Abstract
The present study was intended to investigate the biodegradation of acephate in aqueous media in the presence and in the absence of metal ions [Fe(III) and Cu(II)], and humic acid (HA). Biodegradations were performed using Pseudomonas pseudoalcaligenes PS-5 (PS-5) isolated from the heavy metal polluted site. Biodegradations were monitored by UV–Visible, FTIR, and electron spray ionization–mass spectrometry (ESI–MS) analyses. ESI–MS analysis revealed that PS-5 degraded acephate to two metabolites showing intense ions at mass-to-charge ratios (m/z) 62 and 97. The observed kinetic was the pseudo-first order, and half-life periods (t 1/2) were 2.79 d−1 (of PS-5 + acephate), 3.45 d−1 [of PS-5 + acephate + Fe(III)], 3.16 d−1 [of PS-5 + acephate + Cu(II)], and 5.54 d−1 (of PS-5 + acephate + HA). A significant decrease in degradation rate of acephate was noticed in the presence of HA, and the same was confirmed by UV–Visible and TGA analyses. Strong aggregation behavior of acephate with humic acid in aqueous media was the major cause behind the slow degradation rate of acephate . New results on acephate metabolism by strain PS-5 in the presence and in the absence of metal ions [Fe(III) and Cu(II)] and humic acid were obtained. Results confirmed that Pseudomonas pseudoalcaligenes strain PS-5 was capable of mineralization of the acephate without formation of toxic metabolite methamidophos. More significantly, the Pseudomonas pseudoalcaligenes strain PS-5 could be useful as potential biological agents in effective bioremediation campaign for multi-polluted environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acephate [N-(Methoxy-methylsulfanylphosphoryl)acetamide] is one of the highly effective, low poisonous, and cost-effective organophosphorus insecticides, which is extensively used for protecting crops including the rice, wheat, cotton, tea, tobacco, and vegetable from pests (Kumar et al. 2013, 2014, 2015a, 2016; Singh et al. 2016). Acephate residue in vegetables has been attracted much consideration, because the residue of its metabolite, methamidophos, may be hazardous to human health (Kumar et al. 2015a, b, c, d). Acephate is readily metabolized in the soil under both aerobic and anaerobic conditions. The metabolites formed are O, S-dimethyl phosphoramidothioate (methamidophos), which is a much more potent inhibitor of cholinesterase and O-methyl N acetylphosphoramidate (RE 18,420) (Kumar et al. 2015a; Pinjari et al. 2012; Ramu and Seetharamana 2014). Methamidophos, a toxic metabolite of acephate (much more toxic than acephate), is a widely used organophosphorus insecticide with systemic properties and is effective against chewing and sucking insects (Kumar et al. 2015a; Pinjari et al. 2012; Ramu and Seetharamana 2014) (Supplementary Table 1). The acute dermal toxicity for methamidophos DL50 in rats is 130 mg kg−1 which that of acephate DL50 is 2000 mg kg−1 (Kumar et al. 2015a; Pinjari et al. 2012; Ramu and Seetharamana 2014).
Kumar et al. (2015a, b, c, d) have reported that high-performance liquid chromatography (HPLC), gas chromatography (GC), or gas chromatography–mass spectrometry (GC–MS) are the specific methods for the detection of acephate and methamidophos in the different environmental compartments (Kumar et al. 2015a; Pinjari et al. 2012; Ramu and Seetharamana 2014).
Microbial degradation is generally considered as a safe, cost-effective, and reliable technique for pesticides elimination from the environmental compartments (Kumar et al. 2015a, b, c, d). Several pure bacterial isolates with the ability to use specific organophosphate pesticides as a sole source of carbon, nitrogen, or phosphorus have been isolated (Kumar et al. 2015a, 2016; Pinjari et al. 2012; Ramu and Seetharamana 2014; Wang et al. 2010). Literature has revealed only two studies on the biodegradation of acephate (Pinjari et al. 2012; Ramu and Seetharamana 2014), whereas various studies on biodegradation of methamidophos reported (Kumar et al. 2015a, 2016; Pinjari et al. 2012; Ramu and Seetharamana 2014; Wang et al. 2010).
In the present study, a bacterium Pseudomonas pseudoalcaligenes (strain PS-5) was isolated from the heavy metal contaminated soil, having excellent plant growth promoting activities, capable of degrading acephate under the stress of heavy metals (Fe and Cu) and humic acid (HA). This work suggests the possible application of isolated bacterial strain for remediation of organophosphorus pesticides even under the heavy metal contaminated environment and soil’s organic content.
Materials and methods
Chemicals and reagents
Analytical grade acephate (purity >95%) was gifted by Gautmi Ltd., Hyderabad, India. All other essential chemicals and microbiological media used in various experiments were of analytical grade and purchased from Himedia Laboratories, India.
Soil sample collection and heavy metal analysis
All the soil samples were collected from industrial areas of Jalandhar (India) (Details of sites under supplementary data). Jalandhar is situated at Latitude 31.32560 E and East Longitude 75.57920 E, and at an elevation level of 228 m above the sea level. Soil was sieved through a 2 mm sieve (to remove any large debris) and thoroughly homogenized using a pestle and mortar. The soil samples were kept in a refrigerator at 4 °C till bacterial isolations. Heavy metals analysis was performed using the inductively couple plasma emission spectroscopy (ICPES) having lower limit of detections 0.1 ng/g, previously prescribed by the Lakanen and Ervio (1971).
Isolation and identification of acephate-degrading bacteria
The acephate degrading bacterium was isolated using enrichment culture method, where concentration of acephate was gradually increased from 50 to 200 mg l−1 in 100 ml mineral salt broth medium. The flasks were incubated in an orbital shaker at 150 revolutions per minute (rpm) and 30 °C. After five subcultures, 1.0 ml culture was inoculated in mineral salt agar plates spiked with 200 mg l−1 acephate for the isolation of bacteria. After an incubation of 72 h at 30 °C, bacterial colonies were appeared on the Petri plates. Single colonies were picked and mass multiplied for degradation studies. The isolate was identified on the basis of morphological, biochemical, and 16S rDNA sequence analysis.
Biodegradation of acephate
To decompose the acephate, strain PS-5 was precultured in MM1 medium using different concentrations (25, 50, and 100 ppm) of acephate. Flasks having different concentrations of acephate were incubated overnight at 30 °C on a shaker at 120 rpm. The content of the inoculated flasks containing MM1 medium and acephate was centrifuged, and processed and analyzed. To check the effect of metal ions and humic acid, above experiment was performed with the addition of known amount of Fe, Cu, and humic acid, respectively. Flasks without MM1 medium having different concentrations (25, 50, and 100 ppm) of acephate served as controls. All the experiments were conducted in the triplicate to ensure accuracy.
UV–Visible spectrophotometric study for cell growth and degradation of acephate
To determine the cell growth and degradation of different concentrations of acephate in broth culture, at periodic intervals, an individual flask was sacrificed. Bacterial growth was monitored using UV–Visible spectrophotometer at 600 nm where de-ionized water was used as blank.
ESI–MS and FTIR analysis
To perform the mass (ESI–MS) analysis, 100 mL of the spent medium was clarified by centrifugation at 5000 rpm, followed by filtration through a Whatman 1 filter paper. The clarified medium was extracted thrice with an equal volume of ethyl acetate. The extracted organic phase was allowed to air dry, and remaining residue was dissolved in a minimal volume (250 mL) of water, and about 1 µL taken for mass analysis using a mass spectrophotometer (Waters, Q-TOF Micromass). FTIR analysis was performed on a Shimadzu-8400 s FTIR spectrophotometer using KBr pellets of the dry mass of technical grade acephate and extracted or decomposed metabolites. The FTIR analysis was performed in the mid IR region of 400–4000 cm−1 with a 16 scan speed (Kumar et al. 2015b; Pinjari et al. 2012; Ramu and Seetharamana 2014; Wang et al. 2010).
Degradation kinetics
The decomposition rates of the acephate under different compositions were analyzed according to the pseudo-first-order kinetics as mentioned by the Chai et al. (2010). Equations (1)–(3) correspond to the equations of the concentration variation with time plotted on the basis of the linear regression results obtained by plotting Time (in day) vs Log Ct (in ppm):
where [C] is the acephate concentration at time t (ppm); [C]0 the initial acephate concentration (ppm); k obs is the pseudo-first-order constant (day−1) (Chai et al. 2010).
Study of aggregation behavior of acephate with humic acid
To check the aggregation behavior of acephate with humic acid, a reaction of humic acid and acephate was run by taking 20 mL mixture (10 mL of 100 ppm humic acid + 10 mL of 100 ppm acephate) in round bottom flask (RBF), and stirred (at 200 rpm) for 8 h at 25 °C. The pH of reaction solution was 7.5 (~as that of strain growth condition and biodecomposition experiment) maintained using NaOH (0.01 M) solution. At different times (usually every 5 min during the first 20 min of reaction and every 10 min afterwards), an aliquot of the solution was withdrawn, gently filtered with a 0.45 µm pore size membrane to separate the aggregates from the supernatant, and the humic acid that remained in solution was quantified by taking a spectrum in the 200–600 nm wavelength range with an Shimadzu 1800 UV–Vis spectrophotometer equipped with a 1 cm quartz cell. The product formation or degree of association was evaluated by plotting the degree of progress of the reaction, ‘α’ as a function of time (t). ‘α’ was defined as: α = (C − C 0)/C, where C 0 was the initial humic absorbance (at 300 nm) and C is the acephate–humic acid absorbance [(at 300 nm) measured spectrophotometrically] at a certain reaction time. An ‘α’ vs. ‘t’ curve was called an acephate–humic acid interaction curve. After 8 h, the volume of water was reduced to minimum volume (one-fourth), and allowed for total evaporation in open. After 10 days, totally dry complex was washed with methanol (to wash unreacted/excess acephate). Final complex was dried at 50 °C for 72 h and allowed for the thermal analysis (TGA). Thermogravimetric analysis (DSC/TG/DTG on the same instrument) was carried out in the temperature ranging from 50 to 750 °C in a flow of nitrogen atmosphere by Shimadzu TG 50H thermal analyzer. The experimental conditions were: platinum crucible, nitrogen atmosphere with 30 mL min−1 flow rate, and heating rate of 10 °C min−1 (Brigante et al. 2009; Mazzei and Piccolo 2012).
Statistical analysis
Experimental data were analyzed using MS Excel worksheet for calculation of means and standard errors. Analysis of variance ANOVA was carried out using SPSS 16 statistical software.
Results and discussion
Heavy metal ion analysis
Based on the ICPES heavy metal ion analysis, site 4 was the most contaminated site. Observed heavy metal ions content and physicochemical compositions of soil samples were reported in supplementary data under Table 2a, b. At significant level (P ≤ 0.05), comparatively, higher heavy metal concentrations were observed for sites 1, 4, 6, and 7. Highest concentrations of Arsenic (As) were observed in the soil samples of sites 2, 4, and 7. Highest concentrations of Lead (Pb) and Cadmium (Cd) were observed in the soil samples of sites 4, 5, and 7. Maximum concentrations of Chromium (Cr), Copper (Cu), and Zinc (Zn) were observed in the soil samples of sites 1 and 4. In comparison to the reported data (Charlesworth et al. 2003), significant differences were found. Soils of all sites were contaminated with one or more heavy metals, as site 4 was contaminated with As, Pb, Cd, Cr, Cu, and Zn, site 1 with Cr, Zn, and Cu, and site 7 with As. Site 4 was the most contaminated site having Zn and Cu more than recommended standards (Charlesworth et al. 2003; Kim et al. 1998; Kumar et al. 2015b; Leharne et al. 1992; Wei and Yang 2010). Hence, site 4 was selected for the further study.
Isolation and identification of acephate degrading bacterial strain
Various biochemical tests and 16S rDNA analyses were performed to characterize the isolate. Qualitative identifications of isolate were performed through various biochemical tests, namely gram staining, indole test, MR test, citrate test, and catalase test (Supplementary Data Table 3).
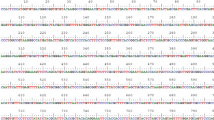
16S rDNA sequence analysis was used to identify the bacterial isolate. Homology search using BLAST revealed congruence of this sequence with 16S rDNA sequence of Pseudomonas pseudoalcaligenes (GenBank Accession No. NBRC110610). Phylogenetic tree constructed using Mole-Blast showed close relationship of this bacterial isolate (Genbank accession number KJ588061.1) with Pseudomonas pseudoalcaligenes isolates (Fig. 1).
Biodegradation study of acephate
At 100 ppm, more than 95% degradation of acephate was observed within 14 days, while the cell density of strain PS-5 increased from 0.15 to 0.62 (OD at 600 nm) within 14 days (Fig. 2a). An increase in optical density (OD) at 600 nm has demonstrated the consumption of acephate as a source of carbon and phosphorus (Chai et al. 2010; Kumar et al. 2015b; Pinjari et al. 2012; Ramu and Seetharamana 2014; Wang et al. 2010).
Growth kinetics and acephate utilization by Pseudomonas pseudoalcaligenes PS-5 on MM1 growth media. a Growth kinetics monitored at 600 nm on MM1 supplemented with acephate (white squares) and % acephate left (blue balls) (Inoculum + 100 ppm acephate). b Growth kinetics monitored at 600 nm on MM1 supplemented with acephate in the presence 100 ppm of Fe(III) ion (white squares) and % acephate left (blue balls) (Inoculum + 100 ppm acephate + 100 ppm Fe(III)). c Growth kinetics monitored at 600 nm on MM1 supplemented with acephate in the presence 100 ppm of Cu(II) ion (white squares) and % acephate left (blue balls) (Inoculum + 100 ppm acephate + 100 ppm Cu(II). d Growth kinetics monitored at 600 nm on MM1 supplemented with acephate in the presence 100 ppm of humic acid (white squares) and % acephate left (blue balls) (Inoculum + 100 ppm acephate + 100 ppm humic acid)
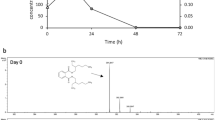
Mass spectrometric method (ESI–MS) considered as the best technique to determine the pesticides in their active and decomposed form (Chai et al. 2010; Kumar et al. 2015a; Pinjari et al. 2012; Prasad et al. 2013; Ramu and Seetharamana 2014; Wang et al. 2010). Observed mass/charge ratio (m/z) of acephate at m/z 183, i.e., {[M + H]+} and [M + H]+H2O at m/z 198, three other metabolites were characterized at m/z 113, m/z 147, and m/z 130 (Fig. 3; Scheme 1). In addition, oxidation of protonated acephate molecule (at m/z 165) was noticed, consequently successive OD (at 600 nm) increase for first 7 days followed by decrease in peak at m/z 165. After 14th day experiment, percentage decrease at m/z 183 has confirmed >95% decomposed of acephate (Fig. 3).
FTIR analysis was performed to support the biodegradation mechanism of acephate analyzed with the use of ESI–MS analysis. The collected FTIR spectra of acephate and its biodegradation metabolites were significantly different from each other, indicating the conversion of acephate to different metabolites. The FTIR spectrum of acephate (Fig. 4a) displayed peaks at 1224 cm−1 for P=O stretching for the presence of phosphorous compound, at 710 cm−1 for C–S stretching for sulfur compounds, at 3115 cm−1 for N–H stretching, at 1699 cm−1 for CO of −CO–NH– stretching, and at 2829 cm−1 for C–H stretching of alkanes (Chai et al. 2010; Kumar et al. 2015a; Pinjari et al. 2012; Prasad et al. 2013; Ramu and Seetharamana 2014; Wang et al. 2010). On the other hand, FTIR spectra of biodegradation metabolites (Fig. 4b) have shown peaks at 1250 cm−1 for O–H stretching of the hydroxy compounds and at 1646 cm−1 for NH2 stretching of amine group. The observed changes in peak pattern and shift of the functional groups have confirmed the degradation of acephate into different metabolites. The results of FTIR analysis have strongly supported the formation of metabolites as explained in mass analysis.
There were the various reports regarding biodegradation organophosphorus pesticides including the methyl parathion, ethoprophos, and chlorpyrifos (Chai et al. 2010; Kumar et al. 2013, 2015a; Manzanilla-Cano et al. 2004; Pinjari et al. 2012; Ramu and Seetharamana 2014; Sarkouhi et al. 2012, 2016; Wang et al. 2010). Only a few reports are available on bacterial-promoted biodegradation of acephate (Chai et al. 2010; Kumar et al. 2015a; Pinjari et al. 2012; Prasad et al. 2013; Ramu and Seetharamana 2014; Wang et al. 2010). Previous researchers (Pinjari et al. 2012; Ramu and Seetharamana 2014) reported that the removal of acephate took place with the formation of metabolites like methamidophos (major metabolite) including the O-methyl phosphoramidate, O, O-dimethyl phosphoramidate, and O, S-dimethyl phosphorothioate (DMPT). Recently, an acephate-degrading strain Pseudomonas sp. Ind01 was isolated, capable to utilize acephate to methamidophos and acetic acid, but it failed to hydrolyze methamidophos and other tested organophosphorus compounds including the paraoxon and parathion. It promotes only the first step of acephate mineralization in a soil microbial community (Pinjari et al. 2012). In another study, Pseudomonas aeruginosa strain Is-6 was isolated, which was capable of complete mineralization of the acephate to methamidophos and acetic acid, hydrolyze methamidophos, and other tested organophosphorus compounds, such as methamidophos, Dimethoate, Parathion, Methyl parathion, Chlorpyrifos, and Malathion (Ramu and Seetharamana 2014). Above-mentioned both studies have revealed that acephate decomposed through the toxic metabolite methamidophos (Pinjari et al. 2012; Ramu and Seetharamana 2014). In the present study, Pseudomonas pseudoalcaligenes strain PS-5 was capable of mineralization of the acephate without formation of toxic metabolite methamidophos. More significantly, Pseudomonas pseudoalcaligenes strain PS-5 was capable of mineralization of the acephate under the stress of heavy metals (Fe and Cu), and humic acid.
Biodegradation study of acephate in the presence of metal ions
With the addition of Fe(III) or Cu(II) (100 ppm), OD (at 600 nm) as well as the decomposition rate was initially decreased as compared to samples without Fe(III) or Cu(II) (Fig. 2). Significant effects of Fe(III) and least effect of Cu(II) were observed (Fe(III) > Cu(II). Reduction in the decomposition rates was attributed to complexation mechanism of acephate in the presence of Fe(III) and Cu(II).
In the presence of Fe(III), ESI–MS spectrometric study has revealed almost similar fragmentation pathway with decreased % age intestines as compared to that of acephate only. Percentage decrease in peak at m/z 183 has confirmed that up to 14 days more than 85% of acephate was decomposed (Fig. 3). In addition to above, after 14 days, more than 90% increase in intensity in the peak at m/z 147 was observed, that was due to formation of ferric ion metal complex [Fe(N–P = O(S))], which further reduced to Fe(OH)2 at m/z 89 and consistence with recent studies (Kumar et al. 2015c, d; Manzanilla-Cano et al. 2004, 2007; Sarkouhi et al. 2012, 2016). Recent studies have revealed that phosphate can promoted the mineral dissolution, probably be due to the different affinities between metals (Fe >> Co > Ni > Cu) and phosphates (Manzanilla-Cano et al. 2004, 2007; Sarkouhi et al. 2012, 2016).
Since, iron is an essential metal ion to all the living beings, but within prescribed limits. Formation of stable complex till 14th day at m/z 147 was due to strong interaction of iron with O and N donor sites of decomposed metabolite. These interactions were logical and expected as per HASB principle (Kumar et al. 2015c, d; Manzanilla-Cano et al. 2004, 2007; Sarkouhi et al. 2012, 2016). The reason behind slow biodecomposition was the complex formation behavior of Fe(III) with acephate through N and O atoms of acephate. Here, Fe(III) may form six membered ring through the O of P=O and C=O, which is kinetically as well as thermodynamically stable (Manzanilla-Cano et al. 2004, 2007; Sarkouhi et al. 2012, 2016).
Biodecomposition of acephate in the presence of cupric ion (at 100 ppm) was depicted in Fig. 3. In the presence of Cu(II), ESI–MS spectrometric study has revealed the almost similar fragmentation pathway with decreased in intestines as compared to that of acephate only. Percentage decrease at m/z 183 has confirmed more than 95% decomposition of acephate in the presence of Cu(II) ion. In addition to above, increase in intensity (>96%) at m/z 97 was observed, may be due to formation of cupric ion metal complex [CuSH2)], which was further reduced to Cu(OH)2 at m/z 97 (Kumar et al. 2015c, d; Manzanilla-Cano et al. 2004, 2007; Sarkouhi et al. 2012, 2016). The formation of stable complex at m/z 97 was most prominently due to Cu–SH2 complex formation. Here, phosphate to Cu complex formation was very consistence with recent studies and with an order mentioned above (Kumar et al. 2015c, d; Manzanilla-Cano et al. 2004, 2007; Sarkouhi et al. 2012, 2016).
As per HSAB principle, soft metal ion Cu(II) interacted with soft ligand S and leads to formation of stable complex. Once Cu(II) interacted with S, the methyl group would be cleaved and trans effect would allow the retention of NH2 group with P and copper. In net shell, Cu(II) will facilitate the regular decomposition through –N–CO– bond. Amongst the Co(II), Ni(II), Cu(II), Zn(II), and Pb(II) divalent metals, it was observed that, out of these metal ions, only Cu(II) possesses most suitable rank for all three catalytic mechanisms and serves as the most effective catalyst for the OPs (Kumar et al. 2015c, d, Manzanilla-Cano et al. 2004, 2007; Sarkouhi et al. 2012, 2016).
The present study has revealed the absence of the methamidophos (m/z = 141.7) and the presence of other intermediates as reported by previous researchers (Pinjari et al. 2012; Ramu and Seetharamana 2014). It indicated the potential of PS-5 bacterium to mineralize acephate without formation of toxic metabolite methamidophos under the multi-polluted environments (i.e., the presence of heavy metals (Fe(III) and Cu(II) and humic acid. Recently, 31P-NMR results have indicated that Ag(I) and Hg(II) ion can promote the degradation of organophosphorus pesticides and other metal ions formed complex with organophosphorus pesticides (Sarkouhi et al. 2012, 2016).
Biodegradation study of acephate in the presence of humic acid
With addition of humic acid (100 ppm), OD (at 600 nm) as well as decomposition rate was initially decreased as compared to samples without humic acid (Fig. 2). It attributed to the aggregation mechanism in the presence of humic acid (Arias-Estevez et al. 2008; Astray et al. 2010; Albers et al. 2009; Brigante et al. 2009; Mazzeia et al. 2013; Mazzei and Piccolo 2012; Piccolo and Celano 1994). That is why only 75% acephate was decomposed up to 14 days as confirmed by ESI–MS analyses (Fig. 3).
To prove the aggregation mechanism of acephate with humic acid, UV–Visible study at 300 nm was performed (Fig. 5a, b). It noticed that within 1 h, up to 75% aggregation has took place (Fig. 5b). The unit value of degree of aggregation ‘α’ represents the 100% aggregation. To check the stability of acephate–humic acid aggregates, TGA study at 10 °C/min was performed. The increase in stability was observed by TGA study due to aggregation of acephate to humic acid (Fig. 5c). An increase in decomposition temperature or thermal stability (more than 100 °C) was observed, and consequently decrease in mass loss (>60%) of acephate–humic acid complex as compared to acephate only. The maximum decomposition of acephate and humic acid occurred at temperature range 151–300 °C, whereas decomposition of their complex or acephate–humic acid aggregates was occurred at 450 °C onward (Fig. 5c). It highlighted the extra stability in the presence of humic acid due to the formation of acephate–humic acid aggregates. A similar kind of fact on soils was proved by Chai et al. 2010 with other mechanism; they mentioned that mineralization process can decrease on humic acid rich soils. Humic acid is a polymeric molecule having number of groups (election rich/poor) including NH, COOH, CO, OH, etc., and it can bind with xenobiotics or pesticides through the various mode of bonding (Arias-Estevez et al. 2008; Astray et al. 2010; Albers et al. 2009; Brigante et al. 2009; Mazzeia et al. 2013; Mazzei and Piccolo 2012; Piccolo and Celano 1994). In the aggregation mechanism, variable adsorption of the organophosphorus pesticides on humic substances considerably depends on their macromolecular structure and leads to the formation of relatively stable complexes (Albers et al. 2009; Mazzei and Piccolo 2012) (Fig. 5).
Humic compounds are known as colloids that interact with pesticides through the hydrogen bonding, charge transfer, hydrophobic bonding, and van der Waals bonding to form complexes of various stabilities (Arias-Estevez et al. 2008; Brigante et al. 2009; Mazzeia et al. 2013; Mazzei and Piccolo 2012; Piccolo and Celano 1994). High aggregation of the humic molecules with acephate content was probably due to an increasing extent of hydrogen-bonding interactions between the P=O and C=O groups of acephate and humic polymer (Arias-Estevez et al. 2008; Astray et al. 2010; Albers et al. 2009; Brigante et al. 2009; Mazzeia et al. 2013; Mazzei and Piccolo 2012; Piccolo and Celano 1994). In recent studies, very slow aggregation rate at pH 4 and 5 has been noticed and the rate was sharply increased by increasing pH (~8), where complete aggregation achieved in 30 min (Brigante et al. 2009; Mazzeia et al. 2013; Mazzei and Piccolo 2012; Piccolo and Celano 1994). In the current study, at pH 7.5, complete aggregations was observed within 60 min. Formation of extra stable acephate-humic complex/aggregations was governed by thermal gravimetric (TGA) study, which has shown 60% decrease in mass loss of acephate with the addition of humic acid to acephate. In the presence of humic acid, there was formation of phosphate metabolites at m/z 79 and 81 up to 28 day with 100% intensity.
Taking into account that humic acids in water solution are micelle-like aggregates, these kinetic results have been rationalized in terms of micellar pseudo phase model (Arias-Estevez et al. 2008; Astray et al. 2010). These observations are explained with the establishment of weak interactions between organophosphorus pesticides and humic molecular superstructures and represent evidence of host–guest complex formation (Smejkalova and Piccolo (2008); Smejkalova et al. (2009)). In particular, hydrogen bond appears mostly responsible for the host–guest or micelle-like aggregates interaction (Mazzei and Piccolo 2012). Organophosphorus pesticides and humic matter are more protonated at pH 5.2 to pH 7, and a larger number of H-bonds may be formed among complementary functions (Piccolo and Celano (1994). The present study at ~pH 7 indicated that acephate may spontaneously and significantly bind to soluble humic matter by non-covalent interactions and rate of decomposition of acephate was decreased.
Kinetics study of acephate under various conditions
Rates of decomposition of acephate in the absence/presence of Fe(III) or Cu(II) or humic acid were analyzed according to pseudo-first-order kinetics (Table 1 and supplementary Fig. S1). Order of the observed decomposition rates of acephate under different conditions/composition was: Inoculum + 100 ppm acephate > Inoculum + 100 ppm acephate + 100 ppm Cu(II) > Inoculum + 100 ppm acephate + 100 ppm Fe(III) >> Inoculum + 100 ppm acephate + 100 ppm humic acid.
In the presence of Fe(III), Cu(II) and humic acid, significant influence upon decomposition was observed. As compared to the Inoculum + 100 ppm acephate, an increase of half-life time of acephate of 13.26, 23.65, and 99.64% was observed for Inoculum + 100 ppm acephate + 100 ppm Cu(II), Inoculum + 100 ppm acephate + 100 ppm Fe(III), and Inoculum + 100 ppm acephate + 100 ppm humic acid, respectively. In recent studies, decomposition of acephate was measured by various authors (Pinjari et al. 2012; Ramu and Seetharamana 2014), but no one has observed the decomposition kinetics of acephate. In recent pesticide decomposition study in the presence of metal ion (Ag(I)), two organophosphorus pesticides, namely, chlorpyrifos and phoxim, have been decomposed which followed the first-order exponential decay kinetics, and having half-life (t 1/2) of chlorpyrifos and phoxim are 693 and 1155 min, respectively Sarkouhi et al. (2016).
Conclusion
A strain Pseudomonas pseudoalcaligenes PS-5 was isolated from the heavy metal contaminated soils capable to decompose the acephate in the absence and presence of the heavy metal ions Cu(II) and Fe(III), and humic acid (HA). In the presence of soil components (mainly humic acid), the rate of acephate decomposition was reduced significantly. UV–Visible and TGA analyses have confirmed that the slow decomposition rate of acephate in the presence of humic acid was because of strong aggregation behavior of acephate with humic acid in aqueous media. This study may contribute new basic knowledge about the biological treatment, which constitutes an effective alternative to available physicochemical methods for the purification of wastewater containing organophosphate or organophosphate–humate complexes or organophosphate–metal complexes. Hence, Pseudomonas pseudoalcaligenes strain PS-5 was capable of mineralization of the acephate without formation of toxic metabolite methamidophos. More significantly, Pseudomonas pseudoalcaligenes strain PS-5 was capable of mineralization of the acephate under the stress of heavy metals (Fe and Cu), and humic acid.
References
Albers CN, Banta GT, Hansen PE, Jacobsen OS (2009) The influence of organic matter on sorption and fate of glyphosate in soil—comparing different soils and humic substances. Environ Pollut 157:2865–2870. doi:10.1016/j.envpol.2009.04.004
Arias-Estevez M, Astray G, Cid A, Fernández-Gándara D, García-Río L, Mejuto JC (2008) Influence of colloid suspensions of humic acids upon the alkaline fading of carbocations. J Phys Org Chem 21:555–560. doi:10.1002/poc.1317
Astray G, García Río L, Lodeiro C, Mejuto JC, Moldes O, Morales J, Moyano F (2010) Influence of colloid suspensions of humic acids on the alkaline hydrolysis of N-methyl-N-nitroso-p-toluene sulfonamide. Int J Chem Kinet 42:316–322. doi:10.1002/kin.20481
Brigante M, Zaninia G, Avena M (2009) Effect of pHanions and cations on the dissolution kinetics of humic acid particles. Colloids Surf A 347:180–186. doi:10.1016/j.colsurfa.2009.04.003
Chai LK, Wong MH, Mohd-Tahir N, Hansen HCB (2010) Degradation and mineralization kinetics of acephate in humid tropic soils of Malaysia. Chemosphere 79:434–440. doi:10.1016/j.chemosphere.2010.01.046
Charlesworth S, Everett M, Mccarthy R (2003) A comparative study of heavy metal concentration and distribution in deposited street dusts in a large and a small urban area: Birmingham and Coventry West Midlands UK. Environ Int 29:563–573. doi:10.1016/S0160-4120(03)00015-1
Kim JY, Myung JH, Ahn JS (1998) Heavy metal speciation in dusts and stream sediments in the Taejon area Korea. J Geochem Explor 64:409–419. doi:10.3390/ijerph13080820
Kumar V, Upadhyay N, Wasit AB, Singh S, Kaur P (2013) spectroscopic methods for the detection of organophosphate pesticides—a preview. Curr World Environ 8:313–319. doi:10.12944/CWE.8.2.19
Kumar V, Upadhyay N, Kumar V, Kaur S, Singh J, Singh S, Datta S (2014) Environmental exposure and health risks of the insecticide monocrotophos—a Review. J Bio Env Sci 5:111–120
Kumar V, Upadhyay N, Kumar V, Sharma S (2015a) A review on sample preparation and chromatographic determination of acephate and methamidophos in different samples. Arab J Chem 8:624–631. doi:10.1016/j.arabjc.2014.12.007
Kumar V, Singh S, Singh J, Upadhyay N (2015b) Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull Environ Contam Toxicol 94:807–815. doi:10.1007/s00128-015-1523-7
Kumar V, Upadhyay N, Manhas A (2015c) Designingsynthesescharacterizationcomputational study and biological activities of silver-phenothiazine metal complex. J Mol Str 1099:135–140. doi:10.1016/j.molstruc.2015.06.055
Kumar V, Kumar V, Upadhyay N, Sharma S (2015d) Interactions of atrazine with transition metal ions in aqueous media: experimental and computational approach. Biotech 5:791–798. doi:10.1007/s13205-015-0281-x
Kumar V, Kumar V, Kaur S, Singh S, Upadhyay N (2016) Unexpected formation of N-phenyl-thiophosphorohydrazidic acid O, S-dimethyl ester from acephate: chemical biotechnical and computational study. 3. Biotech 6:1–11. doi:10.1007/s13205-015-0313-6
Lakanen E, Ervio R (1971) A comparison of eight extractants for the determination of plant available micronutrients on soil. Acta Agralia Fennica 123:223–232
Leharne S, Charlesworth D, Choudhry B (1992) A survey of metal levels in street dusts in an inner London Neighbourhood. Environ Int 18:263–270. doi:10.1016/0160-4120(92)90109-H
Manzanilla-Cano JA, Barceló-Quintal MH, Reyes-Salas EO (2004) Electrochemical monitoring of methylparathion degradation in an acid aqueous medium in presence of Cu(II). J Environ Sci Health B 39:577–588. doi:10.1081/PFC-200026805
Manzanilla-Cano JA, Barceló-Quintal MH, Rendón-Osorio RB, Flores-Rodríguez J (2007) Effect of Fe(III) on acid degradation of methylparathion. J Environ Sci Health B 425:15–22. doi:10.1080/03601230701391740
Mazzei P, Piccolo A (2012) Quantitative evaluation of noncovalent interactions between glyphosate and dissolved humic substances by NMR spectroscopy. Environ Sci Technol 146:5939–5946. doi:10.1021/es300265a
Mazzeia P, Oschkinatb H, Piccolo A (2013) Reduced activity of alkaline phosphatase due to host–guest interactions with humic superstructures. Chemosphere 93:1972–1979. doi:10.1016/j.chemosphere.2013.07.015
Piccolo A, Celano G (1994) Hydrogen bonding interactions of the herbicide glyphosate with water soluble humic substances. Environ Toxicol Chem 13:1737–1741. doi:10.1002/etc.5620131104
Pinjari B, Novikov B, RezenomYH Russell DH, Wales ME, Siddavattam D (2012) Mineralization of acephate a recalcitrant organophosphate insecticide is initiated by a pseudomonad in environmental samples. PLoS ONE 7:31963–31970. doi:10.1371/journal.pone.0031963
Prasad R, Upadhyay N, Kumar V (2013) Simultaneous determination of seven carbamate pesticide residues in gram wheat lentil soybean fenugreek leaves and apple matrices. Microchem J 111:91–97. doi:10.1016/j.microc.2012.12.014
Ramu S, Seetharamana B (2014) Biodegradation of acephate and methamidophos by a soil bacterium Pseudomonas aeruginosastrain Is-6. J Environ Sci Health B 49:23–34. doi:10.1080/03601234.2013.836868
Sarkouhi M, Shamsipur M, Hassan J (2012) 31P-NMR evaluation of organophosphorus pesticides degradation through metal ion promoted hydrolysis. Environ Monit Assess 184:7383–7393. doi:10.1007/s10661-011-2507-7
Sarkouhi M, Shamsipur M, Hassan J (2016) Metal ion promoted degradation mechanism of chlorpyrifos and phoxim. Arab J Chem 9:43–47. doi:10.1016/j.arabjc.2012.04.026
Singh S, Singh N, Kumar V, Datta S, Wani AB, Singh D, Singh K, Singh J (2016) Toxicity monitoring and biodegradation of the fungicide carbendazim. Environ Chem Lett 14(3):317–329. doi:10.1007/s10311-016-0566-2
Smejkalova D, Piccolo A (2008) Host-guest interactions between 2,4-dichlorophenol and humic substances as evaluated by 1H NMR relaxation and diffusion ordered spectroscopy. Environ Sci Technol 42:8440–8445. doi:10.1021/es801809v
Smejkalova D, Spaccini R, Fontaine B, Piccolo A (2009) Binding of phenol and differently halogenated phenols to dissolved humic matter as measured by NMR spectroscopy. Environ Sci Technol 43:5377–5382. doi:10.1021/es900559b
Wang L, Wen Y, Guo X, Wang G, Li S, Jiang J (2010) Degradation of methamidophos by Hyphomicrobium species MAP-1 and the biochemical degradation pathway. Biodegradation 21:513–523. doi:10.1007/s10532-009-9320-9
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils urban road dusts and agricultural soils from China. Microchem J 94:99–107. doi:10.1016/j.microc.2009.09.014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, S., Kumar, V., Upadhyay, N. et al. Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and Fe(III)], and humic acid. 3 Biotech 7, 262 (2017). https://doi.org/10.1007/s13205-017-0900-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0900-9