Abstract
Schinus terebinthifolia leaf lectin (SteLL) was reported to be an antimicrobial and antitumor agent. In this work, we evaluated the immunomodulatory activity of SteLL on mice splenocytes and also determined its native molecular mass and putative sequence similarities with plant proteins. The effects of SteLL (12.5 μg/mL) on viability, cytosolic Ca2+ concentration ([Ca2+]cyt), cytosolic and mitochondrial levels of reactive oxygen species (ROS), and mitochondrial transmembrane potential (ΔΨm) of mice splenocytes were determined. In addition, the culture supernatants were collected for quantification of interleukins (IL), tumor necrosis factor (TNF), interferon-gamma (IFN-γ) and nitric oxide (NO). SteLL showed a native molecular mass of 12.4 kDa and tandem mass spectrometry (MS/MS) ions search revealed similarities with adenosine triphosphate (ATP) synthase and F1-ATPase from plants (4% and 6% coverage, respectively). SteLL was not toxic to splenocytes, did not alter the [Ca2+]cyt and ROS levels, and slightly reduced ΔΨm. The presence of SteLL stimulated the cells to release pro-inflammatory cytokines (IL-17A, TNF-α, IFN-γ and IL-2) and also of IL-4, an anti-inflammatory cytokine that can prevent exacerbated inflammation. SteLL induced decrease in the secretion of NO. In conclusion, SteLL has biotechnological potential as an immunomodulator agent for use in studies employing cultures of immune cells. In addition, the anti-infectious and antitumor properties of the leaves may involve the immunomodulation property of SteLL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schinus terebinthifolia Raddi (Anacardiaceae), the Brazilian pepper tree, is a medicinal plant whose leaves are widely used for treatment of digestive and urinary tract infections, oral candidiasis, and tumors (Morton 1978; Lindenmaier and Putzke 2011). These biological effects are probably due to the direct action of the leaf components on the infectious agents and tumor cells, but the modulation of the immune system can also be an important component involved in them. In addition, leaves are used for treatment of skin wounds (Ribas et al. 2006) and both innate and adaptive immune response contribute to the healing process (Strbo et al. 2014). This context raises the hypothesis that S. terebinthifolia leaf preparations may have immunomodulatory properties. Further, faced with the complex task of finding new agents for infection control, the search for molecules that have both antimicrobial activity and ability for immunomodulation is considered a promising new route for expanding the therapeutic possibilities (Haney and Hancock 2013).
Among the compounds present in the leaves of S. terebinthifolia whose activity has been scientifically proven, there is a thermostable and chitin-binding lectin called SteLL, which is toxic to microorganisms (Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella enteritidis, Staphylococcus aureus and Candida albicans) of medical importance (Gomes et al. 2013) and exerted antitumor activity in vivo in sarcoma 180-bearing mice (Ramos et al. 2019).
Lectins are proteins that specifically recognize free carbohydrates or glycoconjugates (Coelho et al. 2017). The binding of lectin domains to carbohydrates occurs through reversible interactions (hydrogen bonds, van der Waals forces and hydrophobic interactions). Different cellular processes can be triggered when a lectin interacts with glycan moieties present at cell membrane, resulting in several biological properties (Procópio et al. 2017). Lectins can exert immunomodulatory effects through binding to glycans present on the surface of immune cells, stimulating or suppressing the production of growth factors, chemokines, cytokines, and nitric oxide as well as can lead to activation of lymphocytes. This property of lectins can be explored in pre-clinical studies to modulate immune responses at a tumor microenvironment or sites containing foreign antigens aiming to inhibit carcinogenesis, cease infectious process or even reduce autoimmune response, for example (Patriota et al. 2019). In addition, immunomodulatory lectins, such as concanavalin A, have biotechnological value as powerful tools for using in assays requiring immune cells activated or expressing a specific profile (Dwyer and Johnson 1981; Patriota et al. 2019).
The previous evidences that SteLL is an active principle of the antimicrobial and antitumor properties of leaves prompted the evaluation of its immunomodulatory activity when isolated. In this sense, it was evaluated the ability of SteLL to affect the viability and cause cellular stress in BALB/c mice splenocytes as well as to modulate the production of cytokines and nitric oxide (immunomodulatory activity). Further, SteLL was characterized for native molecular mass and putative sequence similarities with plant proteins.
Materials and methods
Plant material
Schinus terebinthifolia is popularly known as “aroeira vermelha” or “aroeira da praia” in Portuguese, and “Brazilian peppertree” in English. The name of the plant species was checked with the website theplantlist.org. The leaves of S. terebinthifolia were collected in the campus of the Universidade Federal de Pernambuco, UFPE (8° 02′ 55.6″ S 34° 56′ 48.3″ W), Recife, Pernambuco, Brazil. A voucher specimen (number 73,431) is archived in the herbarium Dárdano de Andrade Lima from the Instituto Agronômico de Pernambuco (Recife, Brazil). The access to the plant material was recorded (ACB2499) in the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado.
Lectin isolation
The leaves were placed to dry at 28 °C for 15 days and then powdered using a blender. The resulting flour (10 g) was then suspended in 0.15 M NaCl (100 mL) and homogenized for 16 h at 28 °C using a magnetic stirrer. The leaf extract was obtained after filtration through gauze and centrifugation (12,000g, 15 min, 4 °C). SteLL was isolated from the leaf extract as previously described by Gomes et al. (2013). Protein concentration according to Lowry et al. (1951) and the carbohydrate-binding property of SteLL was checked by the hemagglutinating activity (HA) assay as described by Procópio et al. (2018). The specific HA was calculated as the ratio between HA and the protein concentration (mg/mL).
Gel filtration chromatography
Native molecular mass of SteLL was determined by submitting the protein (2 mL; 1 mg) to gel filtration chromatography on a HiPrep 16/60 Sephacryl S-100 HR column coupled to the ÄKTAprime plus system (GE Healthcare Life Sciences, Sweden). The chromatography was performed using 0.15 M NaCl at a flow rate of 0.5 mL/min. The fractions collected (3.0 mL) were monitored for absorbance at 280 nm. The relative molecular mass of SteLL was calculated by comparing its migration time with those of the marker proteins: bovine serum albumin (66 kDa), ovalbumin (45 kDa), and lysozyme (14 kDa).
Mass spectrometry analysis
SteLL was submitted to polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulphate (SDS-PAGE) according to Laemmli (1970). The region of the gel containing the polypeptide band was excised and submitted to discoloration by washing thrice (15 min each) with 400 μL of a solution (1:1, v/v) of 50% (w/v) acetonitrile and 25 mM ammonium bicarbonate pH 8.0. Next, it was added 100% acetonitrile and the gel was dehydrated by vacuum concentration. Afterwards, the material was incubated with 10 μL of 10 mM dithiothreitol (in 100 mM ammonium bicarbonate) for 1 h at 56 °C. After new washing step with 100 mM ammonium bicarbonate, 10 μL of 50 mM iodoacetamide (in 100 mM ammonium bicarbonate) was added and the assay was incubated at 28 °C in the dark. Iodoacetamide was removed by washing with 100 mM ammonium bicarbonate and the material was dehydrated again with 100% acetonitrile. The polypeptides were then digested for 16 h at 37 °C with 5 μL of 20 mg/mL trypsin (Promega, Madison, WI, USA) in 50 mM ammonium bicarbonate. The solution was then transferred to a sterile tube and peptides were extracted thrice by adding 30 μL of 1% (w/v) trifluoroacetic acid in 30% (w/v) acetonitrile. The resulting supernatants were pooled, dried under the vacuum concentrator, and the peptides were analyzed using an electrospray ionization quadrupole time-of-flight (ESI-QUAD-TOF) mass spectrometer (Waters, Milford, MA, USA) and the spectra obtained were compared with those present in the NCBInr database using the tandem mass spectrometry (MS/MS) ions search of the MASCOT software.
Mice splenocytes
All the experimental procedures were approved by the Ethics Committee on Animal Use of UFPE (Process number 0048/2016). Female BALB/c mice (24- to 32-day-old) from the vivarium of the Laboratório de Imunopatologia Keizo Asami (UFPE) were used to obtain the splenocytes. The mice were anesthetized with 2% xylazine (10 mg/kg) and 10% ketamine hydrochloride (115 mg/kg) and euthanized by cervical dislocation. The spleens were removed and put in centrifuge tubes containing RPMI 1640 medium (Gibco, Invitrogen, Carlsbad, CA) supplemented with fetal calf serum. A homogenate was obtained using a dounce tissue grinder and then transferred to centrifuge tubes containing 10 mL of incomplete RPMI medium and covered with Ficoll-Paque™ Plus (GE Healthcare Life Sciences, Sweden) with density adjusted to 1.077 g/mL. After centrifugation (2500g, 25 °C, 25 min), the cell layer containing immune cells was recovered, washed twice with phosphate-buffered saline (PBS), and submitted to another centrifugation step (500g, 25 °C, 10 min). The quantification of cells was done using a Neubauer chamber and the viability was tested through the trypan blue exclusion method. Splenocytes were only used if the viability was higher than 98%.
Treatments
In 24-well plates, splenocytes (106 cells) were incubated with SteLL (3.12–50 µg/mL for cytotoxicity assay; 12.5 µg/mL in the other assays) in supplemented RPMI 1640 medium for 24 h in an incubator (5% CO2). Untreated cells (control) were cultured under the same conditions. Six replicates were performed for all the experiments.
Evaluation of cytotoxicity
The possible induction of apoptotic or necrotic cell death by SteLL was investigated using the “FITC Annexin V Apoptosis Detection Kit I” from BD Biosciences (San Jose, CA, USA). Treated and control cells were collected by centrifugation (450g, 10 min, 25 °C), washed with PBS and centrifuged again. The pellets were resuspended in binding buffer for a concentration of 1 × 106 cells/mL. Next, 100 µL were transferred to a culture tube and it was added 5 µL of annexin V (AnnV) conjugated with fluorescein isothiocyanate (FITC) and 5 µL of propidium iodide (PI). The cells were then analyzed in the FACSCalibur cytometer using the Cell Quest Pro software (BD Biosciences) and FL1 vs. FL3 dot plots. A minimum of 10,000 events was collected. AnnV-negative/PI-positive cells were considered necrotic, and AnnV-positive/PI-negative cells were considered to be at the early stage of apoptosis. Double negatives were considered viable cells.
Determination of cytosolic Ca2+ concentration ([Ca2+]cyt)
Treated and control cells were collected by centrifugation (300g, 26 °C, 5 min), washed with PBS and centrifuged again. The pellets were transferred to 24-well plates and incubated (5% CO2, 37 °C) for 40 min with 5 µM Fluo-3AM (Thermo Fisher Scientific, Waltham, MA, USA), 1 µM pluronic acid F-127 (Sigma-Aldrich, St. Louis, MO, USA) and 30 µg/mL bovine serum albumin (Sigma-Aldrich). Subsequently, the cells were washed with PBS, collected by centrifugation (300g, 26 °C, 5 min) and transferred to cytometer tubes. The fluorescence emission at 525 nm was recorded using an excitation wavelength of 395 nm. A minimum of 10,000 events were collected. The [Ca2+]cyt was calculated according to the equation
where A is the sample fluorescence; B is the minimal fluorescence value (measured using cells incubated for 2 min with 8 mM ethylenediamine tetraacetic acid); C is the maximal fluorescence (measured using cells incubated for 2 min with 1 µM ionomycin); and Kd value is 390 nm (Tsien 1988; Degasperi et al. 2006; Melo et al. 2010).
Determination of cytosolic and mitochondrial levels of reactive oxygen species (ROS)
Treated and control splenocytes were washed with PBS, centrifuged (300g, 26 °C, 5 min) and the pellets were transferred to 24-well plates and incubated (5% CO2) in the presence of 5 µM dihydroethidium (Sigma-Aldrich) for 40 min (for measurement of cytosolic ROS) or 5 µM MitoSox Red (Thermo Fisher Scientific) for 10 min (for quantification of mitochondrial ROS). Thereafter, the cells were washed with PBS, collected by centrifugation (300g, 26 °C, 5 min) and the fluorescence at 620 nm was measured in the flow cytometer using an excitation wavelength of 488 nm. A minimum of 10,000 events were collected.
Measurement of the mitochondrial transmembrane potential (ΔΨm)
Treated and control cells were washed with PBS and collected by centrifugation (300g, 26 °C, 5 min). The pellets were transferred to 24-well plates and incubated (37 °C, 5% CO2) for 30 min with 100 nM MitoStatus (BD Biosciences). After this period, the cells were washed with PBS, centrifuged (300g, 26 °C, 5 min) and transferred to cytometer tubes. The intensity of the fluorescence at 620 nm was analyzed using an excitation wavelength of 488 nm. A minimum of 10,000 events were collected.
Quantification of released cytokines
Supernatants of treated and untreated cultures were collected to determine the levels of interleukins (IL-2, IL-4, IL-6, IL-10, and IL-17A), tumor necrosis factor (TNF), and interferon-gamma (IFN-γ) using the Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences, USA). The assays were performed according to the manufacturer’s instructions. Individual cytokine standard curves (0–5000 pg/mL) were generated and the range of detection was between 2 and 5000 pg/mL. Six experiments were performed.
Quantification of nitric oxide (NO)
The supernatants from untreated and treated cultures were also used to quantify the amount of released NO according to the Griess method (Ding et al. 1988). NO concentration was estimated using a standard curve (3.12–100.0 µmol/mL). Six experiments were performed for statistical analysis.
Statistical analysis
Statistical analysis of the results from cytotoxicity assay was performed using two-way analysis of variance (ANOVA). The statistical differences in the data from the other assays were analyzed by the Student’s t test using the GraphPad Prism 8 software. All the results were analyzed with a significance level of 95% (p < 0.05).
Results and discussion
This work was designed to test the hypothesis that SteLL may be an immunomodulatory agent found in S. terebinthifolia leaves. This hypothesis was raised due to: the broad use of S. terebinthifolia leaves in folk medicine; the biotechnological potential of other lectins as immunomodulatory agents; the need for new therapeutic strategies with greater selectivity for altered endogenous cells or foreign antigens; the previous reports on the antimicrobial and antitumor activity of SteLL.
SteLL showed specific HA of 10,284, which assures that its carbohydrate-binding sites were active. Gel filtration chromatography showed a native molecular mass of 12.4 kDa for SteLL (Fig. 1). This value is closer to the mass of 14 kDa previously reported for SteLL by SDS-PAGE (Gomes et al. 2013; Ramos et al. 2019) and confirms that it is a monomeric protein in the native state. Similar profile was detected by us in SDS-PAGE (data not shown) and the SteLL polypeptide band was submitted to mass spectrometry analysis. MS/MS ions search revealed similarities (Table 1) with sequences of an adenosine triphosphate (ATP) synthase beta chain from Polytomella sp. (4% coverage), F1-ATPase alpha subunit from Burmannia biflora (6% coverage), and a predicted protein from Hordeum vulgare subsp. vulgare (5% coverage). Plant lectins are broadly found in storage vacuoles and extracellular compartments, but it has increased the reports of their presence in cytoplasm and nucleus playing important roles in the cell physiology; for example, the 70-kDa heat shock proteins (HSP70) are carbohydrate-binding proteins composed of structural domains among which one is involved in ATPase activity. However, many of these lectins still do not have their functional roles in the plant clarified as well as what would be their receptors (Lannoo and Van Damme 2010). Our results stimulate future researches in order to discover the role of SteLL in the leaves of S. terebinthifolia.
After obtaining information about putative sequence similarities for SteLL, we investigated the cytotoxicity of this lectin to the mice splenocytes since it is expected that a potential immunomodulatory agent would stimulate cells in vitro without alter their viability as well as would be capable of stimulating protective immunity in vivo against infectious agents or tumors without causing damage to the host (Santos et al. 2016). Interestingly, SteLL did not cause apoptosis or necrosis at the conditions employed in our experiment (Fig. 2). In this sense, the concentration of 12.5 µg/mL was selected for the next investigations since there are recent reports of lectins that exerted immunomodulatory effects at this same concentration (Brito et al. 2017; Procópio et al. 2018; Patriota et al. 2017).
Investigation of the cytotoxic effect of Schinus terebinthifolia leaf lectin (SteLL) on BALB/c mice splenocytes after incubation for 24 h. Cytotoxic effect was assessed by flow cytometry using annexin V (AnnV) and propidium iodide (PI). AnnV−/PI+ cells were considered necrotic and AnnV+/PI− cells were considered apoptotic. Bars represent the mean ± standard deviation of six experiments
We also evaluated whether SteLL, at the same concentration used in the cytotoxic assay, would be able to cause damages in the splenocytes that, although not enough to lead to cell death, could impair their functionality. In addition, the analysis performed allows to detect if some type of activation of these cells was occurring. Therefore, we assessed the effects of SteLL on cytosolic and mitochondrial reactive oxygen species (ROS) levels, cytosolic calcium concentration, and mitochondrial membrane potential.
The ROS are not only by-products of cellular metabolism that mediate cell death and mitotic failure. They can interfere with signal transduction pathways, changing the metabolism through the oxidation of cellular proteins that are linked to immune cell activation (Murphy and Siegel 2013). In turn, the increase in cytoplasmic free calcium influx may constitute an early element of signaling cascades that trigger defense against pathogens; however, it can result from oxidative stress, causing influx into mitochondria (disrupting the normal cell metabolism and leading to death) and nuclei (modulating gene transcription and nucleases that control cell apoptosis) (Blume et al. 2000; Ermak and Davies 2002). Thus, it can be inferred that if a small increase in [Ca2+]cyt and the amount of ROS in the cytoplasm and mitochondria can be signs that an immune cell was being activated. On the other hand, a strong increase would point to the occurrence of cellular damage (Mittal et al. 2014).
SteLL did not alter [Ca2+]cyt (Fig. 3a) nor affected the levels of cytosolic (Fig. 3b) and mitochondrial ROS (Fig. 3c) in the splenocytes. This result agrees with the absence of cells in apoptosis or necrosis after treatment with SteLL and shows that the presence of these samples did not stress the splenocytes nor activate them. When alteration of the ΔΨm of splenocytes was evaluated, a slight reduction was observed in the cells incubated in the presence of SteLL with regard to the control (Fig. 3d). In spite of this, the absence of alterations in the concentrations of ROS and calcium and the non-occurrence of apoptosis ensure that the functioning of the cells treated with SteLL has not been compromised.
Evaluation of the effects of 24-h treatment of BALB/c mice splenocytes with Schinus terebinthifolia leaf lectin (SteLL) at 12.5 µg/mL on cytosolic calcium concentration (a), cytosolic (b) and mitochondrial (c) reactive oxygen species (ROS) production, and membrane mitochondrial potential (ΔΨm) (d) after incubation for 24 h. Bars represent the mean ± standard deviation of six experiments
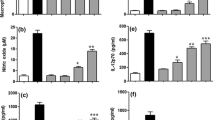
Since SteLL was not cytotoxic to the splenocytes, we assessed whether the ability of these cells to produce cytokines would be modulated by this lectin. As expected, in view of the results showed above, all the cytokines investigated were secreted by the splenocytes, ensuring that the cells were functional. When compared with the control cells, SteLL stimulated the release of IL-17A, TNF-α, IFN-γ, IL-4 and IL-2 (Fig. 4). Even though the release of IL-4 has been stimulated, the data indicate a predominant pro-inflammatory (Th1 and Th17) response. SteLL did not interfere with IL-10 release, which could have counterbalanced this profile through its regulatory properties.
The IL-2 is a 15-kDa protein that interacts with the IL-2Rα, IL-2Rβ, and IL-2Rγ receptors and stimulates the growth and proliferation of T and B lymphocytes through the Janus kinases/signal transducer and activator of transcription proteins (JAK/STATs) pathway. It also induces the production of other cytokines including IFN-γ and TNF-β, which result in the activation of monocytes, neutrophils and natural killer cells. IL-2 has been widely used in cancer therapies, for treatment of metastatic melanoma, renal cell carcinoma, and myelogenous leukemia (Brune et al. 2006; Rosenberg 2014; Mitra and Leonard 2018). IL-17 is a pro-inflammatory cytokine whose antitumor activity depends on IFN-γ production. IL-17 is activated by IL-23, which is the key factor for the expansion and maintenance of the Th17 population. IL-23 is closely associated with IL-12, which is involved in the production of IFN-γ by Th1 cells and antitumor immunological activity (Rosenberg 2014; Mitra and Leonard 2018; Shrihari 2017).
TNF-α is a cytokine associated with Th1 response that together with macrophage inflammatory protein 1α (MIP-1α), H2O2, and NO represents cytotoxic factors against microorganisms and tumor cells. Together with the chemokines (C-X-C motif) ligands (CXCL) 8, 1, and 2, TNF-α recruits and activates N1 phenotypic neutrophils, which have antitumor activity. In addition, the induction of apoptosis by TNF ligands and the release of IFN-γ inhibit the tumor cell proliferation (Shrihari 2017).
The IL-4 is a glycoprotein with anti-inflammatory properties that induces the differentiation of B lymphocytes to produce immunoglobulins G and E, which are important in allergic process and responses to helminthic infestations. IL-4 inhibits ROS production, which may a reason for the maintenance of ROS levels in splenocytes treated with SteLL, even with the reduction in mitochondrial membrane potential and the stimulus of a pro-inflammatory status (Rosenberg 2014; Mitra and Leonard 2018).
Other plant lectins have been described as immunomodulatory agents. The lectin from the inflorescences of Alpinia purpurata stimulated the release of Th1 (IFN-γ, TNF-α, and IL-6) and Th17 (IL-17A) cytokines and nitric oxide by human lymphocytes. In contrast, this lectin also increased the expression of IL-10, an anti-inflammatory mediator (Brito et al. 2017). In the same way, the lectin from Microgramma vacciniifolia frond increased TNF-α, IFN-γ, IL-6, IL-10, and nitric oxide production by human lymphocytes, inducing a predominant Th1 response (Patriota et al. 2017). In this sense, these lectins not only induced a pro-inflammatory response, but also stimulate the production of a cytokine of Th2 response, similarly to SteLL. The lectin isolated from the leaf pinnulae of Calliandra surinamensis did not induce apoptosis or necrosis of mice splenocytes and promoted increase of IL-2 and TNF-α production (Procópio et al. 2018). The authors stated that these results stimulate the evaluation of antitumor activity of this lectin.
Previous report showed that SteLL is an antitumor agent by causing apoptosis of sarcoma 180 cells with an IC50 (concentrations that reduced cell viability to 50%) of 8.30 μg/mL (Ramos et al. 2019). The authors also demonstrated that SteLL at 1 mg/kg and 5 mg/kg reduced in 73.6% and 57.6%, respectively, the weight of sarcoma 180 tumors in Swiss female mice. It was also suggested that the in vivo antitumor activity of SteLL may result in the activation of anti-cancer immune responses. The immunomodulatory activity demonstrated by us in the present paper strengthens this hypothesis.
When SteLL was used as treatment, due to its predominantly pro-inflammatory response, one could expect that the splenocytes would have increased the NO release, which did not occur (Fig. 4h). Hiroi et al. (2013) reported that IL-4 inhibits the NO production induced by IFN-γ. On this way, the increased expression of IL-4 by splenocytes treated with SteLL can be a reason for the slight decrease in the levels of NO, corresponding to other indication of the regulatory role of IL-4 in the status induced by SteLL.
Conclusion
The findings presented here demonstrate that SteLL is able to affect the release of specific cytokines by mice splenocytes without causing cellular stress. SteLL induced a predominant pro-inflammatory (Th1 and Th17) response, but regulated by IL-4. Thus, the popularly recognized anti-infectious and antitumor properties of S. terebinthifolia leaves may involve the immunomodulatory property of SteLL. In addition, the data reported here points out SteLL as a plant-derived prototype with biotechnological potential as an immunomodulator agent for use in studies with cultures of immune cells.
Abbreviations
- ΔΨm:
-
Mitochondrial transmembrane potential
- [Ca2+]cyt :
-
Cytosolic Ca2+ concentration
- AnnV:
-
Annexin V
- ATP:
-
Adenosine triphosphate
- CBA:
-
Cytometric bead array
- CXCL:
-
Chemokine (C-X-C motif) ligand
- ESI-QUAD-TOF:
-
Electrospray ionization quadrupole time-of-flight
- FITC:
-
Fluorescein isothiocyanate
- HA:
-
Hemagglutinating activity
- HSP70:
-
70-kDa heat shock proteins
- IC50 :
-
Concentrations that reduced cell viability to 50%)
- IFN-γ:
-
Interferon-gamma
- IL:
-
Interleukin
- JAK/STAT:
-
Janus kinases/signal transducer and activator of transcription proteins
- MIP-1α:
-
Macrophage inflammatory protein 1α
- MS/MS:
-
Tandem mass spectrometry
- NO:
-
Nitric oxide (NO)
- PBS:
-
Phosphate buffered saline
- PI:
-
Propidium iodide
- ROS:
-
Reactive oxygen species
- SDS-PAGE:
-
Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulphate
- SteLL:
-
Schinus terebinthifolia leaf lectin
- Th:
-
T helper
- TNF:
-
Tumor necrosis factor
- UFPE:
-
Universidade Federal de Pernambuco
References
Blume B, Nürnberger T, Nass N, Scheel D (2000) Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12:1425–1440
Brito JS, Ferreira GRS, Klimczak E, Gryshuk L, Santos NDL, Patriota LLS, Moreira LR, Soares AKA, Barboza BR, Paiva PMG, Navarro DMAF, Lorena VMB, Melo CML, Coriolano MC, Napoleão TH (2017) Lectin from inflorescences of ornamental crop Alpinia purpurata acts on immune cells to promote Th1 and Th17 responses, nitric oxide release, and lymphocyte activation. Biomed Pharmacother 94:865–872
Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann WK, Hogge DE, Nilssom B, Or R, Romero AI, Simonsson B, Spearing R, Stadtmauer EA, Szer J, Wallhult E, Hellstrand K (2006) Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood 108:88–96
Coelho LCBB, Silva PMS, Lima VLM, Pontual EV, Paiva PMG, Napoleão TH, Correia MTS (2017) Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evid Based Complement Altern Med 2017:1594074
Degasperi GR, Zecchin KG, Borecky J, Cruz-Höfling MA, Castilho RF, Velloso LA, Guimarães F, Vercesi AE (2006) Verapamil-sensitive Ca2+ channel regulation of Th1-type proliferation of splenic lymphocytes induced by Walker 256 tumor development in rats. Eur J Pharmacol 54:179–184
Ding AH, Nathan CF, Stuehr DJ (1988) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 141:2407–2412
Dwyer JM, Johnson C (1981) The use of concanavalin A to study the immunoregulation of human T cells. Clin Exp Immunol 46:237–249
Ermak G, Davies KJ (2002) Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol 38:713–721
Gomes FS, Procópio TF, Napoleão TH, Coelho LCBB, Paiva PMG (2013) Antimicrobial lectin from Schinus terebinthifolius leaf. J Appl Microbiol 114:672–679
Haney EF, Hancock REW (2013) Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 100:572–583
Hiroi M, Sakaeda Y, Yamaguchi H, Ohmori Y (2013) Anti-inflammatory cytokine interleukin-4 inhibits inducible nitric oxide synthase gene expression in the mouse macrophage cell line RAW264.7 through the repression of octamer-dependent transcription. Mediat Inflamm 2013:369693
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lannoo N, Van Damme EJM (2010) Nucleocytoplasmic plant lectins. Biochim Biophys Acta Gen Subj 1800:190–201
Lindenmaier DS, Putzke J (2011) Estudo etnobotânico em três comunidades Mbya/Guarani na região central do Rio Grande do Sul, Brasil. Cad Pesq 23:6–18
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 193:265–275
Melo CML, Paim BA, Zecchin KG, Morari J, Chiaratti MR, Correia MTS, Coelho LCBB, Paiva PMG (2010) Cramoll 1,4 lectin increases ROS production, calcium levels, and cytokine expression in treated spleen cells of rats. Mol Cell Biochem 342:163–169
Mitra S, Leonard WJ (2018) Biology of IL-2 and its therapeutic modulation: mechanisms and strategies. J Leukoc Biol 103:643–655
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antiox Redox Signal 20:1126–1167
Morton JF (1978) Brazilian pepper—its impact on people, animals and the environment. Econ Bot 32:353–359
Murphy MP, Siegel RM (2013) Mitochondrial ROS fire up T cell activation. Immunity 38:201–202
Patriota LLS, Procópio TF, Brito JS, Sebag V, Oliveira APS, Soares AKA, Moreira LR, Lima TA, Soares T, Silva TD, Paiva PMG, Lorena VMB, Melo CML, Albuquerque LP, Napoleão TH (2017) Microgramma vacciniifolia (Polypodiaceae) fronds contain a multifunctional lectin with immunomodulatory properties on human cells. Int J Biol Macromol 103:36–46
Patriota LLS, Brito JS, Barboza BR, Paiva PMG, Melo CML, Napoleão TH (2019) A review on the immunomodulatory effects of plant lectins. In: Ng TB, Wong J, Tse R, Tse TF, Chan H (eds) Hemagglutinins: structures, functions and mechanisms. Nova Science Publishers, New York, pp 53–82
Procópio TF, Moura MC, Albuquerque LP, Gomes FS, Santos NDL, Coelho LCBB, Pontual EV, Paiva PMG, Napoleão TH (2017) Antibacterial lectins: action mechanism, defensive roles and biotechnological potential. In: Collins E. (org) (ed) Antibacterials: synthesis, properties and biological activities. Nova Science Publishers, New York, pp 69–89
Procópio TF, Patriota LLS, Barros BRS, Aguiar LMS, Lorena VMB, Paiva PMG, Melo CML, Napoleão TH (2018) Calliandra surinamensis lectin (CasuL) does not impair the functionality of mice splenocytes, promoting cell signaling and cytokine production. Biomed Pharmacother 107:650–655
Ramos DBM, Araújo MTMF, Araújo TCL, Santos-Neto OG, Silva MG, Silva YA, Torres DJL, Patriota LLS, Melo CML, Lorena VMB, Paiva PMG, Mendes RL, Napoleão TH (2019) Evaluation of antitumor activity and toxicity of Schinus terebinthifolia leaf extract and lectin (SteLL) in sarcoma 180-bearing mice. J Ethnopharmacol 233:148–157
Ribas MO, Sou HM, Sartoretto J, Lanzoni TA, Noronha L, Acra LA (2006) Efeito da Schinus terebinthifolius Raddi sobre o processo de reparo tecidual das lesões ulceradas induzidas na mucosa bucal do rato. Rev Odonto Ciênc 21:245–252
Rosenberg SA (2014) IL-2: the first effective immunotherapy for human cancer. J Immunol 192:5451–5458
Santos TAR, Silva AC, Silva EB, Gomes PATM, Espíndola JWP, Cardoso MVO, Pereira VR (2016) Antitumor and immunomodulatory activities of thiosemicarbazones and 1,3-thiazoles in Jurkat and HT-29 cells. Biomed Pharmacother 82:555–560
Shrihari TG (2017) Dual role of inflammatory mediators in cancer. Ecancermedicalscience 11:721
Strbo N, Yin N, Stojadinovic O (2014) Innate and adaptive immune responses in wound epithelialization. Adv Wound Care 3:492–501
Tsien RY (1988) Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci 11:419–424
Acknowledgements
The authors express their gratitude to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 408789/2016-6) for research grants and fellowships (RBZ, PMGP and THN) as well as to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE; APQ-0108-2.08/14; APQ-0661-2.08/15) for financial support. The authors thank the Núcleo de Plataformas Tecnológicas of the Instituto Aggeu Magalhães, Fundação Oswaldo Cruz (FIOCRUZ Pernambuco). We thank Ana Lucia Oliveira Carvalho and Augusto Vieira for technical assistance with mass spectrometry analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
dos Santos, A.J.C.A., da Silva Barros, B.R., de Souza Aguiar, L.M. et al. Schinus terebinthifolia leaf lectin (SteLL) is an immunomodulatory agent by altering cytokine release by mice splenocytes. 3 Biotech 10, 144 (2020). https://doi.org/10.1007/s13205-020-2137-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-2137-2