Abstract
Energy efficiency ratio is significant in completely estimating lignocellulosic biomass pretreatment. In this work, rice straw (RS) was pretreated by ultra-high pressure (UHP), ionic liquid microemulsion (ILM), and a combination of UHP and ILM (ILM + UHP) at mild temperature. The chemical composition, crystalline structure, surface morphology, and enzymatic hydrolysis of untreated and pretreated RS samples were compared. After ILM pretreatment ([Emim]Ac/cyclohexane/Triton X-100/n-butanol = 0.25/0.15/0.45/0.15) at 500 MPa, 50 °C for 4 h, the cellulose content of the regenerated RS increased by 62.5, 66.2% of the lignin was removed, 37.3% of crystallinity index decreased, and the reducing sugar yield of 89.6% was achieved. All results show that the ILM + UHP pretreatments were more effective than sole UHP or ILM treatment at low temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an environmentally friendly renewable liquid biofuel, bioethanol can be obtained from lignocellulosic biomass, which mainly contains cellulose, hemicellulose, and lignin (John et al. 2011; Lynam et al. 2016). Biological degradation of lignin is extremely difficult because the material physically shields the cellulose and hemicellulose parts from the enzymes (Gao et al. 2013a, b). Abundant lignocellulosic biomasses, such as agro-residues (Asakawa et al. 2016; Jiang et al. 2013; Mai et al. 2014), forest residues (Zhao et al. 2016), agricultural weeds (Muktham et al. 2017; Naseeruddin et al. 2017; Singh et al. 2014), and grasses (Aswathy et al. 2010; Gao et al. 2014), have been hydrolyzed to fermentable sugars for subsequent biofuel production. However, the crystalline structure and high lignin content of lignocellulosic biomass provide difficulties for enzymatic hydrolysis, with enzymes failing to reach reaction sites (Teghammar et al. 2010).
Effective pretreatment is fundamental in improving hydrolysis and downstream operations. Pretreatment upstream operations are classified into four different categories, namely, physical, thermal, chemical, and biological, and involve differences in their chemical composition and physical properties (Limayem and Ricke 2012; Mood et al. 2013). During pretreatment, the cellulose and lignin matrix bound by hemicellulose should be broken to reduce cellulose crystallinity and increase the fraction of amorphous cellulose, which is the most suitable form for enzymatic attack (Saini et al. 2015). A sugar yield of 75% was recorded when olive tree biomass was pretreated with dilute sulfuric acid at 180 °C (Cara et al. 2008). Compared with dilute acid pretreatment, ionic liquid (IL) pretreatment is less caustic. ILs have recently become promising efficient solvents for enhancing the hydrolysis of lignocellulosic biomass (Aid et al. 2016; Chang et al. 2017; Hou et al. 2017). More lignin can be removed from switchgrass by IL pretreatment at 160 °C for 3 h compared with dilute acid presoaked for a minimum of 4 h and then heated at 160 °C for 20 min (Li et al. 2010).
Lignocellulosic biomass materials were consistently pretreated by ILs at high temperature (> 100 °C) demanding high energy (Limayem and Ricke 2012; Ninomiya et al. 2014). Meanwhile, toxic byproducts produced at high temperature can be a major hindrance to high-performance pretreatment (Limayem and Ricke 2012). In addition, cellulose recovery notably decreased with pretreatment temperature (Cara et al. 2008). Pretreatment of lignocellulosic biomass at mild temperature is essential to achieve high-energy efficiency ratio and mass balance.
Ultra-high pressure (UHP) processing is widely used as an advantageous nonthermal technique for food treatment at room temperature (Phunchaisri and Apichartsrangkoon 2005; Suarez-Jacobo et al. 2011). The applications of UHP in the extraction of low-molecular weight compounds and nonthermal sterilization are due to its notable properties, such as mild treatment condition and low impurity (Butz et al. 2003; Kim et al. 2012). Yang et al. (2009) subjected longan fruit pericarp to UHP pretreatment at 500 MPa and obtained hydrolysis degrees of control and UHP treatment of only 26.6 and 29.4%, respectively. UHP pretreatment should be combined with other treatments to reach a high degree of hydrolysis. In recent years, IL microemulsions (ILM) have been evaluated as unique, versatile treatment media of lignocellulosic biomass materials. After pretreatment with 1-ethyl-3-methylimidazolium acetate ([Emim]Ac)-based ILM at 70 °C for 12 h, a maximum delignification of 86.1% was observed on pretreated water hyacinths (Xu et al. 2016). However, only 57.5% of the pretreated samples could be recovered, thereby indicating that long time or high temperature for ILM pretreatment can result in considerable cellulose degradation. Hebishy et al. (2015) reported that UHP homogenization can increase the physical stability of oil-in-water emulsions. However, few publications on the enzyme hydrolysis of lignocellulosic biomass by combining ILM with UHP pretreatment are available.
In this study, an efficient pretreatment of rice straw was developed by combining UHP with [Emim]Ac-based microemulsion. The effect of pretreatment combination on the chemical composition, physical property, and enzymatic saccharification of the pretreated rice straw was systematically investigated.
Materials and methods
Materials
The following chemicals and enzymes were purchased from Sigma-Aldrich (St. Louis, MO, USA): Trichoderma reesei cellulase (Celluclast 1.5 L, Product #C2730-50 mL), β-glucosidase (Novo188, Product #C6105-50 mL), glucose assay kit (≥ 99%), and 3,5-dinitrosalicylic acid (DNS, 98%). [Emim]Ac (≥ 99%) was acquired from Lanzhou Institute of Chemical Physics (Lanzhou, China). Cyclohexane (≥ 99%), Triton X-100 (TX-100, ≥ 99%), and n-butanol (≥ 99%) were acquired from Aladdin Ltd. (Shanghai, China). Rice straw (Oryza sativa, RS) comprising 20% lignin, 22% cellulose, 35% hemicellulose, and 10% ash was obtained from a farmland near Zhanjiang City and then dried at 60 °C for 48 h. Ani et al. (2010) reported that the yield of glucose was significantly increased due to the size reduction. Therefore, dried rice straw was powdered to a particle size in the range of < 250 μm (60-mesh size).
Pseudo-ternary phase diagram of microemulsion
The IL-based microemulsion was constructed based on the isotropic properties of various compositions of the IL ([Emim]Ac), surfactant (TX-100), cosurfactant (n-butanol), and cyclohexane. The diagrams were determined by the titration of [Emim]Ac/TX-100/n-butanol mixture with cyclohexane at various temperature (30–50 °C). After each drop was added, the mixture was tempered in a thermostatic bath to guarantee steady-state conditions of the optically clear solution.
Pretreatment process
ILM pretreatment
A 5 g portion of dried rice straw was mixed in ILM (mass ratio of [Emim]Ac:cyclohexane:TX-100/n-butanol = 0.25:0.125:0.625) at a solid–liquid ratio of 1:10 w/v. The mixture was successively heated in an oil bath pan for 2 h each at 30 and 50 °C. After ILM pretreatment, 50 mL of distilled water was added, and the mixture was briefly centrifuged. The treated samples were washed and then dried in an oven at 45 °C for 24 h.
UHP pretreatment
Pressure treatments were conducted using a UHP experimental system (Tianjin Huatai Senmiao Limited Company, Tianjin, China). The system comprised a control panel, pressure vessel, hydraulic intensifier, and pumping system. All parts of the system exposed to high pressure were fabricated from stainless steel. The 0.6 L pressure vessel was designed to withstand a pressure of up to 600 MPa. A copper tube jacket in thermal contact with the outer surface of the vessel wall was connected to the circulator to allow temperature control (Huang et al. 2014).
On the basis of previous reports on the UHP pretreatment of starch (Guo et al. 2015a, b), 5 g of dried rice straw was transferred to vacuum-packed (− 100 kPa) polypropylene bags. The samples were subjected to pressures of 300 MPa at 50 °C, 500 MPa at 30 °C, and 500 MPa at 50 °C for 2 h. The samples were pressurized at a rate of 1 MPa/s. When the UHP pretreatment was completed, pressure was rapidly released to ambient pressure.
ILM + UHP pretreatment
Dried rice straw powder (5 g) was mixed in the ILM at a solid–liquid ratio of 1:10. The mixture was rapidly transferred to polypropylene bags. The vacuum-packed samples were subjected to pressures of 300 MPa at 50 °C, 500 MPa at 30 °C, and 500 MPa at 50 °C for 2 h. After pretreatment, the samples were washed with distilled water and then dried in an oven at 45 °C for 24 h. The pretreatment conditions are listed in Table 1.
Structure and morphology analysis of rice straw
Cellulose, hemicellulose, and lignin contents of pretreated and untreated RS samples were determined according to NREL procedures (Sluiter et al. 2008). The chemical structures of untreated and pretreated rice straw samples were investigated using a Tensor27 Bruker Fourier transform infrared (FT-IR) spectrophotometer through a standard KBr pellet technique. Each spectrum was recorded with 25 scans at a frequency range of 4000-400 cm−1 and a resolution of 4 cm−1.
X-ray diffraction analysis of rice straw samples was performed using an X-ray diffractometer (D8 Advance, Bruker Inc., Germany) at 40 kV and 30 mA voltage. X-ray intensities were collected for 2θ angles ranging from 5° to 45° at a step width of 0.02°. Crystallinity index (CrI, %) of each sample was analyzed using the following equation (Gao et al. 2013b):
where Iam is the intensity of the background scatter at 2θ = 18.2°, and I002 is the intensity of the peak at 2θ = 22.4°. The morphology of the rice straw samples was investigated using scanning electron microscopy (SEM) (S-4800, Hitachi Ltd., Japan). The samples were mounted on circular aluminum stubs with double-adhesive tape and coated with 20 nm of gold.
Enzymatic hydrolysis of rice straw
In a typical hydrolysis reaction, 20 mg of rice straw samples was added to 30 mL of acetate buffer (50 mM, pH 4.8) and incubated at 50 °C for 48 h with shaking at 120 rpm (Gao et al. 2014; Shill et al. 2011). Cellulase and β-glucosidase (1:1) were added at a loading of 20 FPU/g of cellulose for each reaction (Gao et al. 2014; Ninomiya et al. 2014). The total reducing sugar content derived from enzymatic hydrolysis was determined through the DNS method using a UV–vis spectrophotometer (UV-8000, Shanghai, China). Sugar yield was calculated using the following formula (Shill et al. 2011):
Results and discussion
Phase behavior of [Emim]Ac-based microemulsions
The ternary phase diagrams of [Emim]Ac-based microemulsion at 30 and 50 °C are shown in Fig. 1. A large single isotropic region that extends from the IL corner to the cyclohexane corner was observed. The blank region marked “single phase” is the one-phase microemulsion, whereas the shadow region marked “two phase” is a turbid region. A continuous and stable single-phase microemulsion region was always observed within the [Emim]Ac or cyclohexane content range of 0–100% at 30 and 50 °C. Moreover, the single-phase area slightly increased with temperature, thereby indicating that the formation of [Emim]Ac-based microemulsion is preferred at increased temperature. The IL-based microemulsion comprising [Emim]Ac, cyclohexane, and TX-100/n-butanol (3:1) with a mass ratio of 0.25/0.15/0.60 was selected as pretreatment media.
Lignocellulosic composition of pretreated rice straw
Pretreatment media polarity, temperature, and time may influence cellulose loss during the pretreatment. Bahcegul et al. (2012) reported a low recovery of 54.1% for cotton stalks (< 150 μm) pretreated with [Emim]Ac at 150 °C for 30 min. Previously, the microemulsion pretreatment with [Emim]Ac/ethanol/toluene system at 70 °C for 12 h resulted in cellulose recovery of approximately 57.2% (Xu et al. 2016). Herein, more than 80% of rice straw (< 250 μm) was regenerated at the end of each pretreatment (Table 2). Moreover, the high recovery rate may be attributed to the large particle size of the rice straw used in this study.
Lignin is an amorphous heteropolymer that acts as resistance against microbial attack (Hendriks and Zeeman 2009). Yang et al. (2009) reported that UHP pretreatment (100–500 MPa) did not exhibit any significant effect on the lignin composition and cellulose content of longan fruit pericarp. The chemical composition of rice straw samples before and after treatment in this study is summarized in Table 2 on a dry weight basis. Notably, the removal rate of lignin from the rice straw solely pretreated by ILM or UHP can reach 34.1–38.0% and 16.2–22.2%, respectively. The result suggests that the pretreatment of [Emim]Ac-based microemulsions exhibited higher capability to extract lignin from rice straw in comparison with UHP pretreatment in the temperature range of 30–50 °C. Furthermore, the combination pretreatment of [Emim]Ac-based microemulsions with UHP significantly promoted the delignification of rice straw (50.0–66.2%). Total delignification is difficult owing to the lignin location within the lignin–carbohydrate complex, strong poly-ring bonds of C–O–C, C–C, and hydrophobicity (Qiu and Aita 2013). Notably, the extracted lignin content increased with increasing pressure and temperature. In particular, 66.2% of the initial lignin was removed from rice straw, and cellulose content increased by 62.5% after ILM + UHP pretreatment at 500 MPa, 50 °C for 4 h as compared with untreated rice straw.
Structure and morphology properties of pretreated rice straw
The crystalline structure of lignocellulosic biomass is a major obstruction to its application in bioethanol production. Thus, a decrease in the crystallinity of lignocellulosic biomass can significantly enhance the efficiency of the subsequent enzymatic hydrolysis step (Ninomiya et al. 2014; Shill et al. 2011). Li et al. (2014) reported that after pretreatment with quadrol at 80 °C for 3 h, the cellulose crystal structure of Masson pine displayed cellulose I but transformed to cellulose II during the following treatment with anhydrous ILM at 80 °C for 8 h. The diffractograms of rice straw samples pretreated with ILM, UHP, and ILM + UHP display similar patterns to the initial rice straw (Fig. 2). Two typical diffraction peaks observed at 2θ = 16° and 22° for all samples, respectively, correspond to the (101) and (002) lattice planes of crystalline cellulose I (Gao et al. 2013a). The results indicate that minimal or no modification in the crystalline structures of pretreated rice straw occurred upon pretreatment with ILM (0.1 MPa, 50 °C, 4 h), UHP (500 MPa, 50 °C, 4 h), and ILM + UHP (500 MPa, 50 °C, 4 h).
The CrI value of various rice straw substrates is determined by Eq. (2) and illustrated in Table 2. The results show that the untreated and ILM-pretreated rice straw samples exhibit high CrI of 67.6 and 60.9%, respectively. By contrast, the UHP pretreatment at 300‒500 MPa can significantly decrease cellulose crystallinity. For example, when the pretreatment pressure increased from 300 to 500 MPa, the CrI value of the rice straw pretreated by single UHP at 50 °C decreased from 56.3 to 48.3%. Rice straw pretreated with ILM + UHP simultaneously exhibited a significantly lower CrI (42.4–47.9%) than that of untreated samples, thereby indicating an effectively destroyed crystalline structure (Chen et al. 2017).
FT-IR spectroscopic analysis was conducted to determine the structural changes in pretreated rice straw. As shown in Fig. 3, the wavenumbers between 3500 and 3100 cm−1 are related to the OH stretching vibrations involved in intramolecular hydrogen bonds formed between O(2)H···O(6) and O(3)H···O(5) of cellulose (Li et al. 2014). The OH stretching band of hydroxyl groups at approximately 3485 cm−1 shifted to an increased wavenumber of 3636 cm−1 due to the combined pretreatment of ILM (300 MPa, 50 °C) + UHP (500 MPa, 50 °C). This shift indicated decreased crystallinity (Aslanzadeh et al. 2011). However, no significant changes were observed in the FT-IR spectra of rice straw pretreated at 30 °C. Li et al. (2010) reported the characteristic peaks of cellulose at 2922, 1427, and 1373 cm−1 and the stretching vibration of C=O on hemicellulose at 1725 cm−1, which disappeared after pretreatment of quadrol and ILM on Masson pine. Herein, these peaks can be observed in all samples, and this finding agreed with the increased contents of hemicellulose.
Scanning electron microscopy was employed to examine the morphology changes of the pretreated rice straw. As shown in Fig. 4, the compact framework and relatively flat surface exhibited by untreated rice straw was nearly disrupted after each pretreatment. Several irregular cracks (approximately 100 nm) and porous are distinctly observed on the surface of rice straw pretreated by ILM and UHP, respectively. These cracks were probably due to the removal of lignin and the decrease in cellulose crystallinity (Qiu and Aita 2013). Therefore, the combination pretreatment of ILM + UHP yields additional accessible surface area of the pretreated rice straw to cellulase and β-glucosidase.
Enzymatic hydrolysis of pretreated rice straw
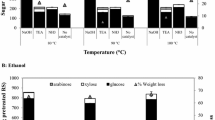
Enzymatic hydrolysis data of untreated and pretreated rice straw are summarized in Fig. 5. The sugar yield of untreated rice straw samples was only 31.6% at the end of hydrolysis for 48 h. After ILM + UHP treatment at 500 MPa and 50 °C for 4 h, the highest sugar yield of 89.6% for the pretreated rice straw was observed compared with those of the ILM-treated and UHP-treated samples (46.7 and 76.8%, respectively). The enzymatic hydrolysis of rice straw after different pretreatments in this work at 30–50 °C follows the order of none < ILM < UHP < ILM + UHP.
The ILM + UHP pretreatment severed the hydrogen bond net structure of cellulose in rice straw and destroyed the crystalline structure, which is beneficial to the loose and disordered morphology properties of the rice straw. Therefore, the combination pretreatment carried out at room/mild temperature can be effectively used to enhance the enzymatic hydrolysis of rice straw. Moreover, the rice straw pretreated with 300 MPa at 50 °C exhibited a higher enzymatic degradability than the samples pretreated with 500 MPa at 30 °C.
The theoretical yields of ethanol obtained from pretreated rice straw are calculated and listed in Table 2. The maximum ethanol yield from ILM + UHP treatment at 500 MPa and 50 °C for 4 h is approximately 20/100 g biomass, whereas butanol yield is 16/100 g biomass.
Conclusion
The feasibility of minimizing the energy intake of lignocellulosic biomass pretreatment was demonstrated by combining ILM and UHP pretreatment at 300–500 MPa, 30–50 °C. The ILM pretreatment exhibited considerable lignin extraction capability, and the selected UHP pretreatment produced porous, amorphous regenerated rice straw. After the combination pretreatment at 500 MPa and 50 °C for 4 h, the lignin content of the regenerated rice straw decreased by 66.2% compared with that of untreated rice straw, the CrI value decreased to 42.4%, and the maximum sugar yield reached 89.6%. The combination pretreatment of ILM and UHP exhibits high potential application in future bioethanol production.
References
Aid T, Hyvarinen S, Vaher M, Koel M, Mikkola JP (2016) Saccharification of lignocellulosic biomasses via ionic liquid pretreatment. Ind Crop Prod 92:336–341
Ani Y, Huang YC, Chen SH (2010) Effect of particle size on the rate of enzymatic hydrolysis of cellulose. Carbohydr Polym 79:192–199
Asakawa A, Oka T, Sasaki C, Asada C, Nakamura Y (2016) Cholinium ionic liquid/cosolvent pretreatment for enhancing enzymatic saccharification of sugarcane bagasse. Ind Crop Prod 86:113–119
Aslanzadeh S, Taherzadeh MJ, Horvath IS (2011) Pretreatment of straw fraction of manure for improved biogas production. BioResources 6:5193–5205
Aswathy US, Sukumaran RK, Devi GL, Rajasree KP, Singhania RR, Pandey A (2010) Bio-ethanol from water hyacinth biomass: an evaluation of enzymatic saccharification strategy. Bioresour Technol 101:925–930
Bahcegul E, Apaydin S, Haykir NI, Tatli E, Bakir U (2012) Different ionic liquids favor different lignocellulosic biomass particle sizes during pretreatment to function efficiently. Green Chem 14:1896–1903
Butz P, FernandezGarcia A, Lindauer R, Dieterich S, Bognar A, Tauscher B (2003) Influence of ultra high pressure processing on fruit and vegetable products. J Food Eng 56:233–236
Cara C, Ruiz E, Oliva JM, Saez F, Castro E (2008) Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification. Bioresour Technol 99:1869–1876
Chang KL, Chen XM, Wang XQ, Han YJ, Potprommanee L, Liu JY, Liao YL, Ning XA, Sun SY, Huang Q (2017) Impact of surfactant type for ionic liquid pretreatment on enhancing delignification of rice straw. Bioresour Technol 227:388–392
Chen BY, Zhao BC, Li MF, Liu QY, Sun RC (2017) Fractionation of rapeseed straw by hydrothermal/dilute acid pretreatment combined with alkali post-treatment for improving its enzymatic hydrolysis. Bioresour Technol 225:127–133
Gao J, Chen L, Yan ZC, Wang L (2013a) Effect of ionic liquid pretreatment on the composition, structure and biogas production of water hyacinth (Eichhornia crassipes). Bioresour Technol 132:361–364
Gao J, Chen L, Yuan K, Huang HM, Yan ZC (2013b) Ionic liquid pretreatment to enhance the anaerobic digestion of lignocellulosic biomass. Bioresour Technol 150:352–358
Gao J, Chen L, Zhang J, Yan ZC (2014) Improved enzymatic hydrolysis of lignocellulosic biomass through pretreatment with plasma electrolysis. Bioresour Technol 171:469–471
Guo Z, Zeng S, Lu X, Zhou M, Zheng M, Zheng B (2015a) Structural and physicochemical properties of lotus seed starch treated with ultra-high pressure. Food Chem 186:223–230
Guo Z, Zeng S, Zhang Y, Lu X, Tian Y, Zheng B (2015b) The effects of ultra-high pressure on the structural, rheological and retrogradation properties of lotus seed starch. Food Hydrocolloid 44:285–291
Hebishy E, Buffa M, Guamis B, Blasco-Moreno A, Trujillo AJ (2015) Physical and oxidative stability of whey protein oil-in-water emulsions produced by conventional and ultra high-pressure homogenization: effects of pressure and protein concentration on emulsion characteristics. Innov Food Sci Emerg 32:79–90
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Hou QD, Ju MT, Li WZ, Liu L, Chen Y, Yang Q (2017) Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems. Molecules 22:490–514
Huang WY, Ji HW, Liu SC, Zhang CH, Chen YL, Guo MH, Hao JM (2014) Inactivation effects and kinetics of polyphenol oxidase from litopenaeus vannamei, by ultra-high pressure and heat. Innov Food Sci Emerg 26:108–115
Jiang LQ, Fang Z, Li XK, Luo J, Fan SP (2013) Combination of dilute acid and ionic liquid pretreatments of sugarcane bagasse for glucose by enzymatic hydrolysis. Process Biochem 48:1942–1946
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193
Kim HS, Kim BY, Baik MY (2012) Application of ultra high pressure (UHP) in starch chemistry. Crit Rev Food Sci 52:123–141
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M, Vogel KP, Simmons BA, Singh S (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101:4900–4906
Li L, Yang RD, Liu DT, Yang F (2014) Influence of combined pretreatment of quadrol and anhydrous ionic liquid microemulsion on the physicochemical property of masson Pine. J Appl Polym Sci 131:985–987
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust 38:449–467
Lynam JG, Chow GI, Hyland PL, Coronella CJ (2016) Corn stover pretreatment by ionic liquid and glycerol mixtures with their density, viscosity, and thermogravimetric properties. ACS Sustain Chem Eng 4:3786–3793
Mai NL, Ha SH, Koo YM (2014) Efficient pretreatment of lignocellulose in ionic liquids/co-solvent for enzymatic hydrolysis enhancement into fermentable sugars. Process Biochem 49:1144–1151
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi G, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sust Energy Rev 27:77–93
Muktham R, Taha M, Shahsavari E, Bhargava SK, Bankupalli S, Ball AS (2017) Pongamia pinnata, seed residue-a low cost inedible resource for on-site/in-house lignocellulases and sustainable ethanol production. Renew Energy 103:682–687
Naseeruddin S, Desai S, Rao LV (2017) Ethanol production from lignocellulosic substrate Prosopis juliflora. Renew Energy 103:701–707
Ninomiya K, Yamauchi T, Ogino C, Shimizu N, Takahashi K (2014) Microwave pretreatment of lignocellulosic material in cholinium ionic liquid for efficient enzymatic saccharification. Biochem Eng J 90:90–95
Phunchaisri C, Apichartsrangkoon A (2005) Effects of ultra-high pressure on biochemical and physical modification of lychee (Litchi chinensis Sonn.). Food Chem 93:57–64
Qiu Z, Aita GM (2013) Pretreatment of energy cane bagasse with recycled ionic liquid for enzymatic hydrolysis. Bioresour Technol 129:532–537
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3. Biotech 5:337–353
Shill K, Padmanabhan S, Xin Q, Prausnitz JM, Clark DS, Blanch HW (2011) Ionic liquid pretreatment of cellulosic biomass: enzymatic hydrolysis and ionic liquid recycle. Biotechnol Bioeng 108:511–520
Singh S, Khanna S, Moholkar VS, Goyal A (2014) Screening and optimization of pretreatments for Parthenium hysterophorus as feedstock for alcoholic biofuels. Appl Energy 129:195–206
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. NREL/TP-510–42618. National Renewable Energy Laboratory (NREL), Golden
Suarez-Jacobo A, Ruefer CE, Gervilla R, Guami BS, Roig-Sagues AX, Saldo J (2011) Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem 127:447–454
Teghammar A, Yngvesson J, Lundin M, Taherzadeh MJ, Horvath IS (2010) Pretreatment of paper tube residuals for improved biogas production. Bioresour Technol 101:1206–1212
Xu F, Chen L, Wang AL, Yan ZC (2016) Influence of surfactant-free ionic liquid microemulsions pretreatment on the composition, structure and enzymatic hydrolysis of water hyacinth. Bioresour Technol 208:19–23
Yang B, Jiang YM, Wang R, Zhao MM, Sun J (2009) Ultra-high pressure treatment effects on polysaccharides and lignins of longan fruit pericarp. Food Chem 112:428–431
Zhao X, Xiong L, Zhang M, Bai F (2016) Towards efficient bioethanol production from agricultural and forestry residues: exploration of unique natural microorganisms in combination with advanced strain engineering. Bioresour Technol 215:84–91
Acknowledgments
This work was supported by Fund for National Natural Science Foundation of China (21706040), Guangdong Province Innovation School Project (2015KQNCX061, GDOU2015050240), Fund of Key Laboratory of Aquatic Product Processing, Ministry of Agriculture, China (B16387), and Fund of Scientific Research Start-up Funds of Guangdong Ocean University (E15178).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Gao, J., Zheng, C., Tan, T. et al. Enhanced saccharification of rice straw using combined ultra-high pressure and ionic liquid microemulsion pretreatments. 3 Biotech 8, 208 (2018). https://doi.org/10.1007/s13205-018-1216-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1216-0