Abstract
In the present study, role of various physicochemical parameters influencing the production of antimicrobial pigment prodigiosin from Serratia nematodiphila RL2 was determined and optimized. The pigment-producing strain was isolated and based on molecular characterization (16S rRNA sequencing), was identified as S. nematodiphila RL2. The pigment produced by S. nematodiphila RL2 was characterized by thin layer chromatography (Rf 0.94), spectrophotometrically (λmax 535 nm) and identified as prodigiosin. Optimization of production parameters of prodigiosin revealed, nutrient broth medium supplemented with lactose and yeast extract at 1% concentration each, have a positive effect on the bacterial growth (10.25–4.6 mg/ml DCW) as well as pigment production (0.46–0.6 mg/ml). Prodigiosin production (0.64 mg/ml) increases optimally after 46–48 h of incubation, at 35 °C at pH between 6 and 7 with addition of metal ions such as Uranyl acetate. An increase of 65% in prodigiosin production (0.46–0.76 mg/ml) was observed after optimizing the various production parameters than unoptimized conditions. Antimicrobial activity of the prodigiosin was also evaluated and found to be effective antimicrobial agent against bacterial pathogens including Listeria sp., Pseudomonas sp., Yersinia sp. and Shigella sp. Present study indicate that S. nematodiphila RL2 is a potent source of pigment prodigiosin which can be further explored for production of prodigiosin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigments are the colouring agents that can be produced either by living organisms or chemical reagents. Natural pigments are mainly obtained from plants and microorganisms and represent one of the potential sources of chemicals as well as medicines (Isaka et al. 2002). At physiological temperature, Serratia spp. may express a pink/red pigmentation, known as prodigiosin, a kind of secondary metabolite produced by other bacteria also (Lewis et al. 2000). Prodigiosin is a secondary metabolite alkaloid with a unique tripyrrole chemical structure (Grimont et al. 1977; Khanafari et al. 2006) and has been shown to be associated with extracellular vesicles or present in intracellular granules (Matsuyama et al. 1986; Kobayashi and Ichikawa 1991). Prodigiosin has no defined primary role in the growth and physiology of producing strains, but has been reported to have antimicrobial (Ibrahim et al. 2014), antimalarial (Isaka et al. 2002), immunomodulating (Giri et al. 2004) and anticancer activities (Montaner et al. 2000).
Keeping the importance of this pigment in mind, the present study was performed to explore the sources of prodigiosin, to optimize various physicochemical parameters for the production of prodigiosin from S. nematodiphila RL2 and exploring its potential as a potent antimicrobial agent.
Materials and methods
Isolation and identification of S. nematodiphila RL2
The pigment-producing organism was isolated from the soil sample collected from the Lahul region of Lahul and Spiti district of Himachal Pradesh, India. Listeria sp., Pseudomonas sp., Yersinia sp. and Shigella sp. were obtained from Indira Gandhi Medical College (IGMC), Shimla, India. Various biochemical tests and 16S rRNA sequencing followed by comparative analysis of sequence was performed for identification of strain.
Optimization of growth conditions for pigment production by S. nematodiphila RL2
Selection of the medium
Four different media, i.e. Nutrient broth, Luria–Bertani broth, Tomato juice broth and Yeast potato dextrose broth were tested for the growth of S. nematodiphila RL2 and prodigiosin production. The initial pH for all media was adjusted to 7.0 and incubation was at 35 °C for 50 h.
Carbon source
Effect of carbon source was studied on the growth and pigment production. For this, organic and inorganic carbon sources (sucrose, galactose, glucose, maleic acid, ammonium acetate, citric acid, glycerol, sodium oxalate and lactose) were used at concentration of 1% (w/v) in the production medium (pH 7.0).
Nitrogen source
To analyse the effect of nitrogen source on the growth of the organism and pigment production, organic and inorganic nitrogen sources (yeast extract, peptone, tryptophan, beef extract, glycine and ammonium sulphate) at concentration of 1% (w/v) were used.
Medium pH
To evaluate the optimum pH for the growth of the organism and production of prodigiosin, the medium pH was varied from 3 to 10.
Temperature
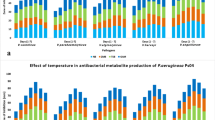
Effect of temperature ranging from 20 to 55 °C was studied on the growth of the organism and production of prodigiosin by S. nematodiphila RL2.
Incubation time
To find out the optimum incubation time for the growth of the organism and production of pigment by S. nematodiphila RL2, culture was incubated up to 50 h and culture samples were withdrawn at an interval of 2 h for assessment of the growth and pigment production.
Metal ions
The effect of metal ions such as UO +22 , Mo+6, Co+2, Fe+3, Cu+2 and Hg+2 at 100 mM on the pigment production as well as on the cell growth was studied.
Production of pigment by S. nematodiphila RL2
Estimation of cell mass
The cells of S. nematodiphila RL2 were harvested from culture broth by centrifugation at 10,000×g for 10 min and corresponding absorbance of harvested cells was measured at 600 nm in a spectrophotometer. Cell pellet was dried in oven overnight and the amount of cell was measured to calculate the dry cell weight (DCW) and absorbance relationship.
Extraction of pigment
The dry cells of S. nematodiphila RL2 were ground using motor pistol, the powder obtained was suspended in acetone and centrifuged at 10,000×g for 10 min. The supernatant containing pigment was collected and absorbance was measured at 535 nm. The supernatant was allowed to evaporate overnight and the amount of obtained dried pigment was measured. The known dried weight of pigment corresponding to their optical density was recorded after suspending it in solvent used (Picha et al. 2015).
Characterization of pigment produced by S. nematodiphila RL2
Thin layer chromatography
The silica gel thin layer chromatography (TLC) was used for effective separation of the impurities from the pigment produced. Totally 10 µl of pigment in acetone was spotted on silica plate. A mixture of dichloromethane, chloroform and acetone (5:5:1%v/v) was used as a mobile phase.
Spectroscopic analysis of pigment
To determine the absorption maxima of the pigment, the pigment was analysed spectrophotometrically in the visible region wave length of 400–700 nm range.
Antibacterial activity of prodigiosin produced by S. nematodiphila RL2
Four bacterial cultures, i.e. Listeria sp., Pseudomonas sp., Yersinia sp. and Shigella sp. were used to test the antimicrobial activity of the pigment produced by S. nematodiphila RL2. A 20 µl of the pigment (0.70 mg/ml) dissolved in acetone was added in test, against a control (acetone without pigment). The antimicrobial activity was analysed by observing clear zone around the well.
Results
Identification of pigment producing microorganism
Based on the various biochemical tests, morphological characteristics (S1) and 16S rRNA gene sequence (S2), the pigment producing strain was identified as Serratia nematodiphila and named as S. nematodiphila RL2. Isolated strain exhibited maximum similarity (99.52% sequence similarity) with the 16S rRNA sequence of Serratia nematodiphila DSM 21420 as shown in Fig. 1 and sequence has been submitted to the NCBI/Nucleotide databases under accession no. MF417388.
Phylogenic position of strain Serratia nematodiphila RL2 among related bacteria was inferred using the neighbor-joining method (Saitou and Nei 1987). The optimal tree with the sum of branch length = 0.38090397 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches (Felsenstein 1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method (Kimura 1980) and are in the units of the number of base substitutions per site. The analysis involved 25 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 1322 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013)
Optimization of parameters for production of pigment prodigiosin from S. nematodiphila RL2
Culture medium
To investigate the effect of nutritional media on the growth and pigment production, it was grown in four different media and nutrient broth showed highest pigment (0.46 mg/ml) along with adequate cell mass production (10.25 mg/ml DCW) as shown in Fig. 2.
Carbon source
To determine the effect of carbon source on the production of pigment, S. nematodiphila RL2 was grown in 50 ml of culture medium with different carbon sources (1%). It was observed that lactose (0.52 mg/ml pigment; 12.8 mg/ml DCW) was most preferred carbon source for this organism, while relatively lower pigment yield was recorded with other carbon sources as shown in Fig. 3.
Nitrogen source
The effect of different nitrogen sources on the production of pigment and growth of the S. nematodiphila RL2 was studied and yeast extract was found to be optimum for the production of pigment (0.6 mg/ml) as well as growth (14.6 mg/ml DCW) of the strain as shown in Fig. 4.
pH
S. nematodiphila RL2 was grown in optimized production medium (nutrient broth) with pH variation from 3 to 10, and it was observed that slight acidic/neutral pH (6–7) was optimum for pigment production (0.6 mg/ml) as well as for cell growth. It was observed that increase in production of pigment occurs up to pH 6 and further increase in pH beyond 7 resulted in suppression of its production as shown in Fig. 5.
Temperature
S. nematodiphila RL2 produced higher amount of pigment as well as cell mass at 35 °C (0.59 mg/ml) and either side variation decrease the production of pigment as shown in Fig. 6.
Time course of pigment production
S. nematodiphila RL2 was grown in nutrient broth and pigment production was assayed after every 2 h up to 50 h. Maximum growth and pigment production was recorded after 46 h of incubation which lasts up to 48 h (0.64 mg/ml pigment) as shown in Fig. 7.
Effect of metal ion
The effect of various metal ions on the production of prodigiosin was studied and it was observed that maximum pigment production was observed with uranyl acetate (0.76 mg/ml) (Fig. 8) while CoCl2 and HgCl2 suppressed the growth as well as production of pigment.
Estimation of cell mass
The known dried cell weight corresponding to their optical density was recorded and the results showed that 1 OD corresponds to the 7.8 mg/ml of cells.
Estimation of pigment
The known dried pigment corresponding to their optical density was recorded and the result showed that 1 OD corresponds to the 0.30 mg/ml of pigment.
Characterization of pigment
Thin layer chromatography
Thin layer chromatography of the pigment extracted from the S. nematodiphila RL2 was performed and a single coloured band was observed (S3). The Rf value of the pigment was calculated and was found to be 0.94.
Spectroscopic analysis
The spectroscopic analysis revealed a single peak at 535 nm which corresponds to absorption maxima of prodigiosin.
Antibacterial activity of pigment
Four bacterial pathogens (Listeria sp., Pseudomonas sp., Yersinia sp. and Shigella sp.) were used to evaluate the antimicrobial activity of the pigment produced by S. nematodiphila RL2. Pigment prodigiosin showed potent antibacterial activity as clear zone of inhibition was observed around the well (S4).
Discussion
Prodigiosin, a pigment having pyrrolylpyrromethane skeleton was first characterized from S. marcescens and reported to be an antifungal, immunosuppressive and anti-proliferative agent (Khanafari et al. 2006). In current study S. nematodiphila RL2 was isolated from the cold desert of Himachal Pradesh having the property to produce prodigiosin. Prodigiosin was characterized by spectrophotometric analysis and thin layer chromatography and spectrophotometric analysis of pigment showed spectrum maxima at 535 nm, corresponds to prodigiosin as reported in literature (Song et al. 2006; Samrot et al. 2011; Silva et al. 2012; Kurbanoglu et al. 2015; Darshan and Manmohini 2016). Thin layer chromatography showed Rf value of 0.94, which was also reported by Someya et al. (2004) in the range of 0.90–0.95, thus concluding that the red pigment as prodigiosin. Davaraj et al. (2009) have also extracted prodigiosin from the standard strain of S. marcescens MTCC 97 having Rf value 0.9–0.95 further supported our observation.
Giri et al. (2004) used the powdered sesame seed in water, nutrient broth and peptone glycerol broth as a growth medium for S. marcescens. It has been reported that Serratia sp. grows well on synthetic media using various compounds as a single carbon source (Furstner 2003) supporting present observations that pigment production was maximum when nutrient broth supplemented with single carbon source, i.e. lactose. Sundaramoorthy et al. (2009) showed that the pigment production was maximum in the presence of maltose, where present study showed that lactose increases pigment production among several carbon sources tested. In the present study, yeast extract as a nitrogen source increases growth as well as pigment production followed by beef extract and peptone. Andrade et al. (2009) reported that the addition of yeast extract as nitrogen source in the medium increased pigment production, such as Corn steep liquor offers a rich source for culture media. Similar results were also shown by Suryawanshi et al. (2014), where yeast extract significantly enhanced the production of prodigiosin, as nitrogen plays an important role in prodigiosin production (Kurbanoglu et al. 2015).
The pH of medium regulates the pigment production as well as cell growth as there was increase in pigment production from 5 to 7 pH was observed, as Patil-Nilam and Chincholkar (2014) investigated the influence of initial pH on the prodigiosin production by S. nematodiphila was highest at 7 pH. Khanafari et al. (2006) reported the reduction of prodigiosin production by S. marcescens mediated by glucose and other metabolizable sugars was due to a lowering in pH observed in cell suspensions. Present investigation showed that heavy metal addition also increases pigment production which can be related to the chelating activity of prodigiosin. Morgenstern et al. (2015) also indicated the role of divalent metal ions in prodigiosin production, in which cobalt divalent ions significantly increases the prodigiosin production by inducing sleeping genes. Temperature is an important factor and it was observed that pigment production was maximum at temperature 30 °C as Giri et al. (2004) showed that there is decrease in pigment production above 30 °C but fatty acid medium such as powdered peanut and seed medium can support the prodigiosin production even up to 42 °C. Sundaramoorthy et al. (2009) also showed significant decrease in prodigiosin production by S. marcescens at 40 °C and comparable pigment production at 25, 30 and 35 °C. Maximum pigment production was observed at 46–48 h interval of incubation, as the prodigiosin is already reported as a secondary metabolite in literature (Williams 1973). Harned (1954) also reported that no significant prodigiosin production was seen after 48 h incubation and supports present findings.
Lee et al. (2011), Priya et al. (2013) and Ibrahim et al. (2014), showed an antibacterial activity of prodigiosin against a range of pathogens by showing inhibition zone which supports the antimicrobial activity of prodigiosin. Ramina and Samira (2009) concluded that the antibacterial activity of prodigiosin is due to its ability to pass through the membrane and causes inhibition of target enzymes, such as DNA gyrase and topoisomerase IV, which are essential for the cell growth. Darshan and Manmohini (2016) showed that prodigiosin from S. nematodiphila can induce programmed cell death which involves DNA fragmentation, ROS generation and expression of caspase like protein in bacterial cells. Arakha et al. (2015) also postulated the antimicrobial activity of prodigiosin may be attributed to the interaction of positively charged prodigiosin with negatively charged bacterial membrane leading to reactive oxygen species production resulting in bacterial cell death. Present investigation also showed that the pigment prodigiosin produced by S. nematodiphila RL2 is potent antibacterial agent against several potent pathogenic strains.
Conclusion
The present investigation had led to the isolation of a bacterial source of prodigiosin, i.e. S. nematodiphila RL2 with a high potential for prodigiosin production. Optimization of culture conditions, pigment production, pigment characterization and antimicrobial activity of pigment was carried out. Culture conditions were optimized in terms of production media, carbon source, nitrogen source, pH, metal ions, temperature and incubation time. Prodigiosin was found to be in higher yield with heavy metal ions under stress condition. Prodigiosin produces from S. nematodiphila RL2 was found to be active against different pathogenic microorganisms showed that it may play important role in microbial competition. These results indicate that S. nematodiphila RL2 has emerged as potent source of prodigiosin and can be further explored for its future applications.
References
Andrade RFS, Luna JM, Rufino RD, Costa Albuquerque CD, Sarubbo LA, Takaki GM (2009) Surface active agent produced by Candida lipolytica using cassava flour wastewater as substrate. In: Current research topics in applied microbiology and microbial biotechnology. World Scientific Publishing Co. Pte. Ltd., Singapore, pp 751–756
Arakha M, Saleem M, Mallick BC, Jha S (2015) The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci Rep 5:9578
Darshan N, Manmohini HK (2016) Prodigiosin inhibits motility and activates bacterial cell death revealing molecular biomarkers of cell death. AMB Exp 6:50
Davaraj NR, Dhanasekaran D, Thajuddin N (2009) Production of prodigiosin from Serratia marcescens and its cytotoxicity activity. JPR 2:590–593
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evol 39:783–791
Furstner A (2003) Chemistry and biology of roseophilin and the prodigiosin alkaloids: a survey of the last 2500 years. Angew Chem Int Ed 42:3582–3603
Giri AV, Anandkumar N, Muthukumaran G, Pennathur G (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol 4:11–18
Grimont PAD, Grimont F, Dulong HLC, Rosnay D, Sneath PHA (1977) Taxonomy of the genus Serratia. J Gen Microbiol 98:39–66
Harned RL (1954) The production of prodigiosin by submerged growth of Serratia marcescens. Appl Environ Microbiol 2:365–368
Ibrahim D, Nazari TF, Kassim J, Lim SH (2014) Prodigiosin—an antibacterial red pigment produced by Serratia marcescens IBRL USM 84 associated with a marine sponge Xestospongia testudinaria. J App Pharm Sci 4:001–006
Isaka M, Jaturapat A, Kramyu J, Tanticharoen M, Thebtaranonth Y (2002) Potent In Vitro Antimalarial Activity of Metacycloprodigiosin Isolated from Streptomyces spectabilis BCC 4785. Antimicrob Agents Chemother 46:1112–1113
Khanafari A, Assadi MM, Fakhr FA (2006) Review of prodigiosin, pigmentation in Serratia marcescens. Online J Biol Sci 6:1–13
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kobayashi N, Ichikawa Y (1991) Separation of the prodigiosin-localizing crude vesicles which retain the activity of protease and nuclease in Serratia marcescens. Microbiol Immunol 35:607–614
Kurbanoglu EB, Ozdal M, Ozdal OG, Algur OF (2015) Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz J Microbiol 46:631–637
Lee JS, Kim YS, Park S, Kim J, Kang SJ, Lee MH, Ryu S, Choi JM, Oh TK, Yoon JH (2011) Exceptional production of both prodigiosin and cycloprodigiosin as major metabolic constituents by a novel marine bacterium, Zooshikella rubidus S1-1. Appl Environ Microbiol 77:4967–4973
Lewis TA, Cortese MS, Sebat JL, Green TL, Lee CH, Crawford RL (2000) A Pseudomonas stutzeri gene cluster encoding the biosynthesis of the CCl4-dechlorination agent pyridine-2,6-bis (thiocarboxylic acid). Environ Microbiol 2:407–416
Matsuyama T, Murakami T, Fujita M, Fujita S, Yano I (1986) Extracellular vesicle formation and biosurfactant production by Serratia marcescens. J Gen Microbiol 132:865–875
Montaner B, Navarro S, Pique M, Vilaseca M, Martinell M, Giralt E, Gil J, Perez-Tomas R (2000) Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Phrmacol 131:585–593
Morgenstern A, Paetz C, Behrend A, Spiteller D (2015) Divalent transition-metal-ion stress induces prodigiosin biosynthesis in Streptomyces coelicolor M145: formation of coeligiosins. Chem Eur J 21:6027–6032
Patil-Nilam G, Chincholkar SB (2014) Probing natural carbon sources for bioactive pigment production from S. nematodiphila 213 C. Int J of Adv Res 2(2)
Picha P, Kale D, Dave I, Pardeshi S (2015) Comparative studies on prodigiosin production by Serratia marcescens using various crude fatty acid sources—its characterization and applications. Int J Curr Microbiol App Sci 2:254–267
Priya KA, Satheesh S, Ashok Kumar B, Varalakshmi P, Selvakumar G, Sivakumar N (2013) Antifouling activity of prodigiosin from estuarine isolate of Serratia marcescens CMST 07. In: Velu RK (ed) Microbiological research in agro ecosystem management. Springer, New Delhi, pp 11–21
Ramina M, Samira YY (2009) The role of red pigment produced by Serratia marcescens as antibacterial and plasmid curing agent. J Duhok Univ 12:268–274
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Samrot AV, Chandana K, Senthilkumar P, Narendra KG (2011) Optimization of prodigiosin production by Serratia marcescens SU-10 and evaluation of its bioactivity. Int Res J Biotechnol 2:128–133
Silva R, Subha K, Bhakta D, Ghosh AR, Babu S (2012) Characterization and enhanced production of prodigiosin from the spoiled coconut. Appl Biochem Biotechnol 166:187–196
Someya N, Nakajima M, Hamamoto H, Yamaguchi I, Akutsu K (2004) Effects of light conditions on prodigiosin stability in the biocontrol bacterium Serratia marcescens strain B2. J Gen Plant Pathol 70:367–370
Song MJ, Bae J, Lee DS, Kim CH, Kim JS, Kim SW, Hong SI (2006) Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J Biosci Bioeng 101:157–161
Sundaramoorthy N, Yogesh P, Dhandapani R (2009) Production of prodigiosin from Serratia marcescens isolated from soil. Ind J Sci Tech 2:32–34
Suryawanshi RK, Patil CD, Borase HP, Narkhede CP, Shinde L, Patil SV (2014) Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl Biochem Biotechnol 173:1209–1221
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Williams RP (1973) Biosynthesis of prodigiosin, a secondary metabolite of Serratia marcescens. Appl Microbiol 25:396–402
Acknowledgements
Authors are highly grateful to University Grants Commission (UGC), New Delhi, India for providing financial assistance to Mr. Mohammad Asif.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Gondil, V.S., Asif, M. & Bhalla, T.C. Optimization of physicochemical parameters influencing the production of prodigiosin from Serratia nematodiphila RL2 and exploring its antibacterial activity. 3 Biotech 7, 338 (2017). https://doi.org/10.1007/s13205-017-0979-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0979-z