Abstract
Extractive butanol fermentation with non-ionic surfactant, a recently explored area, has shown promising results with several advantages but is relatively less investigated. This work reports the extractive fermentation with selected non-ionic surfactants (L62 and L62D) to enhance butanol production using a high-butanol producing strain (Clostridium beijerinckii MCMB 581). Biocompatibility studies with both the surfactants showed growth. Higher concentrations of surfactant (>5%) affected the cell count. 15.3 g L−1 of butanol and 21 g L−1 of total solvents were obtained with 3% (v/v) L62 which was respectively, 43% (w/w) and 55% (w/w), higher than control. It was found that surfactant addition at 9th h doubled the productivity (from 0.13 to 0.31 g L−1 h−1 and 0.17 to 0.39 g L−1 h−1, respectively for butanol and total solvent). Butanol productivity obtained was 2–3 times higher than similar studies on extractive fermentation with non-ionic surfactants. Interestingly, mixing did not improve butanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biobutanol production is a classic case of product inhibition. Butanol normally less than 20 g L−1 becomes toxic to the microorganisms and inhibits cell growth consequently butanol production (Awang et al. 1988). Further, due to low titer of butanol and low productivity, separation of butanol has become a major critical challenge (Abdehagh et al. 2014; Huang et al. 2014). Various methods are reported to relieve butanol toxicity which include gas stripping (Ezeji et al. 2003, 2007; Setlhaku et al. 2013), pervaporation (Qureshi et al. 2001; Setlhaku et al. 2013; Tong et al. 2010), membrane assisted solvent extraction (Jeon and Lee 1987; Tanaka et al. 2012), adsorption (Qureshi et al. 2005; Xue et al. 2016), and liquid–liquid extraction (Dadgar and Foutch 1988; Ha et al. 2010). However, most of these methods focus on separation of butanol as it is formed and not on increasing the initial concentration of butanol in the broth which would reduce the downstream processing cost. Recently, Dhamole et al. (2012, 2015) have for the first time reported extractive fermentation with non-ionic surfactants for butanol production. It addresses two interlinked problems with butanol fermentation, first it relives butanol toxicity to the microbes thus enhancing the butanol production (and hence titer), and second, it concentrates butanol in downstream processing.
Surfactants self-assemble into micelles above their critical micelle concentration and entrap (hydrophobic) butanol into micelles, thus relieving the butanol toxicity to the microorganisms. Surfactant based extractive fermentation has many advantages and is explored for several extractive fermentation (Wang and Dai 2010; Wang et al. 2008a, b). Surfactant reduces the substrate or product toxicity by entrapping the substrate/product. Surfactant based cloud point extraction significantly reduces the process volume and concentrates the product in the surfactant rich phase consequently into reduction in downstream processing cost (Dhamole et al. 2013, 2014). Also, surfactant recovery (>95%) and reuse is possible with surfactants (Dhamole et al. 2012). Despite several advantages, limited studies are reported on extractive fermentation of butanol using non-ionic surfactant (Dhamole et al. 2012, 2015).
Our earlier work (Dhamole et al. 2012, 2015) on extractive fermentation with non-ionic surfactants explored the low butanol producing strains, Clostridium acetobutylicum ATCC No. 824 (NCIM No. 2337) and Clostridium pasteurianum ATCC No. 824 (NCIM No. 2337) (4–5 g L−1). Both the studies showed enhanced butanol production with L62 and L62D. However, the effect of increase in concentration of surfactant on butanol production was studied only till 9% of surfactant. Further, the effect of mixing, time of addition of surfactant, effect of surfactant and its varying concentration on cell count/number and butanol production was not explored. This work investigated extractive butanol fermentation with high-butanol producing strain (as compared to Dhamole et al. 2012, 2015) and also the effect of varying operating conditions (mixing, time of addition of surfactant, effect of surfactant and its varying concentration) on butanol production. Surfactant based system improved butanol production however, the productivity was very low. Butanol fermentation data in presence of surfactant showed delayed butanol production (Dhamole et al. 2012, 2015). Microbes might need some adaptation to the surfactant before starting butanol production. Also, both the studies were carried out using a low butanol producing strain eventually leading to low productivity. Presence of L62D produced maximum butanol of 11.9 g L−1 with productivity of 0.16 g L−1 h−1 using Clostridium acetobutylicum (Dhamole et al. 2015). With Clostridium pasteurianum ATCC No. 824 (NCIM No. 2337) and L62, maximum butanol titer of 10.7 g L−1 with productivity of 0.09 g L−1 h−1 was obtained. The maximum amount of butanol that is produced by C. acetobutylicum and C. pasteurianum in absence of surfactant was respectively, 8 and 4.3 g L−1. Further, the effect of surfactant was observed only on butanol production (in terms of biocompatibility). Impact of these surfactants on cell number or growth was not investigated in both the work. Hence, this work was undertaken to study the enhanced butanol production with a strain producing relatively high amount of butanol and estimate maximum butanol production with such a strain. In addition, understand the effect of surfactant on biomass and improve the productivity of the process. Clostridium beijerinckii MCM B581that produces 10 g L−1 of butanol was used in the present work (Singh et al. 2016).
Materials and methods
Chemicals
Chemicals such as glucose, ammonium sulfate, K2HPO4, CaCO3, MgSO4, FeSO4, yeast extract, and cysteine HCl were purchased from Hi-Media; whereas, butanol, acetone, ethanol, and i-propanol were purchased from Sigma Aldrich. All the chemicals were of analytical grade. Non-ionic pluronic surfactants L62 and L62D were provided by BASF, USA as a gift sample. De-ionized water was used through-out the studies. Both the surfactants (L62 and L62D) are tri-block PEO-PPO-PEO polymeric surfactants and are amphiphilic in nature.
Organism
A laboratory stock of C. beijerinckii isolated (Singh et al. 2016) and deposited at Agharkar Research Institute (ARI, Pune) as MCM B581, was routinely maintained as spore suspension in Pre-Culture medium (PC) (Cheng et al. 2012) and stored at room temperature. The strain is mesophile and shows good growth at 37 °C after incubation period of 18–24 h. The spore suspension was activated by heat shock at 80 °C for 2 min followed by cold shock at −20 °C for 2 min. The activated spores were then transferred to fresh PC medium. This was used for preparing inoculum for studies.
Biocompatibility of the surfactant
Cell count study was carried out in order to analyze surfactant toxicity to the organism. Experiment was performed in 130 mL serum bottles. Surfactants L62 and L62D were added in different concentrations (1–10% v/v) to the Peptone-Yeast Extract- Glucose (PYG) medium (10 g L−1 Peptone; 10 g L−1 Yeast extract; 20 g L−1 Glucose; 3 g L−1 Sodium acetate; 5 g L−1 NaCl; 0.5 g L−1 cysteine HCl) and was sterilized at 121 °C for 15 min. The pH of the media was adjusted to 6.8 and head space of the serum bottles was flushed with N2 gas prior to sterilization. Each bottle was inoculated with 1 mL (containing 1 × 106 cells/mL) actively proliferating cells. Experiment was performed at 37 °C. Cell count was taken using cytometer under phase contrast microscope. Control studies were carried out in absence of surfactant. Cell number was counted at the end of 24 h.
Butanol tolerance test
Clostridium beijerinckii was grown in PYG medium in presence of different concentration of butanol (6–20 g L−1) and 6% (v/v) L62 to study the maximum tolerance of butanol to the microbes in presence of surfactant. Control without any externally added butanol and surfactant was kept for comparison. Samples were collected after 48 h of fermentation and butanol was measured.
Fermentation with non-ionic surfactants: effect of different parameters
All the fermentation studies in this work were carried out using the medium consisting of 80 g L−1 glucose, 2 g L−1 ammonium sulfate, 2 g L−1 K2HPO4, 3 g L−1 CaCO3, 0.55 g L−1 MgSO4, 0.52 g L−1 FeSO4, 6.5 g L−1 yeast extract, 0.5 g L−1 cysteine HCl. This was sterilized at 121 °C for 15 min. The pH of the media was adjusted to 6.5. Serum bottles (130 mL) with working volume of 60 mL were used for fermentation. Anaerobic conditions were maintained by flushing head space of the bottle with N2 gas prior to sterilization. Fermentation was initiated by inoculating a 10% (v/v) highly motile cell of C. beijerinckii (18–24 h old inoculum). All experiments were conducted at a constant temperature of 37 °C.
Effect of surfactant concentration
Fermentation was carried out in presence of different concentrations (3 to 20% v/v) of L62 and L62D. Solvents (i-propanol, acetone, butanol and ethanol) production and glucose consumption was monitored. Biomass growth was measured in terms of protein.
Mixing effect
The fermentation was carried out with 3% (v/v) L62 surfactant in rotary incubator shaker at 37 °C at 0, 60, and 120 rpm for 144 h. Control without surfactant was also run simultaneously.
Time of surfactant addition in fermentation
Samples without surfactants in the media initiated solvent production at 9th h (as shown in Fig. 3a) of the fermentation whereas samples with surfactant in the media initiated solvent production at 24 h and showed very less increment in solvents till 72 h. This shows that addition of surfactants causes an extended lag which ultimately delays the solvent production. Due to this, instead of adding surfactant initially, it was added at 9th h at which microbes had already initiated solvent production, so fermentation was carried out without surfactant addition till 9 h. Sterilized surfactant (3% v/v L62) was added in a controlled manner at 9th h of the fermentation and proceeded till 144 h at 37° C.
Analysis
Solvents were analyzed with Gas Chromatograph (Bruker-450) with capillary column (30 m × 0.32 mm inner diameter Stabilwax®-DA Columns (fused silica), Crossbond® Carbowax® polyethylene glycol) equipped with a flame ionization detector. The standard buffer solution containing known amount of solvents (acetone, methanol, ethanol, i-propanol, n-propanol, i-butanol, n-butanol) was injected. The gas chromatography was operated at an injector temperature of 150 °C, oven temperature 80 °C and detector temperature 200 °C with flow rates of N2-30 mL/min; H2-30 mL/min and Air-300 mL/min, respectively. Glucose concentration in the fermentation media was determined by a standard 3,5-dinitrosalicylic acid (DNSA) assay (Miller 1959). The absorbance was measured using UV–visible spectrophotometer (UV 1601, Shimadzu, Japan). pH of the media was analyzed with calibrated digital pH meter. Initial cell concentration was estimated by optical density at 600 nm. Productivity was calculated as total isopropanol, acetone, butanol, and ethanol (iABE) produced (g L−1) in a given fermentation time (h). Yield was calculated as total gram of iABE produced per gram of glucose utilized.
Results and discussion
Biocompatibility of the surfactant to Clostridium beijerinckii MCMB 581
Earlier butanol fermentation studies with non-ionic surfactant showed maximum butanol production in presence of L62 and L62D (Dhamole et al. 2012, 2015). Since the butanol production (with low butanol producing strain) was enhanced in presence of L62 and L62D, it was decided to carry out biocompatibility studies with both the surfactants with C. beijerinckii MCMB 581. Biocompatibility was decided based on the growth of C. beijerinckii (cell number) at different concentrations of surfactant (1 to 10% v/v) in a growth medium (Table 1). All the flasks were inoculated with 1 mL inoculum containing 106 cells. It was observed that the number of cells increased for all the different concentrations of surfactant with respect to the initially added number of cells. However, as compared to control (7 × 108) the number of cells was slightly less in presence of surfactant. Overall, the cell count marginally decreased with increase in surfactant concentration from 1 to 10% v/v (6 × 108 to 3 × 108). It was seen that surfactant delayed the growth of microbes but did not inhibit it. This could be attributed to covering of cells by the surfactant as was observed under microscope in presence of higher surfactant level. Also, the delayed growth could be attributed to the shock due to surfactant addition. It is anticipated that microbes would take some time for adaptation to surfactant resulting into delayed growth. Thus, it was found that both the surfactants were biocompatible.
Butanol tolerance test
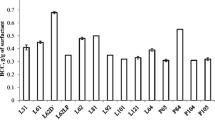
Biocompatibility studies showed that L62 and L62D are biocompatible. However, it is important to relieve the butanol toxicity to the microbes. Hence, butanol tolerance test was carried out in presence of surfactant. Butanol production was observed in presence of different concentrations of externally added butanol (0.6, 0.8, 1.0, 1.4 and 2.0% w/v) and surfactant (L62 and L62D). Butanol production was observed in presence of initially fed butanol 0.8% (w/v) (Fig. 1). However, no butanol was produced in presence of 1.0% (w/v) butanol, though very small amount of glucose (3.6 g L−1) was consumed, indicating butanol tolerance till 1% (w/v) of butanol in presence of surfactant. Thus, it is expected that butanol production will occur even in presence of 1% (w/v) of butanol. Maximum butanol producing capacity of the strain C. beijerinckii MCMB 581 is 11 g/L (as shown in Fig. 3a). More butanol concentration than this amount causes a toxic environment for the cells. Hence, further increase in butanol than 1% eventually kills the cells, thus increase in butanol concentration can be seen up to 1%. Further increase in externally added butanol inhibits butanol production.
Effect of initial butanol (i.e., externally added butanol) on butanol production in presence of 6% (v/v) surfactants (L62 and L62D) and in absence of surfactant at the end of 48 h. Butanol produced in control is 6.6 g L−1 (i.e., when butanol added = 0 g L−1) (Total butanol = Externally added butanol + butanol produced) (L62—bar filled with horizontal lines and L62D—bar filled with inclined lines). Butanol production is inhibited when externally added butanol ≥10 g/L
Effect of different concentrations of surfactants on butanol production
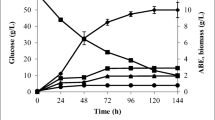
Effect of varying concentrations of surfactant, both L62 and L62D (3% to 20% v/v) on butanol and total solvent production was studied (Fig. 2). It can be seen that total solvent and maximum butanol produced in absence of control is 13.5 and 10.7 g L−1, respectively, whereas in presence of 3% v/v L62, the total solvent and maximum butanol produced is 21 and 15.3 g L−1, respectively. Thus, 43% (w/w) more butanol and 55% (w/w) more total solvents were produced in extractive fermentation with 3% (v/v) L62. Overall L62 was found to be better than L62D in terms of enhancing butanol production and hence remaining studies were carried out with 3% (v/v) L62. The better performance of L62 over L62D could be attributed to the two phase formation with L62D at fermentation temperature consequently into no entrapment of butanol. Therefore, the maximum butanol produced remained unaffected at different concentrations of L62D. Figure 3 shows the typical profiles for butanol, i-propanol, total solvents produced, and glucose utilization in the absence of surfactant and in the presence of 3% (v/v) L62. With 3% (v/v) L62, the butanol and total solvent yield 0.31 and 0.43 g/g glucose. The yield remained unchanged for both the studies. pH decreased during the fermentation from 6.5 to 5. At higher surfactant concentration, butanol production as well as cell number decreased. During cell number studies, it was observed that cells were covered with surfactant initially. Increase in surfactant concentration might be an obstacle for microbes which also caused low microbial motility. Hence, it was anticipated that cells would take more time to get adapted into surfactant contained media. This might have adversely affected the supply of substrate subsequently butanol production. Besides, with increasing surfactant (L62) concentration, cloud point also increases, thus entrapment of butanol into micelles is delayed. Hence, at fermentation temperature, there is decrease in entrapment of butanol with increasing surfactant concentration. This would have lead to reduction in butanol production.
Effect of mixing on butanol fermentation
It is expected that if proper mixing is not provided, then supply of nutrients to microbes and entrapment of butanol would limit the rate of butanol production and its productivity. Hence, it was planned to study the effect of mixing on butanol production in presence of surfactant. Fermentation was carried out at three different shaking speeds (0, 60, and 120 rpm) in an orbital shaker in presence of 3% (v/v) L62. Figure 4 shows the butanol production profile without the addition of surfactant at stationary condition (control) and with addition of surfactant at three different speeds. In presence of 3% L62 at stationary condition, 45% more butanol (14.7 g L−1) was produced as compared to control (with no mixing and in absence of surfactant). It is interesting to note that the butanol production did not increase with increase in mixing speed or when mixing was provided. With 3% (v/v) L62, at 60 and 120 RPM, butanol produced was 10.2 g L−1 as compared to 14.7 g L−1 at stationary conditions. Further, the amount of butanol produced in presence of surfactant at 60 and 120 RPM is close to butanol produced in absence of surfactant at stationary conditions. It is assumed that when mixing was provided, butanol entrapment did not take place which resulted into no capturing of butanol consequently into less amount of butanol production. Also, it can be seen that the delayed fermentation which was observed earlier in presence of surfactant was not observed in these studies when mixing was provided. This clearly indicates that surfactant is free in the broth and they are neither entrapping butanol nor affecting the biomass. It is to be noted that the rate of butanol production (till 48 h) at stationary condition in presence of surfactant was less than control (without surfactant). This again confirms that mixing might have led to inhibition of micelle formation.
Effect of time of addition of surfactant
Extractive fermentation with surfactant enhances butanol production; however, it affects the productivity of the process. Butanol productivity of 0.1 and 0.05 g L−1 h−1 respectively was obtained with L62 and L62D. Butanol production profile in presence of different concentration of surfactant showed that addition of surfactant slowed down the butanol production rate. With increase in concentration of surfactant, the rate of butanol production decreases (Dhamole et al. 2015). In the present study, the cell number data in presence of surfactant and in absence of surfactant also showed that surfactant affects the cell growth. It was observed that in absence of surfactant (i.e., control), butanol production started at 9th h (Fig. 3a) and in presence of surfactant (added at 0th h), butanol production was delayed (started at 24th h). This shows that addition of surfactants at 0 h causes an extended lag which ultimately delays the solvent production. Hence, it was decided to add surfactant at a stage where the microbes are in active state and starts producing butanol (i.e., at 9th h in this work). It was observed that butanol production in control (i.e., without surfactant) reaches a saturation level very quickly (in 72 h). In case of 3% (v/v) L62, it took 120 h to achieve the maximum butanol production of 15.3 g L−1 (Fig. 3). Figure 5 shows the solvent production profile with 3% (v/v) L62 addition at 0 h i.e., at the start of the fermentation (control) and surfactant addition at 9th h. It is clearly seen that addition of surfactant at 9th h significantly enhanced the butanol productivity. The amount of butanol and total solvent produced (with surfactant addition at 9th h) after 48 h, was respectively 14.8 and 18.3 g L−1 with a productivity of 0.31 and 0.38 g L−1 h−1.
With surfactant addition at the start (i.e., at 0 h), the maximum amount of butanol and total solvent production was respectively, 15.3 and 21 g L−1, produced in 120 h with a productivity of 0.13 and 0.17 g L−1 h−1. Thus, addition of surfactant at 9th h doubled the productivity. In comparison with earlier work on extractive fermentation (Dhamole et al. 2012, 2015), productivity is 2–3 times higher (Table 2) than earlier studies.
Conclusions
Both the surfactants (L62 and L62D) were found to be biocompatible. Maximum butanol (15.3 g L−1) and total solvent (21 g L−1) production was achieved with 3% (v/v) L62 using extractive fermentation. Thus, 43% (w/w) more butanol and 55% (w/w) more solvents were obtained with 3% (v/v) L62 than control (without surfactant). Surfactant addition at 9th h doubled the butanol and solvent productivity with respect to surfactant addition at the start. Overall, the productivity was 2–3 times higher than earlier studies on extractive butanol fermentation with non-ionic surfactant. Thus, it can be concluded that enhanced butanol production and higher solvent productivity was achieved using extractive fermentation with surfactant.
References
Abdehagh N, Tezel FH, Thibault J (2014) Separation techniques in butanol production: challenges and developments. Biomass Bioenergy 60:222–246
Awang GM, Jones G, Ingledew W, Kropinski A (1988) The acetone-butanol-ethanol fermentation. CRC Crit Rev Microbiol 15:S33–S67
Cheng C-L, Che P-Y, Chen B-Y, Lee W-J, Chien L-J, Chang J-S (2012) High yield bio-butanol production by solvent-producing bacterial microflora. Biores Technol 113:58–64
Dadgar AM, Foutch GL (1988) Improving the acetone-butanol fermentation process with liquid-liquid extraction. Biotechnol Prog 4:36–39
Dhamole PB, Wang Z, Liu Y, Wang B, Feng H (2012) Extractive fermentation with non-ionic surfactants to enhance butanol production. Biomass Bioenergy 40:112–119
Dhamole PB, Wang B, Feng H (2013) Detoxification of corn stover hydrolysate using surfactant-based aqueous two phase system. J Chem Technol Biotechnol 88:1744–1749
Dhamole PB, Demanna D, Desai S (2014) Extraction of p-coumaric acid and ferulic acid using surfactant-based aqueous two-phase system. Appl Biochem Biotechnol 174:564–573
Dhamole PB, Mane RG, Feng H (2015) Screening of non-ionic surfactant for enhancing biobutanol production. Appl Biochem Biotechnol 177:1272–1281
Ezeji T, Qureshi N, Blaschek H (2003) Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J Microbiol Biotechnol 19:595–603
Ezeji TC, Qureshi N, Blaschek HP (2007) Production of acetone butanol (AB) from liquefied corn starch, a commercial substrate, using Clostridium beijerinckii coupled with product recovery by gas stripping. J Ind Microbiol Biotechnol 34:771–777
Ha SH, Mai NL, Koo Y-M (2010) Butanol recovery from aqueous solution into ionic liquids by liquid–liquid extraction. Process Biochem 45:1899–1903
Huang H-J, Ramaswamy S, Liu Y (2014) Separation and purification of biobutanol during bioconversion of biomass. Sep Purif Technol 132:513–540
Jeon Y, Lee Y (1987) Membrane-assisted extractive butanol fermentation. Ann N Y Acad Sci 506:536–542
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Qureshi N, Meagher M, Huang J, Hutkins RW (2001) Acetone butanol ethanol (ABE) recovery by pervaporation using silicalite–silicone composite membrane from fed-batch reactor of Clostridium acetobutylicum. J Membr Sci 187:93–102
Qureshi N, Hughes S, Maddox I, Cotta M (2005) Energy-efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess Biosyst Eng 27:215–222
Setlhaku M, Heitmann S, Górak A, Wichmann R (2013) Investigation of gas stripping and pervaporation for improved feasibility of two-stage butanol production process. Biores Technol 136:102–108
Singh KG, Lapsiya KL, Gophane RR, Ranade DR (2016) Optimization for butanol production using Plackett-Burman Design coupled with Central Composite Design by Clostridium beijerenckii strain CHTa isolated from distillery waste manure. J Biochem Tech 7(1):1063–1068
Tanaka S, Tashiro Y, Kobayashi G, Ikegami T, Negishi H, Sakaki K (2012) Membrane-assisted extractive butanol fermentation by Clostridium saccharoperbutylacetonicum N1-4 with 1-dodecanol as the extractant. Biores Technol 116:448–452
Tong C, Bai Y, Wu J, Zhang L, Yang L, Qian J (2010) Pervaporation recovery of acetone-butanol from aqueous solution and fermentation broth using HTPB-based polyurethaneurea membranes. Sep Sci Technol 45:751–761
Wang Z, Dai Z (2010) Extractive microbial fermentation in cloud point system. Enzyme Microb Technol 46:407–418
Wang Z, Xu J-H, Chen D (2008a) Whole cell microbial transformation in cloud point system. J Ind Microbiol Biotechnol 35:645–656
Wang Z, Xu JH, Zhang W, Zhuang B, Qi H (2008b) In situ extraction of polar product of whole cell microbial transformation with polyethylene glycol-induced cloud point system. Biotechnol Prog 24:1090–1095
Xue C, Liu F, Xu M, Tang I-C, Zhao J, Bai F, Yang S-T (2016) Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption. Biores Technol 219:158–168
Acknowledgements
Dr. Pradip B. Dhamole would like to thank Department of Biotechnology (Govt. of India) for funding this work (vide Sanction Order No. BT/PR5886/PBD/26/304/2012 dated 26.12.2013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Singh, K., Gedam, P.S., Raut, A.N. et al. Enhanced n-butanol production by Clostridium beijerinckii MCMB 581 in presence of selected surfactant. 3 Biotech 7, 161 (2017). https://doi.org/10.1007/s13205-017-0803-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0803-9