Abstract
Endosulfan contamination is one of the major concerns of soil ecosystem, which causes detrimental effects not only to humans but also to animals and plants. Therefore, the aim of this study was to isolate and identify a novel bacterial strain capable of degrading endosulfan in agriculture contaminated soils. A novel bacterial strain was isolated from the sugarcane field contaminated with endosulfan, and was named as ZAM1 strain. The ZAM1 bacterial strain was further identified as Pseudomonas mendocina based on the biochemical and molecular analysis. 16sRNA sequence analysis of ZAM1 strain shows maximum similarity with known endosulfan-degrading bacteria (Pseudomonas putida), respectively. Enrichment was carried out using the endosulfan as sole sulfur source. The ZAM1 strain was able to use α and β endosulfan as a sole sulfur source. Our results showed that ZAM1 strain degrades endosulfan >64.5% (50 mg/l) after 12 days of incubation. The residues were analyzed by GC–MS analysis and confirmed the formation of metabolites of dieldrin, 2 heptanone, methyl propionate, and endosulfan lactone compounds. Hence, these results indicate that the ZAM1 strain is a promising bacterial source for detoxification of endosulfan residues in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endosulfan is a broad-spectrum cyclodiene organochlorine insecticide and is used to control the numerous insects in a wide variety of crops throughout the globe. Technical grade endosulfan comprises two isomers, α and β in the ratio of 7:3. Endosulfan is recalcitrant to degradation with the half-life of 35–67 days of α-endosulfan and 104–265 days of β-endosulfan. Although endosulfan is toxic to both aquatic and terrestrial life and has been phased out globally because of its acute toxicity, long persistence, and high bioaccumulation (Liao et al. 2004; Sandhu and Brar 2009; Shinggu et al. 2015). In India, endosulfan is applied to sugarcane, cotton, and other crops as an insecticide. Its continual applications resulted in the contamination of soil and water environments at numerous sites, mainly in the close vicinities of agricultural fields, the exposure of endosulfan can cause a varied array of severe and chronic effects including carcinogenesis, estrogenic action, and endocrine disruption, posing a serious threat to environmental and human health (Aggarwal et al. 2008; Schafer and Kegley 2002; Tiemann 2008). As a result there is an increasing concern on environmental endosulfan contamination and in its remediation. Persistent organic pollutants global monitoring network program data obtained through the Global Monitoring Plan (GMP) for POPs persistant organic pollutants showed endosulfan is still abundant in the soil environment and has also been detected in atmospheric dust (Astoviza et al. 2016; Harner et al. 2006), due to its high application and persistent ability and its persistence in the soil was reported across the world (Arias et al. 2011; Guo et al. 2015). Most of the endosulfan production and consumption throughout the world was reported in India. The endosulfan present in soil is naturally oxidized and hydrolyzed to stable compounds of endosulfan, i.e., endosulfan sulfate and endosulfan diol, respectively. Mainly endosulfan sulfate is more persistent than endosulfan diol (Becker et al. 2011; Goebel et al. 1982). It is a highly controversial agrochemical due to its persistent and acute toxicity (Yadav et al. 2015; Jaya et al. 2013) and has significant and serious threats to human health (Desalegn et al. 2011). Despite, it has been extensively used for the protection of commercial crops such as tea, cotton, sugarcane, vegetables, and fruits from broad range of pests. Its contamination also affects the soil composition, structures, functional microbial diversity, and their metabolic activities (Weber et al. 2010; Defo et al. 2011; Vani et al. 2012). High concentration of endosulfan in soil leads to a continuous decline in soil fertility and affects the cultivated crops (Weber et al. 2010; Fox et al. 2007).
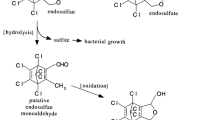
The above-mentioned discussion highlights the role of endosulfan in environmental pollution and researchers have reported the concentration of endosulfan in different matrices such as soil samples, water bodies, air, and soil exchange (Ernst et al. 1991; Qu et al. 2015). It is very obvious that the isomers of endosulfan are extremely toxic to numerous life forms and are also responsible for increasing reproductive toxicity (Choudhary and Joshi 2003), hepatotoxicity (Jamil et al. 2004), and genotoxicity (Liu et al. 2009). Therefore, it is vital to develop technologies for bioremediation of endosulfan present in water bodies in higher concentrations or just above permissible limits (Kumar and Philip 2006a). Number of studies have been exemplified for the biotransformation of endosulfan in soils by functional microbial diversity (Defo et al. 2011, Sutherland et al. 2002a) and specific bacterial strains such as Pseudomonas putida (Singh and Singh 2007; Sunitha et al. 2012), Pseudomonas fluorescens (Jesitha et al. 2015), Rhodococcus sp. (Verma et al. 2011), Achromobacter xylosoxidans, Bordetella sp. (Sand and Singh 2009), Klebsiella, Acinetobacter, Alcaligenes, Flavobacterium, Bacillus (Kafilzadeh et al. 2015), and A. xylosoxidans (Seralathan et al. 2015). Diagrammatic representation of endosulfan biodegradation by Pseudomonas mendocina ZAM1 strain is depicted in Fig. 1. It has been reported in previous studies that a wide range of microbes, mostly bacteria and fungi are capable of utilizing endosulfan as a sole source of carbon and/or sulfur (Sutherland et al. 2002a, b; Sethunathan et al. 2004). Biological pesticide detoxification from soil and water is receiving more attention due to increasing rates of human cancer and other human- and animal-related disorders. Biological pesticide detoxification is the best alternative technology of previously reported detoxification methods such as incineration and landfill. The present study was designed to isolate and characterize soil bacteria competent of degrading endosulfan. The essence of degradation and metabolism of endosulfan by the isolated bacterial strain was investigated using gas chromatography and mass spectroscopy for identification of breakdown products.
Materials and methods
Chemicals and reagents
Technical (95.5%) and analytical grade (99.5%) endosulfan (molecular formula: C9H6Cl6O3S, molecular weight: 406.9, chemical name: 6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepin) was obtained from Bayer Crop Science, India. Culture media, agar powder, peptone broth, and nutrient broth were purchased from a local distributor, Hi-Media (Mumbai, India). Aluminum oxide, potassium permanganate, and iodine reagents were purchased from Merck Ltd. (Mumbai India). Other remaining chemical reagents and solvents used were of high quality.
Soil sample collection
Soil samples were collected from four different locations, sugarcane-, paddy-, cashew nut-, and melon-growing fields of Villupuram District, Tamil Nadu, India. Standard method was followed for composite soil sample collection from different locations. All soil samples were obtained from 10 cm below the surface and stored in sterilized bag at 4 °C.
Culture enrichment
Enrichment culture technique was used for the isolation of bacterial strain capable of utilizing endosulfan as a sole S source. Homogenous mixture of soil sample (25 g) was added to 1L of media (composition gram per liter: 4.5 KH2PO4, 5.8 K2HPO4, 2.0 (NH4)2SO4, 0.16 MgSO4, 0.02 CaCl2, 0.002 Na2MoO4, 0.001 MnCl2, and 0.001 FeSO4) and supplemented with 5% endosulfan and incubated in shaking incubator for 15 days at 140 rpm at 28 °C. The most tolerant bacterial strains were able to grow in basal salt medium with endosulfan as the sole source of sulfur and enrichment of endosulfan was increased up to 20% in the media.
Isolation and purification of the endosulfan-degrading bacterial strain
Enriched culture was diluted up to fivefold by serial dilution with normal saline water. Bacterial isolates were isolated from the enriched culture by spreading 0.1 ml of aliquot on endosulfan-amended minimal media plates. After 48 h of incubation, different colonies appeared with distinguished morphology. These colonies were re-streaked on the same media plates for culture purification and preservation. The culture was stored on nutrient agar slants supplemented with endosulfan at 4 °C.
Endosulfan tolerance assessment
The bacterial isolates were tested for tolerance against endosulfan by dilution methods using minimal media (Ingredients in g/l: 1 KH2PO4, 1K2HPO4, 1 NH4NO3, 0.2 MgSO4·7H2O, 0.02 CaCl2·2H2O, 0.01 FeSO4·7H2O, and 15 agar). During plate preparation, endosulfan was amended in media with rising concentrations of 0, 25, 50 100, 200, 300, 400, and 500 μg/ml. Test bacterial strains were spot inoculated on the surface of solid agar with the help of inoculation loop. All inoculated plates were incubated in incubation chamber at 28 ± 2 °C for 3 days. The endosulfan-tolerant bacterial strain was grown on higher amount of endosulfan-amended plates and it was declared as the highest or maximum tolerance level (MTL) of this strain.
Biochemical and molecular characterization of ZAM1 strain
The bacterial strain ZAM1 was exhibiting maximum tolerance and biodegradation potential of endosulfan in the growing medium. Initially it was identified using biochemical methods and further characterized by standard protocol (Cappuccino and Sherman 2008). For presumptive identification, biochemical tests have been performed. It included citrate utilization, catalase test, oxidase test, gelatin liquefaction, nitrate reduction, indole production, methyl red, Voges–Proskauer, and carbohydrates utilization test such as glucose, sucrose, mannitol, and hydrolysis of starch followed standard methods are shown in Table 1. Molecular characterization of selected bacterial strain was done by 16S rRNA gene sequencing. Sequencing was carried out commercially at Macrogen private sequencing service, Seoul, South Korea. During sequencing universal forward primer 518 F 5′CCAGCAGCCGCGGTAATACG3′ and reverse primer 800R 5′TACCAGGGTATCTAATCC3′ were used for DNA amplification. The program BLASTn was run to obtain known homologous sequences from the website of NCBI (http://www.ncbi.nlm.nih.gov/BLAST) and maximum similarity of ZAM1 strain was determined. 16S rRNA gene sequence data were deposited in the GenBank sequence database to obtain unique accession number. Furthermore, all related sequence analysis of ZAM-1 strain was aligned using Clustal W 1.6 program (www.ebi.ac.uk/clustalW). After alignment, phylogenetic tree was constructed by neighbor-joining method with the help of MEGA 6 software at 1000 bootstraps (Kumar et al. 2004).
Screening of efficient microbial isolates for their capability to utilize endosulfan for growth
Experiments were performed in a batch culture and endosulfan concentration was decided according to the tolerance. Stock solutions of endosulfan were prepared in dimethyl sulfoxide (DMSO). Overnight-grown bacterial culture was inoculated on minimal salt media, which amended up to 500 μg/ml endosulfan in Erlenmeyer flask and incubated in rotary shaker at 140 rpm for different time intervals and at 28 ± 2 °C. Further growth patterns were determined by turbidometrical analysis of optical density of bacterial culture in media measured by UV–Visible spectrophotometer at 600 nm.
Effect of pH and initial concentration on degradation
Effect of pH and initial concentration on endosulfan degradation was observed by conducting experiments from the least initial endosulfan concentration starting from 0 to 400 μg/ml was amended in MSM medium. Batch experiment was done for 100 h. Effect of pH on endosulfan degradation was estimated at different ranges from 4 to 9. The pH of the media was measured as per the standard method using a pH meter (APHA 1998).
Extraction and GC–MS analysis of degraded sample
Bacterial-mediated degradation of endosulfan in the medium was assessed by GC–MS and the metabolites were extracted using chromatographic solvent n-hexane and acetone mixture at different time points. 5 ml of sample was collected at each time point and centrifuged at 8000 rpm for 20 min then passed through a 0.45-µm membrane filter (Millipore). The filtrate sample was extracted using 3:1 ratio of hexane acetone (v/v) and samples were kept in a water bath for evaporation at 50 °C and then remaining residues were dissolved in 2 ml of hexane. A Shimadzu GCMS-QP2010, Kyoto, Japan with chromatographic column (size 0.18 mm I.D.× 0.53 mm I.D., d.f. 3.0 µm + resistance tube 0.82 m × Columns: Rxi®-624sil MS, 30 m *Particle Size Analyzer, 10 nm–300 µm) was used in this study. The injector port temperature was set to 280 °C in a splitless mode with the splitter activated after 1 min injection mode and volume:pulsed injection (300 kPa hold for 1 min) in the split mode (1:10), 6 µL. Solvent-extracted-filtered samples were analyzed using a specific column OV-101 and internal temperature set at 200 °C. During experiment nitrogen gas was used as a carrier with 25 ml/min flow rate. Further machine injector and detector temperatures were set at 220 and 275 °C, respectively. The MS analysis of bacterial metabolites from the endosulfan-degraded sample was done using a CP-Sil-8CB capillary column equipped with MS. Sample injector temperature was set according to program at 0 min from 100 to 300 °C and increased to 180 °C/min for 45 min. The compounds present in metabolites were identified on the basis of retention time and spectra further matched with the NIST library.
Results and discussion
Soil endosulfan assessment
Endosulfan concentration was detected in the composite mixtures of soil samples of sugarcane fields. Endosulfan level in the soil sample was analyzed in the range between 2.7 and 52 mg/Kg α-endosulfan, from 1.5 to 112 mg/kg β-endosulfan, and 3.3 to 161 mg/Kg endosulfan sulfate. The retention time for compound moieties such as α-endosulfan, β-endosulfan, and endosulfan sulfate was found to be 19.84, 20.89, and 18.82 min, respectively. In our results, β-endosulfan concentration was twofold higher then α forms and endosulfan sulfate concentration was three times higher than α form due to its persistent nature.
Characterization and enrichment of endosulfan-degrading bacterial strain
Endosulfan-contaminated soil samples were transferred to enrichment media. Culture enrichment of indigenous bacterial strain was followed for 15 days with sub-culturing after every 5 days and supplemented with sulfur-free minimal media (Sutherland et al. 2002c; Siddique et al. 2003). Bacterial metabolite samples were analyzed by GC–MS. α-Endosulfan and endosulfan sulfate were found extensively in the enriched culture. Fresh composite soil sample was enriched with endosulfan to obtain the competent bacterial strain which can tolerate higher concentrations of endosulfan (Kalyani et al. 2009).
Biochemical and molecular identification of ZAM1 strain
Colony morphology of ZAM1 strain on LB (Luria–Bertani) agar was circular, smooth, and creamy in color. Gram staining confirmed it as a Gram-negative, rod-shaped bacteria, aerobic in nature, and biochemical tests further confirmed the isolate as P. mendocina strain. The results of biochemical tests are indicated in Table 1. Molecular characterization of ZAM1 strain by 16S rRNA gene sequencing revealed maximum homology with P. putida (JX173499), P. putida (EU862561), and P. plecoglossicida (KF782813) in BLASTn analysis. Maximum homology (84%) in nucleotide sequence was matched with accession number JX173499 of P. putida. Phylogenetic tree of 1000 bootstrap was constructed from the partial 16S rRNA gene sequences of the ZAM1 and its maximum related sequences. In phylogenetic analysis, the strain ZAM1 showed maximum likelihood with Pseudomonas species (Fig. 2). The 16S rRNA gene sequence of ZAM1 strain has been submitted to GenBank, NCBI database and was authenticated with a unique Accession Number HG514307.
Assessment of endosulfan biodegradation by ZAM1 strain
The endosulfan degradation result suggests that the growth of microbial isolate ZAM1 was accompanied by the omission of endosulfan. The initial growth rate was higher because of nutrient availability in the medium and afterwards it turns stagnant. Endosulfan removal was significant after 12 days of incubation, while the growth curve study of ZAM1 strain at different concentrations is depicted in Fig. 3. Among 73 indigenous bacterial isolates, ZAM1 strain was highly efficient for endosulfan degradation. Similarly, in another study Gram-negative bacteria were an excellent degrader of a wide range of xenobiotic and organic compounds in soil environment (Bajaj et al. 2010). Pseudomonas mendocina ZAM1 strain degraded the maximum endosulfan concentration in a very short incubation period (in vitro), in comparison to previous studies which reported the bacterial strains for endosulfan degradation, which utilized carbon and sulfur compounds as electron transfer agents (Awasthi et al. 2003; Kumar and Philip 2006a). The prolific growth of bacterial strain during incubation in endosulfan-amended medium was confirmed by increasing optical density with incubation time and it happened due to the increasing number of bacterial cells.
Effect of pH on the biodegradation of endosulfan
The effect of pH on endosulfan degradation was estimated by varying the pH from 4 to 9. The maximum endosulfan degradation, 64.5%, occurred at pH 6.5 and degradation was declined when pH of the media decreased or increased. The effect pH on degradation is depicted in Figs. 4a, b, c, and 5. This has happened due to the surface modification and change in catalytic activity of the enzymes involved in degradation and best growing capacity of bacterial strain at the optimum condition. During endosulfan degradation, pH of the medium decreased with increase in incubation time, due to dehalogenation of endosulfan and subsequently decreasing pH due to the formation of acidic compounds. Previous studies reported similar phenomenon of endosulfan biodegradation with decreasing pH which might be due to the formation of organic acids or HCl in medium by microorganisms (Kullman and Matsumura 2006; Fox et al. 2007).
Effect of endosulfan concentration on biodegradation of endosulfan
The effect of initial endosulfan concentration on degradation was estimated and the percentage of degradation was increased with increase in concentration up to 50 µg/ml and further decrease in degradation rate with increasing concentration (Fig. 6).
Metabolite formation and GC–MS analysis
Progression of the strain ZAM1 was monitored when it was supplied with α-ES and ES sulfate as the sole source of sulfur in the minimal medium. The growth of the ZAM1 strain in minimal medium containing α-ES and ES sulfate as substrate reached a maximum at OD 600 nm in 100 h and declined at day 12 (Fig. 4a, b, c). During the growth, a decline in pH from 7 to 5 was observed. This could be due to the release of metabolites and the presence of dead cells in the medium. Based on GC–MS analysis, the metabolites formed in the supernatant obtained from the growing culture media of ZAM1 strain supplemented with endosulfan showing signature fragmentation patterns obtained from the compounds available in the NIST library and without inoculation (control) is depicted in Fig. 7a, b. Metabolites formed during the degradation of ES in the bacterial culture inoculated at pH 6.5 after 100-h incubation were analyzed and shown in Fig. 8a–d. Extract composition contains different compounds including α and β forms of endosulfan, endosulfan hydroxyl carboxylate dieldrin, octane, and heptanone methyl propionate. Endosulfan transformation into number of compounds was confirmed by the MS fragmentation pattern. Total ion chromatogram indicates a peak at the particular retention time (RT), which indicates the base peak of major ion peak and defines the m/z ratio. The base peak at different retention times from 3.44 to 21.6 which indicates the presence of unique compound at particular m/z ratio in the fragmentation pattern is shown in (Fig. 9). A peak at the 16.95 RT was shown in the fragmentation pattern that perfectly matched endosulfan lactone in the NIST library. The compound in MS fragmentation profile was showing the significant base peak at 69 and major ion peaks at 338 m/z and 18.8 RT, a peak was observed with less area, which matched with endosulfan sulfate. The fragmentation profile showed the base peak at 113 and with major ion peaks at 85 which are characteristic to octane and methyl propionate. Control experiment done without bacterial strain inoculation and metabolites released during degradation analyzed by MS spectra are depicted in Fig. 10a–d. The formation of organic acid in metabolites was confirmed by GC–MS analysis. These results significantly pointed out the role of ZAM1 bacterial strain in the hydrolytic pathway of endosulfan conversion and biodegradation (Kirby et al. 2001; Kim et al. 2001), while endosulfan sulfate was formed during the oxidative pathway of endosulfan biodegradation (Kullman and Matsumura 2006; Kwon et al. 2002). During first 1 h of incubation there was only slight degradation of endosulfan, this represents a lag phase of bacterial strain while it got accelerated as the incubation proceeds, most likely due to the activation of metabolic pathway which releases enzymes in the growing media.
a Mass spectrometry analysis after degradation showing CH3–(CH2)6–CH2 after 3.44 RT. b Mass spectrometry analysis after degradation showing CH3–CH2–COO–CH3; CH3–(CH2)4–CH2 after 5.0 RT. c Mass spectrometry analysis after degradation showing 2-heptanone after 6.54 retention time (RT). d Mass spectrometry analysis after degradation showing 2-heptanone after 8.05 retention time (RT)
a Mass spectrometry analysis after degradation showing after 9.44 retention time (RT). b Mass spectrometry analysis after degradation showing after 14.4 retention time (RT). c Mass spectrometry analysis after degradation showing after 15.03 retention time (RT). d Mass spectrometry analysis after degradation showing 16.02 retention time (RT)
a Mass spectrometry analysis after degradation showing α-endosulfan lactone after 16.95 RT. b Mass spectrometry analysis after degradation showing endosulfan hydroxycarboxylate after 17.16 RT. c Mass spectrometry analysis after degradation showing α-endosulfan after 17.75 retention time (RT). d Mass spectrometry analysis after degradation showing endosulfan sulfate after 18.08 RT
Pseudomonas species are one of the most important groups of Gram-negative bacteria and have been previously documented as an excellent degrader of a wide range of xenobiotic and recalcitrant compounds present in soil and water environment (Filonov et al. 2006; Thangadurai and Sumathi 2014). The ZAM1 bacterial strain degraded endosulfan isomers, i.e., 64.5%, which was higher than of previously documented bacterial strains utilizing endosulfan as sulfur or carbon source, depicted in Table 2 (Awasthi et al. 2003; Sutherland et al. 2002a; Kumar and Philip 2006b). This might be due to their prolific growth during the incubation period as evident from the higher optical densities or the presence of an enzymatic system responsible for the degradation. There was a parallel decrease in pH of the culture medium as the biodegradation proceeded. This dramatic reduction of pH of the bacterial culture might be due to dehalogenation of endosulfan and subsequent formation of acidic substances. These results confirmed the findings of previous studies (Sutherland et al. 2002a; Awasthi et al. 2003).
Conclusion
The Pseudomonas mendocina ZAM1 strain having a history of endosulfan application was isolated from endosulfan-contaminated soil and it was identified and characterized for its efficiency of endosulfan degradation. The metabolite formation in the growth medium was confirmed by gas chromatography and mass spectroscopy. P. mendocina ZAM1 has a tremendous potential to be used in bioremediation of endosulfan-contaminated habitats especially with lower concentrations and without generating endosulfan sulfate, a more persistent metabolite, and this strain can be used for the safe treatment of endosulfan-contaminated soil and water.
References
Aggarwal M, Naraharisetti SB, Dandapat S, Degen GH, Malik JK (2008) Perturbations in immune responses induced by concurrent sub chronic exposure to arsenic and endosulfan. Toxicol 251:51–60
APHA (1998) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Arias AH, Marcelo TP, Jorge EM (2011) Multi-year monitoring of estuarine sediments as ultimate sink for DDT, HCH and other organochlorinated pesticides in Argentina. Environ Monit Assess 172:17–32
Arshad M, Hussain S, Saleem M (2008) Optimization of environmental parameters for biodegradation of alpha and beta endosulfan in soil slurry by Pseudomonas aeruginosa. J Appl Microbiol 104(2):364–370
Astoviza MJ, Cappelletti N, Bilos C, Migoya CM, Colombo JC (2016) Massive airborne endosulfan inputs related to intensive agriculture in Argentina’s Pampa. Chemoshpere 44:1459–1466
Awasthi N, Singh AK, Jain RK, Khangarot BS (2003) Degradation and detoxification of endosulfan isomers by a defined co-culture of two Bacillus strains. Appl Microb Biotechnol 62:279–283
Bajaj A, Pathak A, Mudiam MR, Mayilraj S, Manickam N (2010) Isolation and characterization of a Pseudomonas sp. strain IITR01 capable of degrading α-endosulfan and endosulfan sulfate. J Appl Microbiol 109:2135–2143
Becker L, Scheringe M, Schenker U, Hungerbuhler K (2011) Assessment of the environmental persistence and long-range transport of endosulfan. Environ Pollut 159:1737–1743
Cappuccino J, Sherman N (2008) Microbiology, a laboratory manual. Pearson Education Inc., New York
Choudhary N, Joshi SC (2003) reproductive toxicity of endosulfan in male Albino Rats. Bull Environ Contam Toxicol 70:0285–0289
Defo MA, Njine T, Nola M, Beboua FS (2011) Microcosm study of the long term effect of endosulfan on enzyme and microbial activities on two groundnut soils of Yaounde-Cameroon. Afr J Agric Res 6:2039–2050
Desalegn B, Takasuga T, Harada KH, Hitomi T, Fujii Y, Yan HR, Wang P, Senevirathna S, Koizumi A (2011) Historical trends in human dietary intakes of endosulfan and toxaphene in China, Korea and Japan. Chemosphere 83:1398–1405
Ernst WR, Doe JK, Julien G, Hennigar P (1991) Toxicity to aquatic organisms of off -target deposition of endosulfan applied by aircraft. Environ Toxicol Chem 10:103–114
Filonov AE, Puntus IF, Karpov AV et al (2006) Assessment of naphthalene biodegradation efficiency of Pseudomonas and Burkholderia strains tested in soil model systems. J Chem Technol Biotechnol 81:216–224
Fox JE, Gulledge J, Engelhaupt E, Burow ME, McLachlan JA (2007) Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc Natl Acad Sci USA 104:10282–10287
Goebel H, Gorbach S, Knauf W, Rimpau RH, Huttenbach H (1982) Properties, effects, residues and analytics of the insecticide endosulfan. Residue Rev 83:40–41
Goswami S, Dileep KS (2009) Biodegradation of α and β endosulfan in broth medium and soil microcosm by bacterial strain Bordetella sp. B9. Biodegradation 20(2):199–207
Guo FZ, Zhang LS, Wei JL, Li YB, Shi ZX, Yang YM, Zhou XQ, Sun ZW (2015) Endosulfan induced the arrest of the cell cycle through inhibiting the signal pathway mediated by PKC-α and damaging the cytoskeleton in spermatogonial cells of mice in vitro. Toxicol Res 4:508–518
Harner T, Pozo K, Gouin T, Macdonald AM, Hung H, Cainey J, Peters A (2006) Global pilot study for persistent organic pollutants (POPs) using PUF disk passive air samplers. Environ Pollut 144:445–452
Jaya R, Mohineesh Ray R, Dogra TD, Raina A (2013) Acute oral toxicity and histopathological study of combination of endosulfan and cypermethrin in Wistar Rats. Toxicol Int 20:61–67
Jamil K, Shaik AP, Mahboob M, Krishna D (2004) Effect of organophosphorus and organochlorine pesticides (monocrotophos, chlorpyriphos, dimethoate, and endosulfan) on human lymphocytes invitro. Drug Chem Toxicol 27:133–144
Jesitha K, Nimisha KM, Manjusha CM, Harikumar PS (2015) Biodegradation of endosulfan by Pseudomonas fluorescens. Environ Process 2:225–240
Kalyani SS, Sharma J, Singh S, Lata DP (2009) Enrichment and isolation of endosulfan-degrading microorganism from tropical acid soil. J Environ Sci Health B 44:663–672
Kumar M, Philip L (2006a) Endosulfan mineralization by bacterial isolates and possible degradation pathway identification. Bioremediat J 10:179–190
Kumar S, Tamura K, Nei M (2004) MEGA3, integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Kirby ML, Barlow RL, Bloomquist JR (2001) Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicol Sci 61:100–106
Kafilzadeh F, Ebrahimnezhad M, Tahery CY (2015) Isolation and identification of endosulfan-degrading bacteria and evaluation of their bioremediation in Kor River, Iran. Osong Public Health Res Perspect 6:39–46
Kim YK, Kim SH, Choi SC (2001) Kinetics of endosulfan degradation by Phanerochaete chrysosporium. Biotechnol Lett 23:163–166
Kullman WS, Matsumura F (2006) Metabolic pathways utilized by Phanerochaete chrysosporium for degradation of the cyclodiene pesticide endosulfan. Appl Environ Microbiol 62:593–600
Kwon GS, Kim JE, Kim TK, Sohn HY, Koh SC, Shin KS, Kim DG (2002) Klebsiella pneumoniae KE-1 degrades endosulfan without formation of the toxic metabolite, endosulfan sulfate. FEMS Microbiol Lett 215:255–259
Kumar M, Philip L (2006b) Bioremediation of endosulfan contaminated soil and water optimization of operating conditions in laboratory scale reactors. J Hazard Mater 136:354–364
Li W, Dai Y, Xue B, Li Y, Peng X, Zhang J, Yan Y (2009) Biodegradation and detoxification of endosulfan in aqueous medium and soil by Achromobacter xylosoxidans strain CS5. J Hazard Mater 167(1):209–216
Liu W, Zhu LS, Wang J, Wang JH, Xie H, Song Y (2009) Assessment of the genotoxicity of endosulfan in earthworm and white clover plants using the comet assay. Arch Environ Contam Toxicol 56:742–746
Liao XM, Huang M, Liu CY, Abid WPS (2004) Effects of pesticides on soil biochemical characteristics of a paddy soil. J Environ Sci 16:252–255
Qu C, Xing X, Albanese S, Doherty A, Huang H, Lima A, Qi S, Devivo B (2015) Spatial and seasonal variations of atmospheric organochlorine pesticides along the plain-mountain transect in central China, Regional source vs. long-range transport and air–soil exchange. Atmos Environ 122:31–40
Sand G, Singh DK (2009) Biodegradation of α and β-endosulfan in broth medium and soil microcosm by bacterial Strain bordetella sp B9. Biodegradation 20:199–207
Sandhu HS, Brar RS (2009) Pesticides in textbook of veterinary toxicology. Kalyani Publishers, Ludhiana
Sarfraz H et al (2007) Screening of soil fungi for in vitro degradation of endosulfan. World J Microbiol Biotechnol 23(7):939–945
Schafer KS, Kegley SE (2002) Persistent toxic chemicals in the US food supply. J Epidemiol Community Health 56(11):813–817
Seralathan MV, Sivanesan S, Nargunanathan S, Bafana A, Kannan K, Chakrabarti T (2015) Chemotaxis-based endosulfan biotransformation, enrichment and isolation of endosulfan-degrading bacteria. Environ Technol 36:60–67
Sethunathan N, Megharaj M, Chen ZL, Williams BD, Lewis G, Naidu R (2004) Algal degradation of a known endocrine disrupting insecticide, α-Endosulfan, and its metabolite, endosulfan sulfate, in liquid medium and soil. J Agric Food Chem 52:3030–3035
Shinggu DY, Maitera ON, Barminas JT (2015) Determination of organochlorine pesticides residue in fish, water and sediment in Lake Geriyo Adamawa State Nigeria. Int Res J Pure Appl Chem 8:212–220
Shivaramaiah HM, Kennedy IR (2006) Biodegradation of endosulfan by a soil bacterium. J Environ Sci Health Part B 41(6):895–905
Singh PB, Singh V (2007) Pesticide bioaccumulation and plasma sex steroids in fishes during breeding phase from north India. Environ Toxicol Pharmacol 55:223–237
Siddique T, Okeke BC, Arshad M, Frankenberger WT Jr (2003) Enrichment and isolation of endosulfan degrading microorganisms. J Environ Qual 32:47–54
Sutherland TD, Horne I, Russell RJ, Oakeshott JG (2002a) Gene cloning and molecular characterization of a two-enzyme system catalyzing the oxidative detoxification of b-endosulfan. Appl Environ Microbiol 68:6237–6245
Sutherland TD, Weir KM, Lacey MJ, Horne I, Russell RJ, Oakeshott JG (2002b) Enrichment of a microbial culture capable of degrading endosulfate, the toxic metabolite of endosulfan. J Appl Microbiol 92:541–548
Sutherland TD, Weir KM, Lacey MJ, Horne I, Russell RJ, Oakeshott JG (2002c) Enrichment of a microbial culture capable of degrading endosulphate, the toxic metabolite of endosulfan. J App Microbiol 92:541–548
Sunitha S, Murthy VK, Mahmood R (2012) Degradation of endosulfan by mixed bacterial cultures enriched from endosulfan contaminated soils of Southern India. Int J Biosci Biochem Bioinform 2:31–35
Thangadurai P, Sumathi S (2014) Biodegradation of endosulfan by soil bacterial cultures. Int Biodeterior Biodegrad 94:38–47
Tiemann U (2008) In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor and lindane on the female reproductive tract of mammals, a review. Reprod Toxicol 25:316–326
Vani T, Saharan N, Roy SD, Ritesh R, Pal AK, Siddaiah GM, Kumar R (2012) Alteration in haematological and biochemical parameters of Catla catla exposed to sub-lethal concentration of cypermethrin. Fish Physiol Biochem 6:577–584
Verma A, Ali D, Farooq M, Pant AB, Ray RS, Hans RK (2011) Expression and inducibility of Endosulfan metabolizing gene in Rhodococcus strain isolated from earthworm gut microflora for its application in bioremediation. Bioresour Technol 102:2979–2984
Weber J, Halsall CJ, Muir D, Teixeira T, Small J, Solomon K, Hermanson M, Hung H, Bidleman T (2010) Endosulfan, a global pesticide, a review of its fate in the environment and occurrence in the arctic. Sci Total Environ 408:2966–2984
Yadav IC, Devi NL, Syed JH, Jun L, Zhang G, Jones KC (2015) Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries, a comprehensive review of India. Sci Total Environ 511:123–137
Acknowledgements
Authors are thankful to Mohammed Sathak College of Arts & Science (University of Madras), Chennai, for providing the lab facilities to carry out this work.
Author information
Authors and Affiliations
Contributions
ZAM carried out experimental work; SA, AT, and AA helped in statistical analysis and drafting the manuscript; HAQ and JAB helped in manuscript editing; MO has designed the work and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mir, Z.A., Ali, S., Tyagi, A. et al. Degradation and conversion of endosulfan by newly isolated Pseudomonas mendocina ZAM1 strain. 3 Biotech 7, 211 (2017). https://doi.org/10.1007/s13205-017-0823-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0823-5