture conditions
The bacterial isolates (PS2 and PS10) used for the experiment were Gram-positive rod-shaped PGPRs, and characterized as Bacillus spp. according to 16SrDNA sequencing (Khati et al. 2017a).
Inoculation procedure
The seeds were bacterized in 1 mL of broth (population in broth was maintained up to 106) amended with carboxymethyl cellulose, which act as binding agent.
Seed germination assay
Different treatments were made according to different combinations of nanozeolite and bacterial isolates. The pot trial was conducted with sterilized and unsterilized soil to see the effect of nanozeolite in controlled and natural conditions, respectively (Table 1). Plants were thinned to 10 plants per pot to maintain equal number for further observations.
Analysis of plant sample
The plant materials were sampled at 30 day after sowing.
Physical parameters
Shoot length, root length, leaf area, and number of leaves were measured and mean values were considered for further analysis (Yoshida et al. 1972).
Plant biochemical analysis
Chlorophyll content
Fresh leaves (500 mg) were ground in 10 mL of 80% acetone. The homogenate was centrifuged at 8000 rpm for 10 min and supernatant is used for chlorophyll estimation by taking the absorbance at 645 and 663 nm (Arnon 1949). The total amount of chlorophyll a and chlorophyll b was calculated on the basis of formula:
Total protein extraction and estimation
The total protein content determination according to Bradford method using bovine serum albumin (BSA) as the standard (Bradford 1976). One gram of fresh leaves were ground with 0.2 M Tris (pH 8) in pestle and mortar and the slurry was centrifuged at 10,000 rpm at 4 °C for 20 min. the protein concentration was determined according to the formula:
Gas chromatography–mass spectroscopy
Shade-dried maize leaves (20 days old) were extracted by methanolic extraction and analyzed by gas chromatography–mass spectroscopy (Thermo GC-Trace Ultra Ver. 5.0; Thermo Scientific MS DSQ II). The GC silica column dimension was 30 m 90.25 mm at a flow rate of 1 mL min−1 at 8 °C and then, the temperature was raised to 24 °C. The volatile compounds present in the control, nano silica and bulk silica treated samples were identified by comparing with the standards or the mass spectrum matched with the inbuilt library (Wiley 9).
Physicochemical analysis of soil
The test soil sample was collected after 30 day of treatment. Different physicochemical tests (pH, Organic carbon, available potassium, available phosphorous, nitrate nitrogen and ammoniacal nitrogen) were performed qualitatively with the HiMedia kit.
Soil enzymes assays
Fluorescein diacetate hydrolysis (FDA)
FDA hydrolysis assay was done according to Schurer and Rosswall (1982) method. One g of soil sample was incubated with 50 mL of 60 mM Sodium phosphate buffer pH 7.6 and 0.50 mL of FDA solution (5 mg in 10 mL acetone). The suspension is incubated at 24 °C. Aliquots were withdrawn at regular interval of 1, 2 and 3 h and FDA hydrolysis was stopped by adding 1 mL acetone to a 6 mL aliquot. Slurry was centrifuged (8000 rpm for 5 min) and filtered. Absorbance was recorded at 490 nm.
Dehydrogenase activity
Modified method of Casida et al. (1964) was followed for the dehydrogenase assay of soil samples. To 5 g soil sample 5 mL of 2,3,5-triphenyltetrazolium chloride (TTC) solution (2 g in 100 mL 0.1 M tris buffer pH 7.4) was added. After incubation (37 °C, 120 rpm for 8 h) 25 mL acetone or methanol was added to extract Triphenylformazan (TPF). Centrifugation (4500 rpm for 10 min at 4 °C) to obtained supernatant was done and absorbance recorded at 485 nm.
Alkaline phosphatase activity
One g of each dry soil was mixed with 0.25 mL toluene, 4 mL of Modified universal buffer (MUB) (100 mM, pH 11) and 1 mL p-nitrophenyl phosphatase (pNPP) (25 mM) were added. After incubation at 37 °C for 1 h. 1 mL CaCl2 (0.5 M) and 4 mL of tris buffer (0.1 M, pH 12) were added to stop the reaction. Intensity of color was determined using spectrophotometer at 400 nm (Tabatabai and Bremner 1969).
Statistical analysis
The pot trial was performed on the basis of CRD with three replicate per treatment. The data were statistically treated using general linear model procedure (SPSS, Ver 16.0) to reveal significant effect of nanozeolite treatment with PGPR isolates on maize crop. The results were analyzed using one way analysis of variance (ANOVA).
Results
Seed germination assay
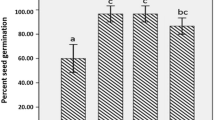
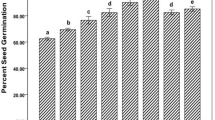
Percentage germination of Zea mays seeds were positively affected by nanozeolite treatment at 50 mg/L concentration (Fig. 1). Briefly, seed germination was highest (94.44%) in NZ + PS2 + SS (nanozeolite with PS2 and sterilized soil), NZ + PS2 + USS (nanozeolite with PS2 and unsterilized soil), NZ + PS10 + USS (nanozeolite with PS10 and unsterilized soil), PS10 + USS (PS10 and unsterilized soil), PS2 + USS (PS2 and unsterilized soil). In case of NZ + PS10 + SS (nanozeolite with PS10 in sterilized soil), NZ + SS (nanozeolite in sterilized soil), PS10 + SS (PS10 in sterilized soil) the percent seed germination was about 88.89, followed by NZ + USS (nanozeolite in unsterilized soil) with 83.33% and PS2 + USS (PS2 in unsterilized soil) with 72.22%. Least germination was found in AC + SS (absolute control) with 44.44 and AC + USS (absolute control with unsterilized soil) with 33.33%. A statistically significant increase in percent germination was recorded at 50 mg/L NZ (nanozeolite) treatment. This indicates that application of nanozeolite together with PGPR isolates enhance the seed germination maximally, but when nanozeolite or PGPR applied individually the seed germination was slightly slower but still better than the control.
Effect of nanozeolite treatment on growth profile of treated seedling
The plant height was significantly increased with nanozeolite treatment in comparison to control. A maximum increase of 1.5 fold was observed in seedling treated with nanozeolite with PS2 in sterilized soil (Figs. 2 and 3). However, no significant change in case of number of leaves was observed and even slight increase in leaf area with a maximum value (30.49 cm2) was observed in case of PS2 treated sterilized soil in comparison to control (24.52 cm2). Total chlorophyll content was also increased by 1.6-folds in comparison to control (Fig. 4). Protein content of plants was also enhanced 1.5–1.8-folds in the treatment of nanozeolite with PS2 in sterilized soil.
GC–MS
The modulations in total organic compounds according to the treatments of nanozeolite and bacterial isolates are elucidated from GC–MS results. A relative abundance of volatile compounds in maize leaf extract with respect to the retention time is presented in chromatograph (Fig. 5). The GC–MS results revealed increased acid esters and alcohols in treated plant samples which are responsible for stress tolerance (Fig. 6). This can be correlated to the better stress adaptation by the plant in the presence of nanocompound alone or in combination with PGPR.
Soil physicochemical properties
Soil physicochemical properties are summarized in Table 2. The pH of soil was alkaline with some variation in all the treatments. The results indicate increase in % OOC (percent oxidizable organic carbon) from M (medium: 0.5–0.75 Kg ha−1) to MH (medium high: 0.75–1 Kg ha−1) nanozeolite treatment with PS2, Nanozeolite alone and PS10 alone in unsterilized soil, but no increase was recorded when nanozeolite treated with PS10, PS10 and PS2 alone in sterilized soil. This indicates that nanozeolite play significant role in increasing OC (organic carbon) content of soil.
Two type of nitrogen estimation was done to target ammoniacal nitrogen and the nitrate nitrogen. Ammoniacal nitrogen was found to be very low in the control soil (without any treatment). Slight increment was observed when NZ treated with PS2 and PS10 (SS) from low to medium. Similarly in PS2 and PS10 alone medium level of AN was found. It means nanozeolite alone do not have significant effect on AN, rather the PGPR isolates alone or in combination with NZ are positively effecting AN status of soil in case of Nitrate nitrogen (NN), soil without any treatment have very low (04 Kg ha−1) NN which was slightly enhanced in NZ treated soil, PS2 treated soil and PS10 (USS), NZ with PS2 (SS) and NZ with PS10 (SS) with low level of NN (about 10 Kg ha−1). Medium level of NN was found when NZ treated with PS2 and PS10 in unsterilized soil.
It is clearly evident from the results that nanozeolite when treated either with PS2 or PS10 gave best result (MH: medium high (56–73 Kg ha−1) as compared to control (medium: 22–56 Kg ha−1), but when applied alone (NZ, PS2 and PS10) good results were only observed in sterilized soil, which indicate that competition with the indigenous microflora suppressed the beneficial traits.
The K content of the soil was found to be low (> 112 Kg ha−1) in most of the cases, but slight increment in the level (from low to medium) was observed in case of nanozeolite when treated with PS2 in sterilized soil, nanozeolite with PS10 in unsterilized soil and PS2 alone in unsterilized soil. The observation shows NZ and PS2 are more beneficial for available potassium status.
Soil enzymes
Significant increase in dehydrogenase activity was observed in all treatments in comparison to untreated soil, with maximum activity in case of PS2 treatment in unsterilized soil (0.050473 U) (Fig. 7). Highest activity of FDA hydrolysis was obtained in nanozeolite treated with PS2 in unsterilized soil (0.912 U). Least enzyme activity was observed in absolute controlled unsterilized (0.338431 U) and absolute control sterilized (0.21817 U), slight increase in the activity was observed in PS2 alone in sterilized soil (0.3307 U) and PS10 alone in sterilized soil (0.3593 U) and a significant increase in activity in case of all the nanozeolite treated soil with or without PGPR isolates. The results suggest the fact that enzyme activity was enhanced by nanozeolite in soil but when combined with PGPR isolates the synergistic effect was two–threefold enhancement in FDA hydrolysis activity.
Increase in enzyme activity from 0.9477 U in case of absolute control in unsterilized soil and 0.731 U in case of absolute control in sterilized soil to a maximum of 1.989 U in case of nanozeolite treatment with PS2 in unsterilized soil. While in other treatments also significant increment (one to two folds) in the alkaline phosphatase activity was observed. This shows enzyme activity was significantly enhanced in nanozeolite treated soil and a synergistic effect was observed in nanozeolite with PS2 and also in nanozeolite with PS10 for smaller extent.
Discussion
Seed germination percent was significantly enhanced in different treatments. Similarly Khodakovskaya et al. (2009) also exposed MWCHTs (Multi walled carbon nanotubes) to tomato seeds and observed enhanced seed germination. MWCNT also enhanced efficiency of water uptake as well as Ca and Fe nutrients uptake which are responsible for enhanced seed germination (Villagarcia et al. 2012). Wheat seed germination was found to be enhanced by ZnO NP (Ramesh et al. 2010; Prasad et al. 2012). RuO2 nanoparticles were found to increase seed germination in Brassica spp. (Singh et al. 2015).
So far the proposed mechanisms could be due to the increased permeability of seed capsule, facilitating the admission of water and di-oxygen into the cells, which accelerates the metabolism and germination process (Zheng et al. 2005). Increase in nitrate reductase activity, higher utilization of fertilizers and enhanced antioxidant system could be another possible mechanism for enhanced seed germination (Lu et al. 2002).
The mechanism of plant height enhancement may be attributed to increased level of gibberellic acid (GA), which is key hormone responsible for shoot elongation (Stepanova et al. 2007). Seif et al. (2011) also reported stem elongation in Borago after silver nanoparticle treatment. Increase in total chlorophyll means increase in photosynthates (Urbonaviciute et al. 2006). Tarafdar (2013) reported that ZnONPs induced a significant improvement in Cyamopsis tetragonoloba plant biomass, shoot and root growth, root area, chlorophyll and protein synthesis, rhizospheric microbial population, acid phosphatase, alkaline phosphatase and phytase activity in cluster bean rhizosphere. Total organic compounds, such as phenols, aldehydes, ketones, etc., are found to be enhanced in maize leaves treated with nanozeolite in combination with PGPR. The other organic compounds are not varied considerably. These results are correlated with the study on the protective mechanism of silicon in rice through phenols (Goto et al. 2003).
Though the physicochemical properties are vulnerably influenced by the change of redox conditions in soils (Lamparter et al. 2009). Therefore, it is necessary to determine the underlying impacts induced by nanocompouds on soil physicochemical properties to optimize their use. Organic carbon is the most important element and a key attribute in assessing soil health, generally correlating positively with crop yield (Bennett et al. 2010). The soil organic carbon also attributes to other important functional process in soil such as nutrient storage, mainly Nitrogen, water holding capacity and also stability of aggregates (Silva and SáMendonça 2007). Above all soil organic carbon supports soil microbial activity which is a key step of enhancing soil health. Nitrogen (N) is the most requires plant nutrients, which is found in several chemical forms in soil (Cantarella 2007) resulting in very dynamic behavior.
Phosphorous (P) in soil is a key nutrient that limit agricultural yields and is present as orthophosphate, but microbial P and organic P are also acts as stocks that can rapidly become available. According to Doran et al. (1999) phosphorous is one of the major indicators of productivity and environment quality of soil. Potassium (K) is also another indicator of soil health and productivity. The results indicate that the nanozeolite along with PGPR isolates enhanced the different minerals nutrient levels which are indicators of soil physicochemical properties.
Similar investigation was also done by Zhou et al. (2012) who studied the dynamic influence of three iron based NPs (Fe0, Fe3O4, and Fe2O3) on soil physicochemical properties such as pH, dissolved organic carbon (DOC), available ammoniacal nitrogen (AAN) available phosphorous and enzymatic activities. They found that the addition of Fe0 NP increased DOC and AAN but significantly reduced AP, while Fe3O4, and Fe2O3NP significantly reduced AAN.
Enzymes are vital activators in life processes, likewise in the soil they are known to play a substantial role in maintaining soil health and its environment. Evaluation of soil health, therefore, requires estimation of these indicators of the same. The soil enzymes play key biochemical functions in the overall process of organic matter decomposition in the soil (Sinsabaugh et al. 1991). According to Kiss et al. (1978) various substrates are catalyzed by soil enzymes and act as nutrients for microorganisms. These enzymes include amylase, dehydrogenase, phosphatase, β-glucosidase and arylesterase.
Dehydrogenases are the most important group of enzymes used as indicator of biological activity in soils (Burns 1978). Dehydrogenases are the major indicators of microbiological redox system and possible measure of microbial oxidative activity (Tabatabai 1982; Trevors 1984).
FDA [3′6′-diacetylfluorescein] hydrolysis can also be used as a measure of microbial activity in soils (Schnurer and Rosswall 1982). FDA is hydrolyzed by a number of different enzymes, such as protease, lipase and esterase.
Phosphatase are the group of enzymes believed to play pivotal role in phosphorous (P) cycling (Speir and Ross 1978; Sylvia et al. 2005). Apart from being good indicator of soil health these enzymes plays key role in soil system (Eivazi and Tabatabai 1977; Dick et al. 2000) by enhancing the P solubilization when deficiency is recorded. Raliya et al. (2015) studied effect of TiO2 nanoparticles on Mung bean and observed the increase in activity of acid phosphatase (67.3%), alkaline phosphatase (72%), phytase (64%) and dehydrogenase (108.7%). Similarly a significant improvement in acid phosphatase (73.5%), alkaline phosphatase (48.7%), and phytase (72.4%) activity in cluster bean rhizosphere was observed over control in 6-week-old plants due to application of nanoZnO (Raliya and Tarafdar 2013).
The results suggest the application of nanocompound is not only beneficial for the plant health parameters but also for the maintenance of soil health which is key to sustainable agriculture system.
Conclusion
The results obtained suggest the application of nanozeolite in combination with PGPR is beneficial to enhance plant health parameters and thus crop productivity. The different enzymes selected for the soil health assessment acted as fingerprints and revealed the status of test soil with respect to different treatments. The present findings are novel and may help in standardization of alternate technology for ensuring world food security and also ensure soil health maintenance.
Abbreviations
- AC:
-
Absolute control
- SS:
-
Sterilized soil
- FDA:
-
Fluorescein diacetate
- OOC:
-
Oxidizable organic carbon
- APH:
-
Available phosphorous
- NN:
-
Nitrate nitrogen
- USS:
-
Unsterilized soil
- AN:
-
Ammoniacal nitrogen
- NZ:
-
Nanozeolite
- APT:
-
Available potassium
References
Aminiyan MM, Sinegani AAS, Sheklabadi M (2015) Aggregation stability and organic carbon fraction in a soil amended with some plant residues, nanozeolite, and natural zeolite. Int J Recycl Organic Waste Agric 4(1):11–22
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bennett LT, Mele PM, Annett S, Kasel S (2010) Examining links between soil management, soil health, and public benefits in agricultural landscapes: an Australian perspective. Agr Ecosyst Environ 139:1–12
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein—dye binding. Anal Biochem 72:248–254
Burns RG (1978) Enzyme activity in soil: some theoretical and practical considerations. In: Bums RG (ed) Soil enzymes. Academic, London, pp 295–340
Casida L, Klein D, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Cantarella H (2007) Nitrogen. In: Novais RF, Alvarez VH, Barros NF, Fontes RLF, Cantarutti RB, Neves JCL (eds) Soil fertility. Brazilian Soil Science Society, Vic¸osa, MG, pp 375–470 (in Portuguese)
Deshpande SB, Fehrenbacher JB, Beavers AN (1971) Mollisols of Tarai region of Uttar Pradesh, Northern India, Morphology and mineralogy. Geoderma 6(3):179–193
Dick WA, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 32(13):1915–1919
Doran JW, Jones AJ, Arshad MA, Gilley JE (1999) Determinants of soil quality and health. In: Pankhurst CE, Doube BM, Gupta VVSR (eds) Biological indicators of soil health. CAB International, Oxon
Eivazi F, Tabatabai MA (1977) Phosphatases in soils. Soil Biol Biochem 9:167–172
Goto M, Ehara H, Karita S, Takabe K, Ogawa N, Yamada Y, Ogawa S, Yahaya MS, Morita O (2003) Protective effect of silicon on phenolic biosynthesis and ultraviolet spectral stress in rice crop. Plant Sci 164:349–356
Khan N, Bano A (2016) Modulation of phytoremediation and plant growth by treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int J Phytorem 18(12):1258–1269
Khati P, Sharma A, Gangola S, Kumar R, Bhatt P, Kumar G (2017a) Impact of agri-usable nanocompounds on soil microbial activity: an indicator of soil health. CLEAN–Soil, Air, Water. https://doi.org/10.1002/clen.201600458
Khati P, Chaudhary P, Gangola S, Bhatt P, Sharma A (2017b) Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech. https://doi.org/10.1007/s13205-017-0668-y
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris S (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 10(3):3221–3227
Kiss S, Dragan-Bularda M, Radulescu D (1978) Soil polysaccharidases: activity and agricultural importance. In: Burns RG (ed) Soil enzymes. Academic, London, pp 117–147
Kloepper JW, Leong J, Teinize M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885–886
Khan AG (2005) Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J Trace Elements Med Biol 18(4):355–364
Lamparter A, Bachmann J, Goebel MO, Woche SK (2009) Carbon mineralization in soil: impact of wetting-drying, aggregation and water repellency. Geoderma 150:324–333
Lin D, Xing B (2008) Root Uptake and Phytotoxicity of ZnO Nanoparticles. Environ Sci Technol 42(15):5580–5585
Lu CM, Zhang CY, Wen JQ, Wu GR, Tao MX (2002) Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci 21:168–172
Mishra VK, Kumar A (2009) Impactof metal nanoparticles on the plant growth promoting rhizobacteria. Digest J Nanomater Biostruct 4(3):587–592
Navrotsky A, Petrovic I, Hu Y, Chen C, Davis ME (1995) Energetics of microporous materials. J Non-Cryst Solids 192:474–477
Petrovic AM (1993) Potential for natural zeolite uses on golf courses. USGA Green Sect 31:11–14
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprsad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale ZnO particles on germination, growth and yield of peanuts. J Plant Nutr 35:905–927
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.). Agric Res 2(1):48–57
Raliya R, Tarafdar JC, Biswasa P (2015) TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiate L.). Biotechnol Rep 5:22–26
Ramesh K, Biswas AK, Somasundaram J, Rao AS (2010) Nanoporous zeolites in farming: current status and issues ahead. Current Sci 99(6):760–764
Schnurer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 6:1256–1261
Seif SM, Sorooshzadeh AH, Rezazadeh S, Naghdibadi HA (2011) Effect of nano silver and silver nitrate on seed yield of borage. J Med Plant Res 5(2):171–175
Silva IR, SáMendonça E (2007) Matériaorgânica do solo = Soil organic matter. In: Novais RF, Alvarez VH, Barros NF, Fontes RLF, Cantarutti RB, Neves JC (eds) Fertilidade do solo = Soil fertility. SociedadeBrasileira de Ciência do Solo, Viçosa, MG, pp 275–374 (in Portuguese)
Sinsabaugh RL, Antibus RK, Linkins AE (1991) An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agric, Ecosys Environ 34(1–4):43–54
Singh A, Singh NB, Hussaina I, Singh H, Singh SC (2015) Plant-nanoparticle interaction: an approach to improve agricultural practices and plant productivity International. J Pharm Sci Invention 2319–6718
Speir TW, Ross DJ (1978) Soil phosphatase and sulphatase. In: Burns RG (ed) Soil enzymes. Academic, London, pp 197–250
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19:2169–2185
Suriyaprabha R, Rathinam GK, Prabu YP, Rajendran V, Kannan N (2014) Effect of silica nanoparticles on microbial biomass and silica availability in maize rhizosphere. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1191
Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA (2005) Principles and applications of soil microbiology, 2nd edn. Prentice Hall, Upper Saddle River, NJ
Tabatabai MA (1982) Soil enzyme. In: Page AL (ed) Methods of soil analysis, part 2. American Society of Agronomy, Madison, WI, pp 903–948
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tarafdar JC, Sharma S, Raliya R (2013) Nanotechnology: interdisciplinary science of applications. Afr J Biotechnol 12(3):219–226
Trevors JT (1984) Effect of substrate concentration, inorganic nitrogen, O2 concentration, temperature and pH on dehydrogenase activity in soil. Plant Soil 77(2–3):285–293
Urbonaviciute A, Samuoliene G, Sakalauskaite J, Duchovskis P, Brazaityte A, Siksnianiene JB, Ulinskaite R, Sabajeviene G, Baranauskis K (2006) The effect of elevated CO2 concentrations on leaf carbohydrate, chlorophyll contents and photosynthesis in radish. Polish J Environ Stud 15:921–925
Villagarcia H, Dervishi E, Silva K, Biris AS, Khodakovskaya MV (2012) Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants. Small 8:2328–2334
Yoshida S, Forno DA, Cock JH (1972) Laboratory manual for physiological studies of rice, 2nd edn. International Rice Research Institute, Los Baños
Yang F, Hong F, You W, Liu C, Gao F, Wu C, Yang P (2006) Influences of Nano-anatase TiO2 on the Nitrogen Metabolism of Growing Spinach. Biol Trace Element Res 110(2):179–190
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Zhou DM, Jin SY, Wang YJ, Wang P, Weng NY, Wang Y (2012) Assessing the impact of iron-based nanoparticles on pH, dissolved organic carbon, and nutrient availability in soils. Soil Sediment Contam Int J 21(1):101–114
Acknowledgement
This research was supported by University Grant Commission (UGC) India and Department of Microbiology, College of Basic Science and Humanity, GBPUA&T Pantnagar.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khati, P., Parul, Bhatt, P. et al. Effect of nanozeolite and plant growth promoting rhizobacteria on maize. 3 Biotech 8, 141 (2018). https://doi.org/10.1007/s13205-018-1142-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1142-1