Abstract
Ultrasound-assisted soaking in aqueous ammonia (USAA) pretreatment with 15 wt% aqueous ammonia under low temperature (~ 60 °C) and short-time (< 12 min) low-frequency (20 kHz, 60–650 W) ultrasound has been investigated for enhancement of enzymatic hydrolysis of corncob. Operational parameters of energy density (2.93–17.07 W/mL) and sonication time (0.34–11.66 min) that affect cellulose recovery, delignification, and sugar recovery yield were studied and optimized. The maximum cellulose recovery, delignification and sugar recovery yield determined at the optimum conditions (energy density 10 W/mL, sonication time 11.66 min) were 83.8, 84.7, and 77.6%, respectively. The corncob pretreated using USAA has a lower hemicellulose content (28.9% vs 31.8%), a slightly lower crystallinity index value (42.7% vs 43.7%), and a larger surface cavity diameter (> 36 μm vs < 20 μm) than that pretreated using soaking in aqueous ammonia (SAA) pretreatment. The USAA pretreatment was proved to be a reliable and effective method for corncob pretreatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulose, a renewable resource alternative to fossil resources, can produce second-generation bioethanol and commodity chemicals simultaneously, and has potential to resolve the food, energy, and environment trilemma (Tilman et al. 2009). Corncob has been recognized as a promising lignocellulosic feedstock for bioethanol production because of its wide distribution, high bulk density, and high collection and transport convenience (Liu et al. 2010). On the basis of these advantages, several energy companies, such as POET, DDCE, and ABENGOA, have chosen corncob as the major raw material for bioethanol production. However, the lignocellulosic material has a supramolecular matrix composed of cellulose, hemicellulose, and lignin, and its non-cellulosic element is a recalcitrant block of lignocellulosic biodegradation. To break this block, pretreatment is necessary. For corncob, many pretreatment methods have been evaluated: dilute acid (Wang et al. 2011; Kahar et al. 2010), sulphite (Cheng et al. 2011), oxalic acid (Lee et al. 2009, 2010), formic acid (Huang et al. 2010; Zhang et al. 2010), and soaking in aqueous ammonia (Du et al. 2012, 2013). Among these methods, soaking in aqueous ammonia (SAA) is superior to the others because of its ability to effectively remove lignin and retain major cellulose and hemicellulose fractions (Kim et al. 2008).
However, our previous research (Du et al. 2012, 2013) has found that SAA method has an underdone delignification (57.3–61.8%) and has no destructive effect on the surface of lignocellulosic materials but swelling. Therefore, there is a need for further improvement of this method to obtain higher delignification and more cracked surface; and hence, ultrasound was integrated with SAA methods. Recently, Xu et al. (2017) has studied ultrasound-assisted aqueous ammonia pretreatment (USAA) for intensification of enzyme hydrolysis for corn cob, whereas with dilute aqueous ammonia concentration of 1.0–4.0 wt%, low solid content of 0.5 g dry basis/25 mL buffer and long ultrasonic time of 1–4 h. In this study, corncob was used as a feedstock, which has a lignin content close to 20%. The ultrasound-assisted soaking in aqueous ammonia (USAA) pretreatment with 15 wt% aqueous ammonia under low temperature (~ 60 °C) and short-time (< 12 min) low-frequency (20 kHz, 60–650 W) ultrasound has been investigated for enhancement of enzymatic hydrolysis of corncob. Furthermore, it is hoped that acoustic cavitation will facilitate subsequent high-solid enzymatic hydrolysis.
Materials and methods
Materials
Corncob (glucan, 34.8%; xylan, 30.3%; lignin, 21.6%) was collected from a farm in Tianjin, China. It was washed with distilled water and air-dried to remove the moisture present. The dried corncob was milled and screened to 20–80 mesh. Cellulase (GC220, 160 FPU/mL) derived from Trichoderma reesei was provided by Genencor International (Palo Alto, CA, USA).
Sonicator
Scientz-IID amplifier (20 kHz, 0–950 W, Scientz Biotechnology Co., Ltd., Ningbo, China) with a Φ6 horn (tip diameter 6 mm, 20 kHz, 60–650 W) was used in this study. The ultrasonic pulse was maintained at 50% (2 s/2 s) and the temperature was controlled using a water bath.
Pretreatment of corncob
SAA pretreatment
Corncob was treated with 15 wt% aqueous ammonia in a screw-capped bottle at 60 °C for 12 h. Solid-to-liquid (S/L) ratios were 1:6. After pretreatment, the solids (SAA-treated corncob, SAACC) were separated from the liquid by filtration under vacuum and washed with deionized water until the pH reached 7.0. The residue obtained was oven dried until constant weight was observed, and then, it was subjected to enzymatic hydrolysis.
USAA pretreatment
Corncob was treated with 15 wt% aqueous ammonia and a 20 kHz sonicator in a screw-capped bottle at 60 °C for 12 h. The S/L ratios were 1:6. The horn was inserted approximately 1 cm under the surface of the sample solution. After pretreatment, the solids (USAA treated corncob, USAACC) were separated from the liquid by filtration under vacuum and washed with deionized water until the pH of the filtrate reached neutral condition. The residue obtained was oven dried until constant weight was observed, and then, it was subjected to enzymatic hydrolysis. The % cellulose recovery in the solid content was calculated using the following equation (Ramadoss and Muthukumar 2014):
where CCC is the amount of cellulose in native corncob and CPT-CC is the amount of cellulose in pretreated corncob measured in (g/g).
The % delignification was calculated using the following equation (Ramadoss and Muthukumar 2014):
where LCC is the amount of lignin present in native corncob and LPT-CC is the amount of lignin in pretreated corncob measured in (g/g).
Response surface methodology
To achieve high enzymatic digestibility after USAA pretreatment, ultrasonic parameters were optimized by the two factors, three levels central-composite design (CCD). The matrix corresponding to the CCD is presented in Table 1. Thirteen experiments were carried out with two variables and each variable varied at three levels for sugar recovery yields. The sugar recovery yield (YS) was calculated according to the following equation (Yoo et al. 2013):
where Rglucan (Rxylan) is the glucan (xylan) remaining after USAA pretreatment measured in (g), Dglucan (Dxylan) is the enzymatic digestibility of glucan (xylan) measured in (%), and Cglucan (Cxylan) is the glucan (xylan) content in untreated corncob measured in (g). A second-order polynomial model fitted for the sugar recovery yield (Y) was shown in the following equation (Yoo et al. 2013):
where A and B are the coded independent variables, and α0, α1, α2, α11, α22, and α12 are regression coefficients for the intercept, linear, quadratic, and interaction effects, respectively. The statistical analysis of the data was performed using Design-Expert software (version 8.0.5b, Stat-Ease, Inc., Minneapolis, USA).
Enzymatic hydrolysis
The enzymatic hydrolysis test was performed to evaluate the pretreatment effectiveness. 1 g of untreated or pretreated corncob sample was added to an Erlenmeyer flask containing 10 mL of 50 mM citric acid-NaOH buffer with a pH value of 4.8, 0.065 mL of GC220 (30 FPU/g glucan), which was then incubated in a shaking water bath at 50 °C and 120 rpm for 24 h. The initial glucan concentration was 10% (w/v) based on 100 mL of total liquid and solid. After enzymatic hydrolysis, the hydrolysates were immediately boiled for 10 min to denature the enzymes and then filtered. The supernatants were taken for fermentable sugar analysis using HPLC.
The glucan and xylan digestibilities were calculated as follows (Yoo et al. 2013):
(0.9 is the conversion factors of glucose to equivalent glucan)
(0.88 is the conversion factors of xylose to equivalent xylan).
Analytical methods
Carbohydrates and lignin contents in the corncob samples were determined according to the NREL procedures LAP 002. The morphologies of corncob samples before and after pretreatment were characterized by an S-4800 field emission SEM (Hitachi High-Technologies Co., Japan) at an acceleration voltage of 5 kV. Before SEM imaging, the samples were platinum sputter coated without critical point drying. X-ray diffraction (XRD) patterns of corncob samples were measured by an X’Pert Pro X-ray diffractometer (PANalytical Ltd., Holland) with a CoKα radiation of 1.789 Å (30 kV, 30 mA) and a scan rate of 12o min−1 from 2θ = 3 − 50 deg. The crystallinity index (CrI) defined as the percentage of crystalline material in corncob was calculated using the Segal method as follows (Wu et al. 2017):
where I002 is the peak intensity with respect to the (002) lattice plane and Iam is the peak intensity with regard to amorphous zone diffraction intensity of 2θ ≈ 18.8o.
Results and discussion
Composition of corncob
The composition of raw corncob was found to be 34.7 ± 0.3% cellulose, 31.3 ± 0.2% hemicellulose, 19.0 ± 0.1% lignin, 1.3 ± 0.3% ash, and 13.7 ± 0.3% others (extractable non-carbohydrates). The hemicellulose part was found to consist of 92% xylan and 8% arabinan and the lignin part was found to consist of 89% Klason lignin and 11% acid soluble lignin. Carbohydrates present in corncob accounted for about 66% of the dried material and this indicates the suitability of corncob as a very promising substrate.
Pretreatment
Corncob was pretreated with two different methods and the composition data obtained are presented in Table 2. SAA pretreatment method produced 73.6 g dry weight per 100 g corncob and showed 96.3% cellulose recovery. The hemicellulose and lignin elimination ratio obtained was 25.2 and 68.2%, respectively. USAA pretreatment showed a yield of 72.3 g dry weight per 100 g corncob. The cellulose recovery obtained was 83.8%, whereas hemicellulose and lignin removal were 33.2 and 84.7%, respectively (Fig. 1). The hemicellulose and lignin content in native corncob was 31.3 and 19.0%, respectively, and after the USAA pretreatment, the amount was reduced to 28.9 and 4.03%, respectively. This is due to the synergistic effect of ammonia and hydroxyl radicals formed during the cavitation which enhanced the depolymerization of lignin and cleavage of lignin–hemicellulose linkages (Ramadoss and Muthukumar 2014). Hence, the dry weight of USAA pretreated corncob was decreased. The USAA pretreatment gave 31.8% more removal of hemicellulose and 24.1% more removal of lignin compared to SAA pretreatment, respectively.
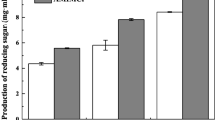
RSM study
The RSM experiments were used to optimize the USAA pretreatment conditions and performed with S/L ratio of 1:6 and 15 wt% aqueous ammonia. The sugar recovery yield (YS) was calculated and used due to its combination of carbohydrate remaining and enzymatic digestibility (see Eqs. (3)–(6)) (Yoo et al. 2013). The responses of sugar recovery yield were subjected to ANOVA analysis. The second-order polynomial equation, describing the sugar recovery yield (YS) as a simultaneous function of energy density and sonication time of USAA pretreatment, is shown below:
Values of p for A, B, A2, and B2 less than 0.05 indicated that these coefficients can significantly affect sugar recovery yield, and for AB greater than 0.1000 indicated that this coefficient cannot significantly affect sugar recovery yield. The F value of 11.63 implies the model is significant. The determination coefficient (R2 = 0.9991) indicated that 99.91% of the variability in the response could be explained by the model. The 3D response surface for sugar recovery yield is shown in Fig. 2. The optimization of energy density and sonication time of USAA treatments to achieve maximum sugar recovery yield was carried out in the range of experimental runs using the Design-Expert software. The maximum sugar recovery yield and optimal conditions were also determined as 77.6% with 10 W/mL energy density, 11.66 min sonication time, 15 wt% NH4OH, 1:6 S/L ratio, and 60 °C reaction temperature.
Morphologies of corncob
The morphological changes induced by pretreatment were imaged by SEM to obtain insight into the principle of different pretreatment method, and the results are shown in Fig. 3. The image of CC shows that it has a relatively smooth and continuous surface, while those of the pretreated corncob samples exhibit an ablated and collapsed surface containing large quantities of cellular structures, indicating that both two pretreatment methods (SAA and USAA) have modified the structure of corncob to some degree. The broken surface and cellular structures can be attributed to the collective effect of NH4OH and ultrasound. Kim et al. (2008) previously reported that ammonia could effectively remove lignin from biomass and swell the substrate. At the same time, the SAA at low temperature (~ 60 °C) has been proved which can effectively retain the hemicellulose in the solids (Rollin et al. 2011). As Wu et al. (2017) and Su et al. (2017) pointed out, ultrasonication as well as the resulting shear forces, shock waves, and microjets can split the interior and surface of corncob. Moreover, cavitation as well as the consequent highly reactive hydroxyl radicals can attack the lignocellulosic cell wall constituents at close proximity, resulting in collapse of the lignocellulose matrix, interrupt of cellulose chains, and modification of lignin network. It can be seen that cavity diameter (36.1–42.0 μm) of the outer surface of the USAA pretreated corncob samples were dramatically amplified, indicating that the ultrasonication intensified the swelling effect of SAA pretreatment.
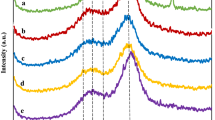
Crystallinity of corncob
Crystallinity of lignocellulosic substrate has been recognized as one of the major properties determining hydrolysis rate (Bansal et al. 2010; Shirkavand et al. 2016). In general, the amorphous fraction of lignocellulosic substrate is related to hemicellulose and lignin, and cellulose is almost considered crystalline. In this work, the effect of pretreatment on the cellulose crystallinity of corncob was investigated through XRD analysis, and the results are shown in Fig. 4. The major diffraction peak corresponding to the cellulose crystalline plane (002) was obtained for 2θ ranging between 21.8° and 27.5°, and the smaller peak corresponding to the amorphous zone (101) was obtained for 2θ = 18.8°. It can be observed that the pretreated corncob samples have higher (002) plane. According to the Segal method, the calculated crystallinity index values are 34.6, 43.7, 42.7, 42.3, and 42.1% for corncob, SAACC, USAACC-6, USAACC-8, and USAACC-10, respectively. It can be seen that the SAA and USAA pretreatment distinctly increases the crystallinity index of corncob. Based on the above composition and SEM analyses, it can be concluded that the change in the crystallinity index is largely due to the increase of cellulose content (Chundawat et al. 2011). USAACC-6, USAACC-8, and USAACC-10 have a slightly lower crystallinity index value than SAACC, indicating that the ultrasound-assisted soaking in aqueous ammonia pretreatment may have a better efficiency in decreasing the crystallinity of corncob.
Conclusions
In this study, corncob was pretreated using USAA method for the fermentable sugar production. The maximum cellulose recovery (83.8%), delignification (84.7%), and sugar recovery yield (77.6%) were obtained at the optimum conditions of energy density 10 W/mL and sonication time 11.66 min. The composition analysis shows that the USAA pretreatment could selectively remove lignin and hemicellulose without degrading cellulose. XRD analysis confirmed the slightly reduction in cellulose crystallinity by USAA and SEM analysis confirmed the dramatic amplification of surface cavity diameter, which is beneficial to fermentable sugar production. The results of this study concluded that USAA was a reliable and effective method for corncob pretreatment.
References
Bansal P, Hall M, Realff MJ, Lee JH, Bommarius AS (2010) Multivariate statistical analysis of X-ray data from cellulose: a new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour Technol 101:4461–4471
Cheng K-K, Wang W, Zhang J-A, Zhao Q, Li J-P, Xue J-W (2011) Statistical optimization of sulfite pretreatment of corncob residues for high concentration ethanol production. Bioresour Technol 102:3014–3019
Chundawat SPS, Donohoe BS, Sousa LdC, Elder T, Agarwal UP, Lu F, Ralph J, Himmel ME, Balan V, Dale BE (2011) Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment. Energy Environ Sci 4:973–984
Du R, Su R, Li X, Tantai X, Liu Z, Yang J, Qi W, He Z (2012) Controlled adsorption of cellulase onto pretreated corncob by pH adjustment. Cellulose 19:371–380
Du R, Huang R, Su R, Zhang M, Wang M, Yang J, Qi W, He Z (2013) Enzymatic hydrolysis of lignocellulose: SEC-MALLS analysis and reaction mechanism. RSC Adv 3:1871–1877
Huang R, Qi W, Su R, He Z (2010) The optimization of fractionating lignocellulose by formic acid using response surface methodology. Energ Source Part A 32:1282–1292
Kahar P, Taku K, Tanaka S (2010) Enzymatic digestion of corncobs pretreated with low strength of sulfuric acid for bioethanol production. J Biosci Bioeng 110:453–458
Kim TH, Taylor F, Hicks KB (2008) Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresour Technol 99:5694–5702
Lee J-W, Rodrigues RCLB, Jeffries TW (2009) Simultaneous saccharification and ethanol fermentation of oxalic acid pretreated corncob assessed with response surface methodology. Bioresour Technol 100:6307–6311
Lee J-W, Rodrigues RCLB, Kim HJ, Choi I-G, Jeffries TW (2010) The roles of xylan and lignin in oxalic acid pretreated corncob during separate enzymatic hydrolysis and ethanol fermentation. Bioresour Technol 101:4379–4385
Liu K, Lin X, Yue J, Li X, Fang X, Zhu M, Lin J, Qu Y, Xiao L (2010) High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour Technol 101:4952–4958
Ramadoss G, Muthukumar K (2014) Ultrasound assisted ammonia pretreatment of sugarcane bagasse for fermentable sugar production. Biochem Eng J 83:33–41
Rollin JA, Zhu Z, Sathitsuksanoh N, Zhang Y-HP (2011) Increasing cellulose accessibility is more important than removing lignin: a comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol Bioeng 108:22–30
Shirkavand E, Baroutian S, Gapes DJ, Young BR (2016) Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew Sust Energ Rev 54:217–234
Su R, Yang R, Jifeng Y, Du R, Huang R, Qi W, He Z (2017) Oscillating cellulase adsorption and enhanced lignocellulose hydrolysis upon ultrasound treatment. Trans Tianjin Univ 23:11–19
Tilman D, Socolow R, Foley JA, Hill J, Larson E, Lynd L, Pacala S, Reilly J, Searchinger T, Somerville C, Williams R (2009) Beneficial biofuels—the food, energy, and environment trilemma. Science 325:270–271
Wang GS, Lee J-W, Zhu JY, Jeffries TW (2011) Dilute acid pretreatment of corncob for efficient sugar production. Appl Biochem Biotech 163:658–668
Wu H, Dai X, Zhou S-L, Gan Y-Y, Xiong Z-Y, Qin Y-H, Ma J, Yang L, Wu Z-K, Wang T-L, Wang W-G, Wang C-W (2017) Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour Technol 241:70–74
Xu Q-Q, Zhao M-J, Yu Z-Z, Yin J-Z, Li G-M, Zhen M-Y, Zhang Q-Z (2017) Enhancing enzymatic hydrolysis of corn cob, corn stover and sorghum stalk by dilute aqueous ammonia combined with ultrasonic pretreatment. Ind Crops Prod 109:220–226
Yoo CG, Nghiem NP, Hicks KB, Kim TH (2013) Maximum production of fermentable sugars from barley straw using optimized soaking in aqueous ammonia (SAA) pretreatment. Appl Biochem Biotech 169:2430–2441
Zhang M, Qi W, Liu R, Su R, Wu S, He Z (2010) Fractionating lignocellulose by formic acid: characterization of major components. Biomass Bioenerg 34:525–532
Acknowledgements
This research was supported by the Research Project of Chongqing Education Commission (No. KJ1500632), Chongqing Technology and Business University (No. 1556035 and 20145601), and the Natural Science Foundation of China (No. 21776212).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Du, R., Su, R., Qi, W. et al. Enhanced enzymatic hydrolysis of corncob by ultrasound-assisted soaking in aqueous ammonia pretreatment. 3 Biotech 8, 166 (2018). https://doi.org/10.1007/s13205-018-1186-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1186-2