Abstract
This work deals with finding a suitable non-ionic surfactant which has high butanol capturing capacity and can be separated at a temperature close to room temperature and does not extract any intermediates or substrate (i.e., glucose). Importantly, it should be biocompatible, and its separation from the aqueous phase is not affected by other fermentation products. Hence, a pool of non-ionic Pluronic surfactants (L31, L61, L62D, L62LF, L62, L81, L92, L101, L121, L64, P65, P84, P104, P105) were selected for the study. Screening of the surfactant was done based on its hydrophile-lipophile balance (HLB) value, butanol capturing capacity (BCC), and cloud point temperature. Among the various surfactant investigated, L62D captured maximum amount of butanol (0.68 g/g of surfactant). Also, the cloud point temperature of L62D is close to room temperature (28.7 °C). Biocompatibility studies were carried out by conducting fermentation in presence of 3 % L62D which resulted in 148 % increase in butanol production as compared to control (without surfactant). Further, the fermentation products did not have strong influence on phase separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biobutanol is gaining importance as a biofuel over ethanol due to its high calorific value and low affinity for water which makes it easy to mix it with gasoline at the refinery itself. However, butanol production through fermentation route suffers a major challenge. Butanol titer >20 g/L produced by biological means inhibits the further production of butanol due to solvent toxicity (product inhibition) [1]. Several methods are explored for separating the butanol and relieving the butanol toxicity to the microbes [2, 3]. These methods include adsorption [4, 5], gas stripping [6], pervaporation [7], perstraction, liquid–liquid extraction [8, 9] etc. Most of these methods focus only on removing butanol as and when it is formed and not on increasing the titer and then separating the product. Low concentration of butanol in the final broth makes the butanol recovery process very expensive [10]; hence, it is desirable to increase the titer of butanol in the fermentation broth itself and then separate it. Another way of increasing the butanol concentration in the starting material is genetic engineering wherein the strain can survive at high concentration of butanol. However, efforts made in this direction have made limited progress [11].
Recently, Dhamole et al. [12] have explored the surfactant based aqueous two-phase system for extractive butanol fermentation which enhanced the butanol production significantly (2.25 times the control). It not only separated butanol but was also concentrated. Conventional method of using organic solvents for extractive fermentation has many limitations, and one of the most important limitations is biocompatibility of the solvent [8, 9]. Most of the solvents tend to rupture the cell wall membrane resulting into decreased production. Thus, despite having high partition coefficient for the desired solute, solvents finds limited applications in biological systems. Non-ionic surfactants are relatively biocompatible. Extractive fermentation with non-ionic surfactants using cloud point extraction is a promising method for fermentation where product inhibition or substrate inhibition occurs [13–15]. Also, it can be used for limited substrate solubility and product degradation [13]. Non-ionic surfactants form micelle that entraps butanol and relieves the butanol toxicity to the microbes. Surfactant rich phase can be separated by incubating the fermentation medium at the cloud point temperature.
Extractive fermentation with non-ionic surfactants has shown improved butanol production [12]. Further, it reduces the process volume significantly and concentrates the product [16, 17]. With L62 as a surfactant, butanol production increased by 225 % as compared to control fermentation. Downstream processing of the butanol showed volume reduction of 4–6 times the original volume and concentration of the product [12]. Dhamole et al. [12] tested limited surfactants randomly, and the surfactant with less butanol capturing capacity which was biocompatible was shortlisted. Hence, the present work was undertaken with an objective to study a large number of surfactants and screen the surfactant with maximum butanol capturing capacity having cloud point close to room temperature and that does not extract intermediates or substrates and is biocompatible. Further, the fermentation products do not influence the phase behavior of the surfactant strongly.
Materials and Methods
Chemicals
All the chemicals (K2HPO4, KH2PO4, glucose, yeast extract, para-amino benzoic acid, thiamine, biotin, MgSO4.7H2O, FeSO4.7H2O, MnSO4.H2O, NaCl, and ammonium acetate) were of analytical grade. Non-ionic Pluronic surfactants listed in Table 1 were provided by BASF, USA as a gift sample. Analytical grade butanol, acetone, butyric acid, and acetic acid were purchased from Sigma- Aldrich. De-ionized water was used throughout the studies.
Organism
Freeze-dried culture Clostridium acetobutylicum ATCC No. 824(NCIM No. 2337) acetone-butanol ethanol producing strain was purchased from National Chemical Laboratory (NCL), Pune, India. The spores were heat shocked at 80 °C for 10 min in a cooked meat medium (Himedia, India) for preparation of the innoculum. This was followed by incubation at 37 °C in an anaerobic chamber for 36 h, and the cells thus obtained were used for fermentation.
BCC
Butanol capturing capacity (g butanol captured/g of surfactant) of all the surfactants were estimated in the present work. All the experiments were carried out in a marked glass tube (15 mL). Fermentation medium consisting of glucose (60 g/L), yeast extract (1 g/L), butanol (10 g/L), and other nutrients (minerals and vitamins) was used as a model system. Surfactant (1 % v/v) was added in to the fermentation medium, and the solution was mixed vigorously to form a homogenous phase. It was then placed in a boiling water bath for 5 min. Surfactant formed a separate phase. Volumes of both the aqueous phase and the surfactant rich phase were noted down, and the sample from the aqueous phase was collected for further studies. Butanol in the aqueous phase was estimated using gas chromatography, and the butanol in the surfactant rich phase was estimated by material balance calculations.
Results are expressed in terms of butanol capturing capacity (BCC) defined as:
where MB = amount of butanol captured by the surfactant, and Ms = amount of surfactant used in the experiments.
Biocompatibility and ABE Fermentation with non-Ionic Surfactants
Fermentation was carried out in a 250-mL flask in presence of 3 % (v/v) L62D. Fermentation medium consisting of 60 g/L of glucose, 1 g/L yeast extract, 1 mL/L buffer stock solution (50 g/L, K2HPO4; 50 g/L, KH2PO4; 220 g/L ammonium acetate), 1 mL/L vitamin stock solution (0.19 g/L para-amino benzoic acid, 0.19 g/L thiamine, 0.19 g/L biotin), and 1 ml/L of mineral stock solution (20 g/LMgSO4.7H2O, 1 g/L MnSO4.H2O, 1 g/L FeSO4.7H2O, and 1 g/L NaCl) was used. The cell growth in the fermentation broth was monitored in time with Hitachi U-2900 spectrophotometer at 600 nm. Control shake flask was incubated to compare cell growth without surfactants with growth in the presence of surfactants.
Analysis
Glucose concentration in a fermentation broth was determined by a standard 3,5-dinitrosalicylic acid method [18]. The fermentation products, acetone, butanol, ethanol, acetic acid, and butyric acid were measured with Agilent technology 7820 GC system equipped with a flame ionization detector (FID). Internal standard method was used to analyze the concentration of products in the samples. Isobutanol and isobutyric acid were used as internal standards for the solvent products and acid products present in the samples. An internal standard buffer solution containing 0.5 g/L isobutanol, 0.1 g/L isobutyric acid, and 1 % phosphoric acid was used to dilute each sample 20 times for acidification and calibration prior to analysis on GC. The gas chromatograph was operated at an injection temperature of 200 °C with 1 μL of the acidified sample injected. Column temperature was held at 80 °C for 3 min, raised to 150 °C at a rate of 30 °C/min, and held at 150 °C for 3.7 min.
Results and Discussions
Screening of Surfactants
The toxicity of surfactants follows the order: ionic surfactant > nonionic surfactant > polymeric nonionic surfactant. Hence, di-block and tri-block polymeric surfactants were selected for the study. Moreover, properties of tri-block copolymers i.e. PEO-PPO-PEO ((PEO) polyethylene oxide, (PPO) polypropylene oxide) can be modified to a great extent to form micelles for enhancing butanol extraction and phase separation at fermentation temperature. PEO and PPO play an important role in hydrophobicity and biocompatibility of the surfactant. In our earlier work, only a few Pluronic surfactants were tested for its BCC and biocompatibility. Hence, a large number of tri-block surfactants were selected in this work (Table 1). Various properties of surfactants i.e., cloud point temperature (CP), average molecular weight, solubility, and hydrophile-lipophile balance (HLB) are included in Table 1.
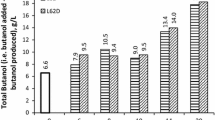
Surfactants with broad HLB value were selected for finding its butanol capturing capacity. It can be seen that with increase in PEO, butanol capturing capacity goes through a maximum (Table 1 and Fig. 1). It is the highest for 20 % PEO and then decreases with increase in amount of PEO. Further, for a constant amount of PEO, butanol capturing capacity goes through a maximum with increase in molecular weight. Fraction of PEO in the surfactant determines its HLB which increases with increase in % PEO. Also, the cloud point increases with increase in amount of PEO. High PEO leads to more solubility, high HLB, and high cloud point. Since amount of PEO influences HLB and cloud point, its effect on butanol capturing capacity was evaluated. It was observed that BCC depends on HLB value. For HLB in the range of 1–7, the BCC was 0.4–0.7 g/g of surfactant whereas for HLB in the range of 12–18, BCC was 0.3–0.4 g/g of surfactant. Surfactants with low HLB value have low amount of PEO which is hydrophilic in nature and hence capture more amount of butanol than surfactants having high HLB. Surfactants having BCC > 0.45 g/g of surfactant were L61, L62D, L62, and L81. It is interesting to note that these surfactants have cloud point in the range of 14–29 °C, which is close to fermentation temperature. Maximum butanol capturing capacity was obtained with L62D as 0.68 g/g of surfactant. Hence, L62D was selected for further studies.
Butanol capturing capacity (BCC) of the surfactants. Butanol was extracted into surfactant rich phase from an aqueous solution containing glucose (60 g/L), yeast extract (1 g/L), butanol (10 g/L), and other nutrients (minerals and vitamins). Surfactant (1 % v/v) was added in to the solution at 100 °C
Estimating Butanol Toxicity Limit for the Strain and Biocompatibility of L62D
Butanol toxicity limit was established by externally adding butanol to fermentation medium and monitoring the butanol produced. It was observed that butanol fermentation was inhibited completely when the butanol concentration was more than 8 g/L in the broth (Fig. 2). It can be seen that acetone, butanol, ethanol, and biomass production decreased with increase in externally added butanol concentration (Fig. 2a to 2c).
Establishing the toxicity level of butanol. Butanol fermentation carried out at different initial butanol concentrations. Butanol values shown are excluding the external butanol level. (2a) Initial Butanol concentration = 2 g/L (b) Initial butanol concentration = 4 g/L and (c) Initial butanol concentration = 6 g/L. Butanol (filled square), ethanol (“x” symbol), acetone (filled triangle), biomass (filled circle)
Biocompatibility of L62D was investigated by carrying out fermentation in presence of L62D and comparing it with control (without surfactant). Butanol production increased substantially (148 % over control) in presence of 3 % L62D (Fig. 3). Yield of butanol also increased from 0.18 (control) to 0.24 (g/g of glucose). In case of control, in the first 36 h, the rate of glucose consumption was 1.01 g/L/h which decreased to 0.17 g/L/h (almost 5 times) in the next 36 h. This is attributed to butanol production which starts inhibiting further glucose uptake. It can be clearly seen that in presence of surfactant, the glucose consumption rate decreased from 0.97 to 0.41 g/L/h (almost 2.3 times); however, it is still higher than the control (Table 2). Thus, glucose consumption rate, butanol production and yield clearly show that L62D is not only biocompatible but also helps in enhancing the butanol production by relieving butanol toxicity.
Distribution of Intermediates (Butyric Acid, Acetic Acid) and Glucose into Surfactant Rich Phase
ABE fermentation involves intermediate products mainly acetic acid and butyric acid. It is important to investigate if any intermediate is entrapped by the surfactants as it may hamper the ABE production directly. Also, it is of interest to know the distribution of primary substrate mainly glucose into the surfactant rich phase. Hence, partitioning of glucose (60 g/L), acetic acid (3 g/L), and butyric acid was studied with L62D at different levels of surfactant (1 to 5 %) (Table 3). It was observed that loss of all the three compounds was less than 4 % of the total amount. Further, if the surfactant is recycled back, the loss will be negligible. Thus, L62D can be used for extractive fermentation as it not only enhances butanol fermentation but also results into negligible loss of intermediates and glucose.
Effect of Fermentation Products on Two-Phase Formation
Since there are other products (acetone and ethanol) besides butanol, it is important to understand the effect of these products on phase separation. Hence, a detailed study was carried to investigate the effect of acetone, butanol, and ethanol on phase separation. Increase in surfactant concentration decreases the cloud point and also the temperature at which two phases are observed.
For acetone, cloud point temperature (36–40 °C) and two-phase separation temperature (40 to 44 °C) increased with increase in acetone concentration from 2 to 10 g/L (Fig. 4a). Acetone is capable of forming the hydrogen bond with water molecules. The hydrogen bond is between the oxygen of the acetone molecule and the hydrogen of water. This makes it a water structure breaker decreasing the hydrophilic interactions. Hence, there is an increasing trend observed in the cloud point with the introduction of acetone into the system. Effect of ethanol on phase separation was similar to that of acetone i.e., the cloud point temperature and two-phase separation temperature increased with increase in ethanol concentration from 2 to 10 g/L (Fig. 4b). This is attributed to the ability of ethanol to form hydrogen bond. This makes water behave as good solvent thereby solving the non-ionic surfactant to some extent which weakens the hydrophobic interactions and in turn does not favor the micellization. Hence, there is an increase in the cloud point and also the phase separation temperature with addition of ethanol.
Conclusions
A pool of non-ionic surfactants was investigated for its suitability as extractant for extractive butanol fermentation. L62D was found to capture maximum butanol (0.68 g/g of butanol) and was also biocompatible. Three percent L62D (v/v) enhanced butanol production by 148 % as compared to control (without surfactant). It is expected that optimized condition would further enhance the butanol production and hence the titer. In addition, L62D did not result into loss of intermediates (butyric acid and acetic acid) as well as primary substrate (i.e., glucose). Thus, it can be used for enhancing the butanol production. Future scope of the work includes optimization of the fermentation conditions and use of a strain capable of producing high amount of butanol (15–20 g/L).
References
Awang, G. M., Jones, G. A., & Ingledew, W. M. (1988). The acetone-butanol-ethanol fermentation. Critical Reviews in Microbiology, 15, S33–S67.
Abdehagh, N., Tezel, F. H., & Thibault, J. (2014). Separation techniques in butanol production: challenges and developments. Biomass and Bioenergy, 60, 222–246.
Huang, H-J., Ramaswamy, S. & Liu, Y., (2014). Separation and purification of biobutanol during bioconversion of biomass. Separation and Purification Technology, 132, 513–540.
Nielsen, D. R., & Prather, K. J. (2009). In situ product recovery of n-butanol using polymeric resins. Biotechnology & Bioengineering, 102, 811–821.
Qureshi, N., Hughes, S., Maddox, I. S., & Cotta, M. A. (2005). Energy-efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess and Biosystems Engineering, 27, 215–222.
Ezeji, T. C., Qureshi, N., & Blaschek, H. P. (2007). Bioproduction of butanol from biomass: from genes to bioreactors. Current Opinion in Biotechnology, 18, 220–227.
Qureshi, N., & Maddox, I. S. (2005). Reduction in butanol inhibition by perstraction: utilization of concentrated lactose/whey permeate by Clostridium acetobutylicum to enhance butanol fermentation economics. Food Bioproducts and Processing, 83, 43–52.
Adhami, L., Griggs, B., Himebrook, P., & Taconi, K. (2009). Liquid–liquid extraction of butanol from dilute aqueous solutions using soybean-derived biodiesel. Journal of the American Oil Chemists, 86, 1123–1128.
Taconi, K., Venkataramanan, K., & Johnson, D. (2009). Growth and solvent production by Clostridium pasteurianum ATCC® 6013™ utilizing biodiesel-derived crude glycerol as the sole carbon source. Environmental Progress and Sustainable Energy, 28, 100–110.
Xue, C., Zhao, J-B., Chen, L-J., Bai, F-W, Yang, S-T. & Sun, J-X. (2014). Integrated butanol recovery for an advanced biofuel: current state and prospects. Applied Microbiology and Biotechnology, 98, 3463–3474.
Qureshi, N., Saha, B. C., Dien, B., Hector, R. E., & Cotta, M. A. (2010). Production of butanol (a biofuel) from agricultural residues: part I - use of barley straw hydrolysate. Biomass and Bioenergy, 34, 559–565.
Dhamole, P. B., Wang, Z., Liu, Y., Wang, B., & Feng, H. (2012). Extractive fermentation with non-ionic surfactants to enhance butanol production. Biomass and Bioenergy, 40, 112–119.
Wang, Z., & Dai, Z. (2010). Extractive microbial fermentation in cloud point system. Enzyme and Microbial Technology, 46, 407–418.
Wang, Z., Xu, J. H., & Chen, D. (2008). Whole cell microbial transformation in cloud point system. Journal of Industrial Microbiology and Biotechnology, 35, 645–656.
Wang, Z., Zhao, F., Hao, X., Chen, D., & Li, D. (2004). Microbial transformation of hydrophobic compound in cloud point system. Journal of Molecular Catalysis B: Enzymatic, 27, 147–153.
Dhamole, P. B., Wang, B., & Feng, H. (2013). Detoxification of corn stover hydrolysate using surfactant based aqueous two phase system. Journal of Chemical Technology and Biotechnology, 88, 1744–1749.
Dhamole, P. B., Demanna, D., & Desai, S. A. (2014). Extraction of p-coumaric acid and ferulic acid using surfactant based aqueous two phase system. Applied Biochemistry and Biotechnology, 174, 564–573.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Journal of Analytical Chemistry, 31, 426–429.
Acknowledgments
Dr. Pradip B. Dhamole would like to thank Department of Biotechnology (Govt. of India) for funding this work (vide Sanction order No. BT/PR5886/PBD/26/304/2012 dated 26.12.2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhamole, P.B., Mane, R.G. & Feng, H. Screening of non-Ionic Surfactant for Enhancing Biobutanol Production. Appl Biochem Biotechnol 177, 1272–1281 (2015). https://doi.org/10.1007/s12010-015-1812-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1812-y