Abstract
This study investigates the potential application of the polyethyleneimine- (PEI) and amidoxime-modified Spirulina (Arthrospira) platensis biomasses for the removal of uranium ion in batch mode using the native biomass as a control system. The uranium ion adsorption was also characterized by attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra, zeta potential analysis, and surface area measurement studies. The effects of pH, biomass amount, contact time, initial uranium ion concentration, and ionic strength were evaluated by using native and modified algal biomass preparations. The uranium ion removal was rapid, with more than 70 % of total adsorption taking place in 40 min, and equilibrium was established within 60 min. From the experimental data, it was found that the amount of adsorption uranium ion on the algal preparations decreased in the following series: amidoxime-modified algal biomass > PEI-modified algal biomass > native algal biomass. Maximum adsorption capacities of amidoxime- and PEI-modified, and native algal biomasses were found to be 366.8, 279.5, and 194.6 mg/g, respectively, in batchwise studies. The adsorption rate of U(VI) ion by amidoxime-modified algal biomass was higher than those of the native and PEI-modified counterparts. The adsorption processes on all the algal biomass preparations followed by the Dubinin–Radushkevitch (D-R) and Temkin isotherms and pseudo-second-order kinetic models. The thermodynamic parameters were determined at four different temperatures (i.e., 15, 25, 35, and 45 °C) using the thermodynamics constant of the Temkin isotherm model. The ΔH° and ΔG° values of U(VI) ion adsorption on algal preparations show endothermic heat of adsorption; higher temperatures favor the process. The native and modified algal biomass preparations were regenerated using 10 mM HNO3. These results show that amidoxime-modified algal biomass can be a potential candidate for effective removal of U(VI) ion from aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is one such heavy metal having both radiochemical and toxicological effects (Brugge, and Goble 2002; Wang et al. 2015). It found in the aqueous environment in the hexavalent form, has biologically dynamic activity and chemical toxicity, leading to potential long-term harm to mammalian reproduction and development with reduced biological fertility, and abnormal and slow embryonic development (Gottlieb and Husen 1982). So, seeking a way for cleaning uranium ion contaminated water effectively and thoroughly has become an important research topic. In general, heavy metal ions from wastewater can be removed by physical, chemical, and biological methods such as adsorption, flocculation, coagulation, precipitation, membrane filtration, and electrochemical techniques (Erkaya et al. 2014; Kim et al. 2014; Li et al. 2014; Kushwaha and Sudhakar 2013; Zhang et al. 2013; Ozer et al. 2012; Ghasemi et al. 2011; Bayramoglu et al. 2006; Plazinski 2012). Adsorption of uranium ion by various adsorbents and microorganisms is reported in the literature (Fryxell et al. 2005; Yuan et al. 2011, 2012; Ding et al. 2012; Khani et al. 2008; Bhat et al. 2008). For example, Fryxell et al. (2005) have used glycinyl-urea- and salicylamide-modified MCM-41 silica particles for actinide (i.e., Th, Np, Am(III), Pu(IV), and U(VI)) adsorption, and the binding is dependent on medium pH. Yuan et al. (2011, 2012) have studied the adsorption of uranium from aqueous solution on the phosphonate- and dihydroimidazole-functionalized silica particle, and the maximum adsorption capacities of the modified silica particles were reported as 303 and 268 mg g−1, respectively. The maximum sorption capacity of the adsorbent for U(VI) ion was found to be 299 mg g−1 at pH 4.0. Yang et al. (2013) reported metal–organic frameworks (MOF-76) as adsorbent for U(VI) removal from aqueous solution. The maximum adsorption capacity of 298 mg g−1 was achieved at pH 3.0. Additionally, the selectivity of MOF-76 for U(VI) ion over a series of competing metal ions was also reported. Bai et al. (2015) prepared different amino group modified metal–organic frameworks for removal of radionuclides from aqueous solutions. The adsorption performances of the adsorbents toward U(VI) ion were studied, and these amine-modified adsorbents were highly efficient in removal of U(VI) compared to pristine materials. The adsorption capacity of diethylenetriamine-grafted materials for U(VI) was 350 mg g−1 at pH 5.5. On the other hand, various biomasses such as algae, fungi, and unicellular bacteria were also studied for adsorption or binding of uranium ion from aqueous solutions (Khani 2011). Among them, algal species either in living or in chemically modified form have been employed for the removal of uranium ion (Erkaya et al. 2014; Singhal et al. 2013; Lee et al. 2014; Cecal et al. 2012). The modification of microbial biomasses with various ligands such as polyethyleneimine (PEI), amidoxime, and chlorine dioxide groups improved their adsorption characteristics (Ding et al. 2014; He et al. 2014). For example, the chlorine dioxide modified microbial biomass (423 mg g−1) demonstrated about 10 % higher uranium adsorption capacity than its native counterpart (He et al. 2014). The maximum adsorption capacity of the Ca-pretreated Cystoseira indica alga was 318.15 mg g−1 at pH 4.5 (Ghasemi et al 2011). Algae are naturally abundant and found in all kinds of aquatic environment. Among algae species, Spirulina platensis is a photosynthetic, filamentous, spiral-shaped, multicellular, and blue-green microalgae. Its chemical composition includes proteins (55–70 %), carbohydrates (15–25 %), essential fatty acids (18 %), vitamins, minerals, and pigments like carotenes, chlorophyll a, and phycocyanin. Spirulina is the most important commercial microalgae for the production of biomass as health food and animal (Ali and Saleh 2012; Chen 2011; Promya, and Chitmanat 2011).
The purpose of the present study is to evaluate the adsorption capacity of the native, and chemically modified (i.e., PEI and amidoxime attached) S. platensis biomass for the removal of U(IV) ion from aqueous solutions. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy, Zetasizer analyses, and analytical methods were used to characterize algal biomass preparations and evaluate the possible binding sites. To evaluate removal mechanism and kinetics, the effects of the removal parameters such as pH, temperature, equilibrium time, and effect of initial uranium (VI) concentrations on the removal efficiency were investigated. The equilibrium and kinetic parameters of the adsorption processes in defined conditions were determined being useful for understanding the mechanisms involved in the removal of U(VI) ion from aqueous medium.
Materials and methods
Material
Diaminomaleonitrile (DAMN, 98 %), hydroxylamine hydrochloride (NH2 · OH · HCl), polyethyleneimine, and glutaraldehyde were purchased from Sigma-Aldrich Corp., St. Louis, MO, USA. All reagents were of analytical purity and used without further purification. All other chemicals were of analytical grade and were purchased from Merck AG (Darmstadt, Germany).
Microorganism and media
The microalgae S. platensis was isolated from Mogan Lake in Ankara. It was grown in the nutrient medium having a common composition of chemicals: NaHCO3, K2HPO4, MgSO4, CaCl2, citric acid, Na2EDTA, Na2CO3, trace metal ions solution, etc. (Chen 2011). This medium is used successfully for most cyanobacteria. S. platensis inoculated culture flasks were placed in a climate cabinet at 28 °C. The culture flasks containing algal cell suspension were subjected to continuous illumination at a light flux: U = 6.0 × 10−3 J cm−2 s−1.
Modification of S. platensis cells with PEI and amidoxime
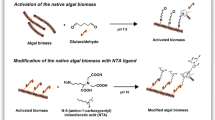
Modification of algal cells was carried out in two sequential steps: In the first step, algal cell surface was activated with glutaraldehyde (GA), and in the second step, the PEI and amidoxime ligands were attached onto GA-activated algal cell surface. For GA activation, S. platensis cells (about 5.0 g) were equilibrated in Tris–HCl buffer (25 mL, 50 mM, pH 8.0) for 2.0 h and activated with glutaraldehyde in the same Tris–HCl buffer (25 mL, 1.0 vol% glutaraldehyde). The glutaraldehyde activation reaction was carried out at 45 °C on water bath shaker for 4 h. After the reaction period, the GA-activated algal biomasses were collected by filtration and cleaned by washing sequentially with distilled water, and acetate buffer (0.1 M, pH 6.0). In order to attach PEI on the GA-activated algal cells, the following procedure was applied. The GA-activated algal cells were incubated with PEI solution (2.0 %) at pH 10 and at 65 °C in a reactor containing wet algal biomass (about 5 g) and were shaken for 6 h. After this period, the PEI-attached algal cells were removed from the medium by filtration and washed with 1.0 M NaCl. It was dried in a vacuum oven at 45 °C under vacuum for 24 h. The leakage of the PEI from the algal cells was followed by incubating the fully wetted algal cells with 10 mL of acetate buffer solution at pH 5.0 for 24 h at room temperature. The leakage experiments were carried out at room temperature at a stirring rate of 50 rpm. PEI released after this incubation was measured at 233 nm in the liquid phase spectrophotometrically.
For amidoxime group attachment, the glutaraldehyde-activated algal biomass (about 5.0 g, dry weight) was transferred ethanol (150 mL) containing DAMN (1.0 g), and the reaction was carried out at room temperature for 3.0 h. The DAMN-incorporated algal cells were collected by filtration and then modified into amidoxime groups. The modification reaction was carried out in ethanol/water mixture (90:10, v/v, 150 mL) containing NH2OH · HCl (2.0 g) and K2CO3 (2.0 g). The reaction mixture was placed in a round-bottomed flask and refluxed at 80 °C for 6 h. The amidoxime group generated algal biomass was collected by filtration and washed with distilled water and ethanol. The product was dried under reduced pressure at 45 °C for 24 h and stored at 4 °C until use. The schematic presentation of the PEI and amidoxime group incorporation on the algal biomass is presented in Fig. 1.
Characterization of native and modified algal biomasses
The ATR-FTIR spectra of the native, and PEI- and amidoxime-functionalized S. platensis biomasses were obtained in the one-bounce ATR mode in a Spectrum 100 FTIR spectrometer (Perkin-Elmer Inc., Norwalk, CT, USA) equipped with a Universal ATR accessory. Samples were scanned from 4000 to 400 cm−1. The surface area of the native, and PEI- and amidoxime-functionalized S. platensis biomass was measured by a surface area apparatus (Quantachrome Nov. 2200 E, USA.) and calculated using the Brunauer, Emmett, and Teller (BET) method. The surface charges of the native, and PEI- and amidoxime-functionalized S. platensis biomass at different pH values (pH 2.0–11.0) were measured by a zeta potential analyzer. During the ζ potential measurement, 0.1 g of dried biomass was transferred into 100 mL of purified water, and it was mixed about 1.0 h. The solution pH was adjusted with NaOH or HCl (0.1 M). After this period, the equilibrium solution pH was recorded, and the algal biomass suspension was used to conduct potential measurement with a Zetasizer instrument (NanoZS, Malvern Instruments Ltd., Malvern, UK). All the ζ potential measurements were conducted three times, and their average values were used.
Bisorption of uranium (VI) ion on native and modified S. platensis biomass
The adsorption of U(VI) ion on the native and modified algal biomass was investigated in a batch system. Uranium acetate (UO2(CH3COO)2) was used for the preparation of stock solution (2000 mg/L in Milli-Q water). A range of U(VI) “UO2 +2 uranyl ion” with different concentrations was prepared from above stock solution. Adsorption experiments were carried out at 25 ± 2 °C while rotated on an orbital shaker at 150 rpm for 2.0 h. A known amount of algal biomass (50 mg) was introduced in a 25-mL medium. After a predetermined time, the algal biomass was removed by filtration, and the concentration of the U(VI) ion in the medium was determined by using a spectrophotometric method (Bayramoglu, et al. 2006). Briefly, the method was based on the formation of colored complexes of U(VI) ion with sodium salicylate in aqueous medium. A calibration curve was constructed using U(VI) ion standards. The absorbance of the solution was measured at 468 nm using an UV/Visible spectrophotometer (PG Instrument Ltd., Model T80+; PRC). For each set of data reported, standard statistical methods were used to determine the mean values and standard deviations. The amount of U(VI) ion adsorbed per unit of algal biomass (mg U(VI) ion/g dry biomass) was obtained by using the following equation:
where q is the amount of U(VI) ion adsorbed onto the unit mass of the algal biomass (mg/g), C o and C are the concentrations of the U(VI) ion before and after adsorption (mg/L), V is the volume of the aqueous phase (L), and m is the amount of the biomass (g). After adsorption process, the used algal biomass in each test was dried in a vacuum oven at 40 °C overnight and the dry weight of the biomass was used in the calculations.
The effect of pH on the adsorption rate was investigated in the pH range 2.0–7.0. The medium pH was measured by Boeco microprocess pH meter, Model BT-600 (Germany), and the adsorption medium pH was adjusted by using 0.1 M HCl or NaOH solutions at 25 °C, and not controlled afterward at 25 °C. To optimize the algal biomass dosage (g/L), batch experiments were conducted using different amounts of algal biomass (between 0.4 and 3.6 g) in a 1.0-L solution at pH 5.0 and at 25 °C. To investigate the influence of ionic strength on U(VI) ion removal, sodium chloride was employed as background electrolyte and varied between 0.0 and 1.0 mol/L.
Desorption and regeneration studies
To carry out the desorption studies, the native and PEI- and/or amidoxime-modified algal biomass (about 50 mg) was transferred in 25 mL of HNO3 (10 mM) solution. After 2.0-h incubation, the suspension was separated and the leached U(VI) ion concentration in the supernatant was determined as described above. For desorption experiments, the algal biomass from adsorption experiments was gently washed with distilled water and transferred in HNO3 (10 mM) solutions and rotated on a shaker at room temperature for 2.0 h. After separation of samples with filtration, the remaining U(VI) ion concentration in the supernatant was measured to evaluate the desorption percentage. The regenerated algal biomass was washed thoroughly with phosphate buffer solution (50 mM, pH 6.0) and then used for the next sorption–desorption cycle.
Results and discussion
Characterization of the native and modified algal biomass
The average specific surface areas of the native, and PEI- and amidoxime-functionalized algal biomass were measured by Brunauer-Emmer-Teller (BET) method and were found to be 1.87, 2.26, and 2.47 m2/g for native, and PEI- and amidoxime-functionalized algal biomass, respectively. PEI and amidoxime modification caused an increase in specific surface area, in comparison to the native algal biomass. The chemical modification appears to provide more surface area for the algal biomass and would favor higher adsorption capacity for U(VI) ion. The increase in the surface area of the modified biomass can be due to the removal of lipid component of the algal cell walls during modification reactions.
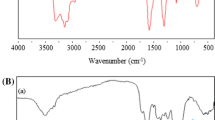
The spectra of native, and PEI- and amidoxime-modified S. platensis biomasses were measured by an ATR-FTIR spectrometer within the range of 400–4000 cm−1 wave number (Fig. 2a–c, respectively). The peaks at 3389, 2917, 1651, 1353, 1031, and 583 cm−1 are observed in the spectrum of the native algal biomass (Fig. 2a). Additionally, the broad spectrum of native algal biomass between 3500 and 3200 cm−1 could be attributed by the overlapping of the –OH and –NH stretching vibration bands. The bands at 1031 and 1084 cm−1 exhibited correlative characteristic of C–O stretching vibrations in alcohol and amine groups on the algal biomass surface. The changes in the functional groups and surface properties of the algal biomass after PEI and/or amidoxime modification are also confirmed by ATR-FTIR spectra (Fig. 2b, c, respectively). ATR-FTIR spectra of the PEI- and amidoxime-modified S. platensis biomasses confirm the heterogeneity of the biosorbents and evidence the presence of different characteristics peaks in agreement with the possible presence of amino, amidoxime, carboxylic, hydroxyl phosphate, and carbonyl groups. After attachment of PEI via glutaraldehyde coupling reaction, the spectrum of the PEI-modified algal biomass exhibits some changes (Fig. 2b). A broad shifted overlapping peak was observed at 3414 cm−1 due to the incorporation of a large number of amine groups by the PEI. The new peaks at 1462 and 1085 cm−1 are attributed to the C–H bending and C–N stretching, respectively. After incorporation of amidoxime groups (Fig. 3b), the appearance of new bands at 1647 and 1069 cm−1 are assigned to C = N and N–O stretching of oxime groups, respectively. The broad band between 3600 and 3300 cm−1 are ascribed to the bounded hydroxyl (–OH) or amine (–NH) groups. FTIR spectra of native and modified algal biomasses also revealed that the surface functional groups (such as –COOH, –NH2, and –OH) on the tested biosorbents are involved in U(VI) biosorption.
Zeta potential studies
PEI and amidoxime are well-known ligands for their metal chelation properties due to the presence of amine groups and are used for modification of biomass surface to increase their adsorption capacities (Bayramoglu et al. 2012; Liu et al. 2011). The modification protocols are schematically presented in Fig. 3. To investigate the change of S. platensis biomass surface charge before and after the surface modification protocols, the zeta potentials as a function of solution pH were measured using native, and PEI- and amidoxime-modified algal biomasses. The change in the zeta potential values of the native, and both modified algal biomass as a function of suspension medium pH is presented in Fig. 3. As observed in this figure, the zeta potential values for all the studied algal biomass decrease with increasing solution pH. The zero zeta potential point value for native algal biomass was found to be at around pH 4.0; on the other hand, the zero potential values for PEI- and amidoxime-modified algal biomass were observed at pH 10.5 and 10.0, respectively. The positive charge density on the PEI- and amidoxime-modified biomass surface significantly decreased with increasing medium pH due to the deprotonation of the amine groups on the ligand molecules. The zeta potential values were studied at pH values between 2.0 and 11.0 with the native and modified algal biomass preparations. The charge density in this pH range changed from −26.7 to 6.9 mV for native algal biomass. On the other hand, PEI- and amidoxime-modified algal preparations were changed from −13.6 to 33.2 and −16.8 to 21.4 mV, respectively. Thus, the medium pH is an important parameter to control on the adsorption of uranyl ion, since it determines the ionization state of the functional groups on the sorbent, as well as the degree of ionization state and speciation of the uranium ion. The zero zeta potential values of the native, and PEI- and amidoxime-modified algal biomass in our study are in accordance with the other studies.
Effect of algal biomass dosage on U(VI) ion adsorption
The effect of algal biomass dosage in the range of 0.4–3.6 g/L on the U(VI) ion removal by the native, and PEI- and amidoxime-modified algal biomass in the initial metal concentration of 1000 mg L−1 and temperature of 25 °C at pH 5.0 is presented in Fig. 4. As seen in this figure, increasing the algal biomass dosage from 0.4 to 2.0 g/L results in significant increase in U(VI) ion removal. At algal biomass dosages >2.0 g/L, the incremental U(VI) ion removal becomes less significant as the amount of U(VI) ion left in solution becomes very low. At algal biomass dose increase, the availability of functional groups and surface area of the biomass increase in the adsorption medium and, thus, provide higher adsorption sites for percent removal of U(VI) ion. It should be noted that with the increased amount of biosorbent, the relative amount of U(VI) ion biosorbed (mg/g) decreased. Therefore, 2.0 g/L of algal biomass was used in further U(VI) ion removal studies.
Effect of pH
Initial pH plays an important role in the adsorption of uranium ion from the aqueous medium. It influences both the speciation of uranium ion in the aqueous medium, and the binding sites present on the surface of algal biomass preparations (Rippka et al. 1979; Bayramoglu and Arica 2009). Many functional groups present on the algal biomass preparation, such as amine, hydroxyl, carboxyl, and phosphate. They not only were involved in the ion exchange of the uranium ion but also disturbed the combining force between functional groups on algal biomass surface and uranium ion in the medium (Donat et al. 2009; Bayramoglu and Arica 2011). The effect of pH on the adsorption process was studied at pH values ranging from 2.0 to 7.0. The results are shown in Fig. 5. As seen in this figure, the maximum uranium ion adsorption was observed at pH 5.0 for all the studied algal biomass preparations. The adsorption profile (mg U(VI) ion/g algal biomass) of the experimental at a uranium concentration (1000 mg/L) for 60 min was in the order of amidoxime-modified > PEI-modified > native biomass. The enhancement in U(VI) ion adsorption capacity upon attachment of PEI and amidoxime could be due to the algal biomass surface become enriched with amino, imino, and hydroxyl groups. The reason for maximum adsorption of uranium ion at pH 5.0 could be due to the presence of ligands like carboxyl, amino, imine and phosphate groups on the surface of the biomass, preparations which have pK a values in the range of 4.0–7.0 (Bayramoglu and Arica 2009; Akar et al. 2013). Further increase in pH may result in precipitation of U(VI) in the form of hydroxide complex whereas lowering the pH may result in excess acetate ions in the medium which prevents the adsorption of U(VI) ion. This finding was also confirmed by the zeta potential studies at different pH values (Fig. 3). According to these results, the amine groups (–NH–, –N=, –NH2) on the PEI- and amidoxime-modified biomass surface were protonated at pH below 10.5 and 10.0, respectively. On the other hand, the carboxyl group of the native biomass was protanated at around pH 4.0. As U(VI) ion existed as cationic at pH 5.0, the electrostatic attraction played an important role in the adsorption process at pH 5.0 with native algal biomass. As indicated above, the pH of solution can affect both the relative distribution of U(VI) species in solution and the surface properties of the tested biosorbents (i.e., native, and PEI-and amidoxime-modified). According to the relative distribution of U(VI) species in solutions, it can be found that UO2 +2 was the dominant species at around pH 5.0. It is thought that the amine and amidoxime groups can act as ligand systems for adsorption of uranyl cations. Thus, the complexation and electrostatic interactions between functional groups of the biomass preparations and U(VI) ion lead to a maximum adsorption capacity at around pH 5.0. In the literature, the best pH for uranium adsorption on the algal biomass preparations is reported to be between 4.0 and 5.0. For example, the optimal pH for adsorption of uranium ion by Cystoseira indica was at pH 4.0 (Ghasemi et al. 2011), at pH 4.5 for Catenella repens, a red alga (Bhat et al. 2008), and at pH 4.5 for Chlamydomonas reinhardtii (Erkaya et al. 2014). As optimum pH for adsorption process was found to be 5.0, all the following experiments were carried out at this pH.
Effect of ionic strength
Another important parameter which affects the U(VI) adsorption is medium ionic strength. Ionic strength of the adsorption medium was changed between 0.0 and 1.0 mol/L by adding a known amount of NaCl solution. As presented in Fig. 6, the adsorbed amount of U(VI) ion decreased with increasing ionic strength (Bai et al. 2013). U(VI) ion adsorption capacities of the native, and PEI- and amidoxime-modified algal biomass decreased by about 42, 59, and 64 % when the NaCl concentration in the adsorption medium was 1.0 mol/L. A decrease observed in the adsorption capacity of algal biomass preparations for U(VI) ion may be attributed to the electrostatic attraction forces between the algal cell surface and uranium ion (Bayramoglu et al. 2009). It should be noted that when the medium ionic strength is increased, repulsive electrostatic interaction between U(VI) ion and functional groups on the algal cell surface becomes dominant. Thus, hydrogen bonding and van der Waals interactions between the biosorbents and uranyl ion could be hidden. Similar results are reported by other researchers (Bayramoglu et al. 2006; Gharieb et al. 2014).
Effect of initial uranium ion concentration on adsorption
The initial concentration provides an important driving force to overcome all mass transfer resistance of uranium ion between the aqueous and solid phases. Hence, a higher initial concentration of uranium ion will increase the adsorption rate. Figure 7 shows the effect of equilibrium concentration of U(VI) ions on the adsorption capacity of the native, and PEI- and amidoxime-modified biomass preparations. The adsorption values increased with increasing equilibrium concentration of U(VI) ion, and a saturation value was achieved at U(VI) ion concentration of around 1000 mg/L, which represents saturation of the active binding sites on the algal biomass preparations. It should be noted that increasing initial U(VI) ion concentration increased the number of collisions between U(VI) ion and the adsorbent, which enhanced the adsorption process. The adsorption of U(VI) ion onto the native, and PEI- and amidoxime-modified algal biomasses was about 194.6, 279.5, and 366.8 mg/g dry biomass, respectively. It should be noted that significant improvements were observed with the PEI- and amidoxime-modified biosorbents in the adsorption of U(VI) ions, comparing to native algal biomass, indicating that the incorporation of amine groups on the surface of algal biomass is beneficial to adsorption capacity. In general, the adsorption process involves mainly on cell surface sequestration. Upon modification, the surface area of algal cell was increased between 1.87- and 1.32-fold and resulted enhancement in the U(VI) adsorption capacity for both modified algal preparations. Bai et al. (2015) prepared chromium-based metal–organic framework (MOF) and its amino derivatives. The amine-functionalized MOFs are much more efficient in U(VI) adsorption compared to bare MOF. And, the adsorbability of amine-functionalized MOFs follows the order of diethylenetriamine > ethanediamine > NH2. Therefore, both PEI- and amidoxime-modified biosorbents could be potentially used for removing U(VI) ion from aqueous solutions.
The equilibrium data for the removal of U(VI) ion using biosorbents into various isotherm models results in a suitable model that can be used for the design of an adsorption process. In the present study, different isotherm models were applied to describe interactions between the U(VI) ion and algal biomass preparations. Modeling of the experimental isotherm data has been done using the Langmuir (Langmuir 1919), Freundlich (Freundlich 1906), D-R (Dubinin and Radushkevich 1947), and Temkin isotherms (Temkin and Pyzhev 1940). The Langmuir model can be described by the following linearized equation:
where q max is the maximum adsorption capacity (mg/g), C e is the equilibrium U(VI) ion concentration in solution (mg/L), and b is the Langmiur constant.
The Freundlich, D-R, and Temkin isotherms are represented as linearized in Eqs. 3, 4, and 5, respectively:
where K F is the Freundlich constant, and n is the Freundlich exponent. 1/n is a measure of the surface heterogeneity ranging between 0 and 1, becoming more heterogeneous as its value gets closer to zero.
In Eq. (4), q m is the theoretical monolayer adsorption capacity (mg/g), K is the constant of the sorption energy (mol2/J2), which is related to the average energy of adsorption per mole of the biosorbate as it is transferred to the surface of the solid from infinite distance in the solution, and ε is Polanyi potential [ε = RT ln (1 + 1 / C e), where T is the solution temperature (K) and R is the gas constant and is equal to 8.314 J/mol · K. The value of mean free energy of adsorption, E (kJ/mol), can be calculated from D-R parameter K [E = (−2 K)−1/2]. In Eq. (5), K T (L/mg) is the equilibrium binding constant corresponding to the maximum binding energy, and q T (mg U(VI)/g algal biomass) is the differential surface capacity for uranyl ion adsorption per unit binding energy.
The Langmuir constants q max and b for U(VI) ion adsorption on the algal biomass preparations were determined from the slope and intercept of the linear plot of specific sorption (C e/q e) against the equilibrium concentration (C e) and are presented in Table 1. The maximum adsorption capacities (q max) for the native, and PEI- and amidoxime-modified algal biomass preparations were determined from the slope to be 114.9, 434.8, and 370.4 mg/g and the Langmuir constants were extracted from the intercept to be 2.61 × 10−4, 2.58 × 10−3, and 2.81 × 10−2 L/mg, respectively. The maximum adsorption capacities (q max) for the native, and PEI-modified algal biomass preparations were not fitted well with experimental capacity. And also, correlation coefficient (R 2) was low between 0.141 and 0.663 for the native and PEI-modified algal biomass preparations (Table 1), indicating that the Langmuir adsorption model cannot be applied in these algal biomass preparations. However, a high Langmuir constant, b (i.e., K a), value for amidoxime-modified algal biomass indicates a high affinity, and also the Langmuir constant q max represents the monolayer saturation at equilibrium when the surface is fully covered with uranyl ion and assists in the explanation of adsorption performance. These results show that the adsorption of uranyl ion on amidoxime-modified algal biomass obeys only the Langmuir model.
The Freundlich model is an empirical equation assuming heterogeneous adsorptive energies on the adsorbent surface. The K F and n values can be evaluated from the intercept and the slope of the linear plot of experimental data of ln q e versus ln C e. For U(VI) ion, the measured K F values of the studied algal preparations showed an easy adsorption. The value of n proposes the favorability of the adsorption systems. According to the theory, n > 1 represents favorable adsorption conditions (Table 1). In addition, the n values of native and PEI-modified algal biomass were lower than those of amidoxime-modified biomass, meaning that the slope (1/n) of the Freundlich plots was steeper and the capacity of sorbent was more susceptible to the modification techniques.
Calculated isotherm parameters in D-R, and Temkin equations are consistent with the experimental q e values for adsorption of U(VI) ions. When R 2 values are considered, it was seen that the D-R isotherm and Temkin isotherm models were also well fitted to the experimental data. From the linear plot of D-R model for the native, and PEI- and amidoxime-modified algal biomass preparations, q m was determined to be 121.6, 270.3, and 330.4 mg/g; the mean free energy 0.041, 0.58, and 0.259 kJ/mol, respectively, indicating a physiosorption process; and the correlation coefficient, R 2, higher than that of Freundlich for all the studied algal biomass preparations. If the E value is below 8 kJ/mol, physical sorption is considered to occur, and in the ranges from 8 to 16 kJ/mol, chemical sorption occurs. In this adsorption system, the magnitude of energy of adsorption (E) in the D-R isotherm is around 0.041–0.259 kJ/mol which indicated physical adsorption and useful for the assessment of the adsorption mechanism. As a result, the D-R and Temkin sorption models seem to provide the best fit with the experimental data and predict values for the adsorption of uranyl ions from aqueous solution on native and PEI-modified algal biomass, whereas for amidoxime-modified algal biomass, all tested sorption models, i.e., Langmuir, D-R, and Temkin isotherms, except Freundlich, fit quite well with the experimental data (correlation coefficient R 2 > 0.942). This was very suitable for describing the adsorption equilibrium of uranyl ions from aqueous solution on amidoxime-modified algal biomass.
Thermodynamic parameters
The effect of temperature on the adsorption of U(VI) was investigated at four different temperatures (i.e., 15, 25, 35, and 45 °C), at pH 5.0. The adsorption of U(VI) ion on the native, and PEI- and amidoxime-modified algal biomass was found to increase with the increase in temperature, indicating that the process is endothermic in nature (Table 2). The reason for an increase in U(VI) ion adsorption on the algal biomass preparations at high temperatures could be attributed to increase on probability of interaction between U(VI) ion and functional groups on algal biomass surface because of an increase in the energy of the system. Both physical and chemical adsorptions may occur on the surface at the same time; a layer of molecules may be physically adsorbed on top of an underlying chemisorbed layer. The same surface can display physisorption at one temperature and chemisorption at a higher temperature. Activation energy is an important parameter in a thermodynamic study as it determines the temperature dependence of the reaction rate. The activation energy (E a) for the adsorption of an adsorbate onto an adsorbent surface in an adsorption process can be determined from experimental measurements of the adsorption rate constant at different temperatures according to the Arrhenius equation. The activation energy (E a) values for the native, and PEI- and amidoxime-modified algal biomass preparations were determined as −10.2, −13.9, and −4.98 kJ/mol, respectively (Table 2). In physical adsorption, the equilibrium is usually rapidly attained and easily reversible, because the energy requirements are small. The activation energy for physical adsorption is usually no more than 4.2 kJ/mol, since the forces involved in physical adsorption are weak.
In order to further support the assertion that physical adsorption is the predominant mechanism, the values of sticking probability (S*) were estimated from the experimental data. The values of activation energy (E a) and sticking probability (S*) were calculated from the experimental data throughout the temperature range from 288 to 318 K by calculating the surface coverage at various temperatures, presented in Table 2. They were calculated using modified Arrhenius-type equation related to surface coverage (θ) as follows (Horsfall and Spiff 2005):
The values of E a and S* were calculated from slope and intercept of the plot of ln(1 − θ) versus 1/T (Table 2). The value of S* which was very close to zero confirmed the dominance of physisorption mechanism (Horsfall and Spiff 2005). Positive value of E a supports the endothermic nature of the adsorption process. This is in accordance with the positive values of ∆H. The results as shown in Table 2 indicate that the probability of U(VI) ion to stick to the biomass surface is very high as 0 < S* < 1 for U(VI) ion from aqueous solution.
The thermodynamic parameters of process, such as enthalpy change (ΔH°), entropy change (ΔS°), and Gibbs free energy change (ΔG°) of U(VI) adsorption on the native and both modified algal preparations can be calculated by using the following equations:
where K c (=q e/C e) obtained from q e/C e is the equilibrium constant in molar unit, and T is the temperature (K). The values of ΔH° and ΔS° were calculated from slope and intercept of van’t Hoff plot of ln K a versus 1/T (data not shown here).
The ΔH° and ΔS° values for the native, and both the PEI- and amidoxime-modified algal biomass preparations were determined as 18.9, 31.7, and 26.4 kJ/mol and 0.101, 0.149, and 0.137 J/mol K, respectively, while the ΔG° values were −11.1, −12.8, and −14.6 kJ/mol at 298 K (Table 2). The amount of U(VI) ion adsorbed increased for the native, and PEI- and amidoxime-modified algal biomass preparations from 171.8 to 261.4, from 224.1 to 368.2, and from 329.4 to 426.9 mg/g, respectively, when the temperature was increased from 15 to 45 °C (Table 2). The positive value of ΔH° showed that U(VI) ion adsorption was endothermic and the magnitude of ΔH° implies that the nature of adsorption was also physical in nature, involving electrostatic and/or hydrophobic interactions. The positive values of entropy ΔS° indicate the increased randomness at the solid-solution interface during the adsorption of U(VI) ion on the active sites of the algal preparations. Also, a positive entropy value represented a degree of freedom of the adsorbed species. The negative values of ΔG° indicate the spontaneous nature of adsorption between 288 and 318 K. The magnitude of ΔG° increased from −10.1 to −13.2, −11.3 to −15.7, and –13.2 to –17.4 kJ/mol with increasing temperature from 288 to 318 K for U(VI) ion from aqueous solution by the native, and PEI- and amidoxime-modified algal biomass preparation, respectively. Generally, the ΔG° value is in the range of 0 to −20 kJ/mol and −80 to −400 kJ/mol for physical and chemical sorption, respectively. The values of ΔG° of the adsorption process altered when the temperature increased from 288 to 318 K; it can be deduced that the adsorption of U(VI) ion on the algal biomass preparations is controlled by physical sorption. The adsorption is spontaneous and endothermic in nature. The increase in randomness at the solid/liquid interface during the adsorption of U(VI) ion on algal biomasses and no remarkable change on entropy occur.
Adsorption time and kinetics
Figure 8 shows the time-dependent adsorption of U(VI) ions on the native, and PEI- and amidoxime-modified algal biomasses which were obtained by monitoring the decrease of the U(VI) ion concentration in the medium. The adsorption capacity of the algal biomass preparations increased with increasing contact time, and a larger amount of uranium was removed by algal biomass preparation within the first 40 min of contact time. Equilibrium was established in 60 min. After this equilibrium period, no significant increase in the quantity of adsorbed U(VI) ions was observed with increase in contact time; thus, it was characterized as the optimum contact time. The equilibrium amount of adsorbed U(VI) ions is much lower for the native algal biomass surface as compared with both modified algal biomasses (i.e., PEI- and amidoxime-modified). The adsorption quantities reach to equilibrium at about 60 min with absorption quantity of about 194.6, 279.5, and 366.8 mg U(VI)/g for native, and PEI- and amidoxime-modified algal biomass, respectively. This observed contact time is very short, and amidoxime-modified fungal biomass is expected to be promising for the recovery of uranium from aqueous medium.
Kinetic models have been studied to analyze the effect of several experimental conditions such as adsorbent weight, initial metal concentration, percentage additive used, and pH, on the rate of reactions and the yield. The kinetic models addressed are pseudo-first-order, and pseudo-second-order, and they have been applied to examine the rate-controlling mechanism of the adsorption process (Ho and McKay 1998).
The pseudo-first-order rate equation is given as
where q e (mg/g) is the amount of metal ion adsorbed at equilibrium, and q t (mg/g) is the amount of adsorbed metal ion adsorbed at time t where k 1 is the first-order adsorption rate constant (min−1). The plot of log(q e − q t) versus t gives a straight line and the pseudo-first-order rate constant can be calculate from the slope value.
The pseudo-second-order equation is given as
where k 2 is the second-order adsorption rate constant (g mg−1 min−1) and q e is the adsorption capacity calculated by the pseudo-second-order kinetic model (mg/g). The constant k 2 is used to calculate the initial sorption rate “h” (mg/(g min)), at t → 0 by using h = k 2 q e. The application of the pseudo-second-order kinetics by plotting t/q t versus t yields the second-order rate constant k 2 (Fig. 9).
Pseudo-first-order model given by Lagergren is rendered the rate of occupation of the adsorption sites to be proportional to the number of unoccupied sites. Pseudo-second-order kinetic model assumed the chemical reaction mechanisms, and the adsorption rate is controlled by chemical adsorption through sharing or exchange of electrons between the sorbrate and biosorbent. Therefore, the adsorption behavior belonging to the pseudo-second-order kinetic model is a chemical process.
The calculated rate constants of both kinetic models are listed in Table 3. As seen in this table, the correlation coefficients (R 2) obtained from the pseudo-second-order model were found to be higher than 0.989, making them larger than those of the pseudo-first-order model (or the other applied models). The results obtained from the pseudo-second-order model were the best for describing the kinetics of U(VI) ion adsorption (Fig. 9 and Table 3); the calculated values of adsorption capacities (q e,cal) obtained from the pseudo-second-order model agreed with the corresponding experimental adsorption capacities (q e,exp). Since, the amidoxime-modified algal biomass has a very high equilibrium adsorption capacity q e, and the adsorption rates for all the studied algal preparations are very fast and the equilibrium times short. The adsorption capacities of the U(VI) ion at 30 min for almost all the initial concentrations reached more than 70 % of the calculated equilibrium adsorption capacities. It should be noted that short equilibrium time coupled with high adsorption capacity indicated a high degree of affinity between the U(VI) and amidoxime-modified algal biomass. The results indicated that the adsorption of investigated U(VI) ions from aqueous solution on native and modified algal biomass followed the pseudo-second-order model well (Table 3).
Desorption and reusability studies
To be useful in metal ion recycling processes, metal ions should be easily desorbed under suitable conditions. Desorption of U(VI) ion from the algal biomass preparations was performed in a batch system. The final U(VI) ion concentrations in the aqueous phases were determined as described above. When HNO3 is used as the elution agent, the coordination of chelated U(VI) ions is disrupted and subsequently U(VI) ion are released from the adsorbent surface into the desorption medium. The desorption time was found to be around 60 min. Desorption ratio was at least 94 % for the studied algal biomass preparations.
In order to determine the reusability of the adsorbed U(VI) by the native, and PEI- and amidoxime-modified algal biomass preparations, consecutive adsorption–desorption cycles were repeated eight times by using the same algal biomass preparations. The adsorption capacity is stable during five cycles of use, and a reduction in U(VI) adsorption capacities was observed after six cycles. In other words, these algal preparations can be used up to five adsorption/desorption cycles without significant reduction in their U(VI) adsorption capacities.
Conclusion
In this study, native, and PEI- and amidoxime-modified algal biomass preparations were used for removal of U(VI) ion from aqueous solutions. The PEI and amidoxime ligands were successfully attached on the algal biomass surface via glutaraldehyde coupling reaction. High-density amine groups are created on the algal cell surface after PEI and amidoxime ligand attachment, and these ligand molecules can provide many active adsorption sites for uranium ion, thus achieving high sorption capacities. Thus, the interaction between the amine groups and the uranium ion played an important role in the adsorption process, which was verified by FTIR analysis and zeta potential studies. Additionally, different important parameters such as pH, ionic strength, biosorbent dosage, temperature, time, and initial metal ion concentration were investigated in order to evaluate the optimum condition for the U(VI) ion adsorption process. Some results were given as follows: The adsorption of U(VI) ion was sensitive to medium pH, and the maximum adsorption was obtained at acidic pH 5.0 for all the studied algal biomass preparation. A higher U(VI) ion adsorption capacity was obtained amidoxime modified algal biomass preparation may be due to the changes in adsorptive characteristics of the algal cell surface as a result of amidoxime ligand attachment. The adsorption capacities, and the theoretical monolayer sorption capacity, of the native, and PEI- and amidoxime-modified algal biomass preparations from D-R isotherm model equation were found to be 121.6, 270.3, and 330.4 mg U(VI)/g dry biomass, respectively. The adsorption isotherms for the native, and PEI- and amidoxime-modified algal preparations were described well by the D-R equations. The adsorption of U(VI) ion on the algal preparations seems to be followed the second-order kinetic equations. Thermodynamic parameters were also evaluated for the algal biomass preparations and revealed that the adsorption of uranium ion is endothermic in nature and spontaneous.
References
Akar T, Celik S, Ari AG, Tunali Akar S (2013) Nickel removal characteristics of an immobilized macro fungus: equilibrium, kinetic and mechanism analysis of the adsorption. J Chem Technol Biotechnol 88:680–689
Ali SK, Saleh AM (2012) Spirulina - An Overview. Int J Pharm Pharm Sci 4:9–15
Bai J, Fan F, Wu X, Tian W, Zhao L, Yin X, Fan F, Li Z, Tian L, Wang Y, Qin Z, Guo J (2013) Equilibrium, kinetic and thermodynamic studies of uranium adsorption by calcium alginate beads. J Environ Radioact 126:226–231
Bai Z-Q, Yuan L-Y, Zhu L, Liu Z-R, Chu S-Q, Zheng L-R, Zhang J, Chai Z-F, Shi W-Q (2015) Introduction of amino groups into acid-resistant MOFs for enhanced U(VI) sorption. J Mater Chem A 3:525–534
Bayramoglu G, Arica MY (2009) Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for adsorption of Cu(II), Zn(II) and Ni(II): Kinetics and equilibrium studies. Bioresour Technol 100:186–193
Bayramoglu G, Arica MY (2011) Preparation of a composite biosorbent using Scenedesmus quadricauda biomass and aginate/polyvinyl alcohol for removal of Cu(II) and Cd(II) ions: Isotherms, kinetics, and thermodynamic studies. Water Air Soil Pollut 221:391–403
Bayramoglu G, Celik G, Arica MY (2006) Studies on accumulation of uranium by fungus Lentinus sajor-caju. J Hazard Mater 136:345–353
Bayramoglu G, Gursel I, Tunali Y, Arica MY (2009) Adsorption of phenol and 2-chlorophenol by Funalia trogii pellets. Bioresour Technol 100:2685–2691
Bayramoglu G, Altintas B, Arica MY (2012) Synthesis and characterization of magnetic beads containing aminated fibrous surfaces for removal of Reactive Green 19 dye: kinetics and thermodynamic parameters. J Chem Technol Biotechnol 87:705–713
Bhat SV, Melo JS, Chaugule BB, D’Souza SF (2008) Adsorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Brugge D, Goble R (2002) The history of uranium mining and the Navajo people. American J Public Health 92:1410–1419
Cecal A, Humelnicu D, Rudic V, Cepoi L, Ganju D, Cojocari A (2012) Uptake of uranyl ions from uranium ores and sludges by means of Spirulina platensis, Porphyridium cruentum and Nostok linckia alga. Bioresour Technol 118:19–23
Chen Y-C (2011) The effect of shifts in medium types on the growth and morphology of Spirulina platensis(Arthrospira plantensis). J Marine Sci Technol 19:565–570
Ding D-X, Tan X, Hu N, Li G-Y, Wang Y-D, Tan Y (2012) Removal and recovery of uranium (VI) from aqueous solutions by immobilized Aspergillus niger powder beads. Bioprocess Biosyst Eng 35:1567–1576
Ding DX, Xin X, Li L, Hu N, Li GY, Wang YD, Fu PK (2014) Removal and recovery of U(VI) from low concentration radioactive wastewater by ethylenediamine-modified biomass of Aspergillus niger. Water Air Soil Pollut 225:2206
Donat R, Cılgi GK, Aytas S, Cetisli H (2009) Thermodynamics parameters and sorption of U(VI) on ACSD. J Radioanal Nucl Chem 279:271–280
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of the activated charcoal. Proc Acad Sci USSR Phys Chem Sec 55:331–333
Erkaya IA, Arica MY, Akbulut A, Bayramoglu G (2014) Adsorption of uranium (VI) by free and entrapped Chlamydomonas reinhardtii: kinetic, equilibrium and thermodynamic studies. J Radioanal Nucl Chem 299:1993–2003
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:385–389
Fryxell GE, Lin Y, Fiskum S, Birnbaum JC, Wu H, Kemner K, Kelly S (2005) Actinide sequestration using self-assembled monolayers on mesoporous supports. Environ Sci Technol 39:1324–1331
Gharieb MM, Al-Fakih AA, Ali MI (2014) Adsorption of Pb(II) and Co(II) ions from aqueous solutions using pretreated Rhizopus oryzae (Bread Mold). Arabian J Sci Eng 39:2435–2446
Ghasemi M, Keshtkar AR, Dabbagh R, Safdari SJ (2011) Adsorption of uranium(VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: Breakthrough curves studies and modeling. J Hazard Mater 189:141–149
Gottlieb LS, Husen LA (1982) Lung cancer among Navajo uranium miners. Chest 81:449–452
He S, Ruan B, Zheng Y, Zhou X, Xu X (2014) Immobilization of chlorine dioxide modified cells for uranium absorption. J Environ Radioact 137:46–51
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Horsfall M, Spiff A (2005) Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by caladium bicolor (wild cocoyam) biomass. Electron J Biotechnol 8:162–169
Khani MH (2011) Statistical analysis and isotherm study of uranium biosorption by Padina sp. algae biomass. Environ Sci Pollut Res 18:790–799
Khani MH, Keshtkar AR, Ghannadi M, Pahlavanzadeh H (2008) Equilibrium, kinetic and thermodynamic study of the adsorption of uranium onto Cystoseria indica algae. J Hazard Mater 150:612–618
Kim I, Lee M, Wang S (2014) Heavy metal removal in groundwater originating from acid mine drainage using dead Bacillus drentensis sp. immobilized in polysulfone polymer. J Environ Manag 146:568–574
Kushwaha S, Sudhakar PP (2013) Sorption of uranium from aqueous solutions using palm-shell-based adsorbents: a kinetic and equilibrium study. J Environ Radioact 126:115–124
Langmuir I (1919) The adsorption of gases on plane surfaces of gas, mica and platinum. J Am Chem Soc 40:1361–1403
Lee KY, Kim KW, Baek YJ, Chung DY, Lee EH, Lee SY, Moon JK (2014) Adsorption of uranium(VI) from aqueous solution by biomass of brown algae Laminaria japonica. Water Sci Technol 70:136–143
Li X, Ding C, Liao J, Lan T, Li F, Zhang D, Yang J, Yang Y, Luo S, Tang J, Liu N (2014) Adsorption of uranium on Bacillus sp. dwc-2: preliminary investigation on mechanism. J Environ Radioact 135:6–12
Liu Y, Liu Y, Cao X, Hua R, Wang Y, Pang C, Hua M, Li X (2011) Adsorption studies of uranium (VI) on cross-linked chitosan: isotherm, kinetic and thermodynamic aspects. J Radioanal Nucl Chem 290:231–239
Ozer T, Erkaya IA, Udoh AU, Duygu DY, Akbulut A, Bayramoglu G, Arica MY (2012) Biosorption of Cr(VI) by free and immobilized Pediastrum boryanum biomass: equilibrium, kinetic, and thermodynamic studies. Environ Sci Pollut Res 19:2983–2993
Plazinski W (2012) Sorption of lead, copper, and cadmium by calcium alginate Metal binding stoichiometry and the pH effect. Environ Sci Pollut Res 19:3516–3524
Promya J, Chitmanat C (2011) The effects of Spirulina platensis and Cladophora algae on the growth performance, meat quality and immunity stimulating capacity of the African Sharptooth Catfish (Clarias gariepinus). Int J Agr Biol 13:1
Rippka R, Deruelles J, Waterbury J, Herdman M, Stanier R (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Singhal RK, Basu H, Pimple MV, Manisha V, Basan MKT, Reddy AVR (2013) Spectroscopic determination of U(VI) species sorbed by the Chlorella (Chlorella pyrenoidosa) fresh water algae. J Radioanal Nucl Chem 298:587–592
Temkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochimica URSS 12:327–356
Wang D, Zhou S, Liu L, Du L, Wang J, Huang Z, Ma L, Ding S, Zhang D, Wang R, Jin Y, Xia C (2015) The influence of different hydroponic conditions on thorium uptake by Brassica juncea var. Foliosa. Environ Sci Pollut Res. doi:10.1007/s11356-014-3914-4
Yang W, Bai Z-Q, Shi W-Q, Yuan L-Y, Tian T, Chai Z-F, Wang H, Sun Z-M (2013) MOF-76: from a luminescent probe to highly efficient UVI sorption material. Chem Commun 49:10415–10417
Yuan L-Y, Liu Y-L, Shi W-Q, Lv Y-L, Lan J-H, Zhao Y-L, Chai Z-F (2011) High performance of phosphonate-functionalized mesoporous silica for U(VI) sorption from aqueous solution. Dalton Trans 40:7446–7453
Yuan L-Y, Liu Y-L, Shi W-Q, Li Z-J, Lan J-H, Feng Y-X, Zhao Y-L, Yuan Y-L, Chai Z-F (2012) A novel mesoporous material for uranium extraction, dihydroimidazole functionalized SBA-15. J Mater Chem 22:17019–17026
Zhang X, Wang J, Li R, Liu Q, Li L, Yu J, Zhang M, Liu L (2013) Efficient removal of uranium(VI) from aqueous systems by heat-treated carbon microspheres. Environ Sci Pollut Res 20:8202–8209
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bayramoglu, G., Akbulut, A. & Arica, M.Y. Study of polyethyleneimine- and amidoxime-functionalized hybrid biomass of Spirulina (Arthrospira) platensis for adsorption of uranium (VI) ion. Environ Sci Pollut Res 22, 17998–18010 (2015). https://doi.org/10.1007/s11356-015-4990-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4990-9