Abstract
This work deals with the incorporation of propylene carbonate (PC) as a plasticizer in conjunction with poly (ethylene oxide) (PEO), silver triflate (AgCF3SO3) and nanocrystalline tin oxide (SnO2) for obtaining the nanocomposite polymer electrolyte system (PEO)50AgCF3SO3:2 wt% SnO2 + x wt% PC (x = 10, 20, 30 and 40) by solution casting method. The present electrical conductivity data extracted by means of complex impedance spectroscopic analysis in the frequency range 20 Hz–1 MHz and over the temperature domain 298–373 K have demonstrated that the maximum electrical conductivity value of 5.9 × 10−5 S cm−1 at 298 K would be possessed by the specimen containing 30 wt% PC incorporated into the optimized nanocomposite system (PEO)50AgCF3SO3:2 wt% SnO2. Silver ionic transference number (tAg+) data evaluated using AC/DC polarization technique have indicated that the highest tAg+ value of 0.52 could be realized in the case of the specimen (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC, whereas the complexation of the plasticizer within the nanocomposite electrolyte has been deduced from the detailed Fourier transform infrared spectroscopic investigation owing to the fact that such results have revealed the appearance of absorption bands corresponding to free triflate ions (CF3SO3−) and PC. Surface morphological features of pure PEO and (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC nanocomposite systems were analysed through scanning electron microscope. The feasibility of a reduction in the degree of crystallinity of the plasticized system has been indicated by the X-ray diffraction data and confirmed from differential scanning calorimetric results obtained in terms of quantification of crystallinity and reduction in the glass transition temperature due to the addition of the chosen plasticizer into the nanocomposite polymer electrolyte matrix. Interestingly, the all solid-state cell based on the nanocomposite polymer electrolyte, namely, (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC and silver anode has exhibited an open circuit voltage of 676 mV and short circuit current of 192 μA at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolytes are rapidly attaining a great deal of technological interest in order to develop high-performance materials for a wide range of applications such as batteries, sensors, electrochromic displays and other energy storage devices (Bruce and Gray FM 1997; MacCallum and Vincent 1987; Scrosati 1993). The development of such materials focuses mainly on increasing ionic conductivity, while maintaining favorable mechanical and electrochemical properties. The high-molecular weight poly (ethylene oxide) (PEO) exhibits good complexation properties, high flexibility and mechanical stability at temperatures up to its melting point. However, the practical uses of PEO are restricted owing to the main drawback involving low levels of conductivity at desired operating temperatures since considerable conductivity has been achieved only at higher temperatures (Chu and Reddy 2003; Pitawala et al. 2008). Unfortunately, the presence of crystalline phases at low temperatures may limit the ionic conductivity of PEO-based electrolytes while high ionic conductivity values at elevated temperatures are associated with the high amorphous phase (Jayathilaka et al. 2002; Kuila et al. 2007). Owing to such a behavior, pure poly (ethylene oxide) (PEO) is until now a challengeable candidate in order to improve its ionic conductivity and electrochemical properties at room temperature. However, the number of charge carrying species and their respective mobility appears to remain as important factors in attaining the sufficient level of ionic conduction and therefore it is obvious that ion association may reduce the mobility of the available number of charge carriers in the case of polymer electrolytes. With a view to overcome such practical problems, several efforts have been made toward improvement of the performance of PEO-based electrolytes in terms of their ionic transport, electrical conductivities and electrode–electrolyte interfacial properties apart from electrochemical and mechanical stabilities of such promising PEO–salt matrix systems at ambient conditions. One of the promising approaches applicable to PEO–salt systems involved introduction of nano-size fillers like Al2O3, SiO2 or other ceramics as reported by many research groups and resulted in considerable improvement of mechanical and ion transport properties (Huchang et al. 2005; Scrosati et al. 2000; Kwang-Sun et al. 2003). On the other hand, another approach to enhance the ionic conductivity of a polymer electrolyte was proven to be the incorporation of low molecular weight as well as high dielectric constant plasticizers like ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), poly (ethylene glycol) (PEG), etc. These plasticizers were found to facilitate both improving the ionic conductivity as well as increasing the amorphous content, thereby dissociating ion aggregates and lowering the glass transition temperature of polymer electrolytes (Kuila et al. 2007; Isebella et al. 2002). However, in the literature only few reports are available pertaining to the combined effect of nanofiller and plasticizer in the case of PEO–salt electrolyte systems in particular (Pitawala et al. 2007; Pradhan et al. 2008). In this scenario, we report the effect of propylene carbonate (PC) as a plasticizer incorporated into the (PEO)50AgCF3SO3:SnO2 nanocomposite polymer electrolyte examined in terms of detailed electrical, structural and morphological characterisations in the present endeavor.
Experimental techniques
Thin film samples of a series of compositions of (PEO)50AgCF3SO3: 2 wt% SnO2 + x wt% propylene carbonate (Aldrich) (where x = 10, 20, 30 and 40) nanocomposite polymer electrolyte system were prepared by solution casting technique (Wang et al. 2005) using poly (ethylene oxide) (PEO, Aldrich) with a high-molecular weight (Mw = 5 × 106 Da) and silver triflate (AgCF3SO3, Aldrich) as starting materials. Prior to use, silver triflate and nanocrystalline SnO2 (size ~100 nm, Aldrich) were dried under vacuum for 1 h at 393 K. Appropriate quantities of PEO and AgCF3SO3 (taken in the O/M ratio, 50:1) were initially dissolved in acetonitrile (Sisco Research Laboratory, India) under constant stirring at 313 K for 4 h. Subsequently, the solution was dispersed with 2 wt% of nano-sized SnO2 particles and made into a suspension corresponding to the optimized stoichiometric composition of the nanocomposite polymer electrolyte system. Varying amounts of the plasticizer namely, propylene carbonate (PC) viz., 10, 20, 30 and 40 wt% were also carefully incorporated into this (PEO)50AgCF3SO3:2 wt% SnO2 suspension. Finally, the prepared mixture of this solution was poured into a glass petri dish and dried in a vacuum oven maintained a pressure of 10−3 torr at 323 K for 24 h in order to remove all the traces of the solvent. All those solvent-free films thus obtained were of thickness of about 90–100 μm and all such plasticized nanocomposite polymer electrolyte specimens were stored in a vacuum dessicator for further analyses. The X-ray diffraction (XRD) patterns were recorded for all the specimens obtained in the form of a thin film of 100 μm thickness using a Bruker D8 Advance X-ray diffraction system with Cu–Kα1 radiation at room temperature (298 K) in the scanning angle range 2θ between 10° and 70°. Thermal analysis of all the specimens was performed in the temperature range 173–373 K with the aid of differential scanning calorimetric (DSC) technique using a Mettler Toledo DSC 822e model instrument under nitrogen atmosphere. The degree of crystallinity (χc) of the present plasticized nanocomposite polymer electrolytes was evaluated from the area of melting peak considered as the melting enthalpy ΔHm of the polymer electrolyte, since the degree of crystallinity (χc) may be related as (Bhide and Hariharan 2007).
where ΔHm (Jg−1) is the melting enthalpy of the polymer sample measured by DSC and ΔH Φm (Jg−1) is the melting enthalpy of 100 % crystalline PEO material (Suthanthiraraj and Sheeba 2007). Surface morphological analysis was carried out by means of scanning electron microscopy (SEM) on freshly prepared thin film specimens of pure PEO and (PEO)50AgCF3SO3: 2 wt% SnO2 + 30 wt% PC with the aid of a Hitachi S-3400 model scanning electron microscope. Each sample for the SEM analysis was carefully chosen as a mechanically stable thin film (obtained directly from petri dish) over which a layer of gold was deposited by sputtering process in order to make the same electrically conducting prior to testing under the magnification of ×500 for the sake of comparison of various images. Fourier transform infrared spectral studies were carried out in transmission mode for all those transparent thin film polymer electrolyte samples by a Perkin Elmer RX1 IR model spectrometer in the wave number region 4,000 to 400 cm−1 with the resolution of 1 cm−1. Accordingly, each sample was taken in the form of a thin transparent film and placed within the sample holder. Electrical characterization was performed using a HP 4284A Precision LCR Meter over the frequency range from 1 MHz to 20 Hz in the temperature domain 298–338 K wherein the freshly synthesized sample, sandwiched between a pair of silver electrodes, could be heated with the aid of a temperature bath maintained within ±1 K. The total electrical conductivity (σ) was calculated using the relation (Suthanthiraraj and Vadivel 2011a, b),
where t is the thickness of the specimen, A the area covered by the silver electrodes in contact with the specimen and Rb is the bulk resistance of the material derived from the intercept of the impedance plot on the real axis. In order to estimate the values of transference number of silver ions, tAg+ (relative to the anions) in the case of the present polymer electrolyte specimens, the relevant technique involving the combination of DC polarization and AC impedance as originally developed by Bruce and co-workers (Bruce and Vincent 1987; Evans et al. 1987) was employed. Accordingly, the plasticized nanocomposite polymer electrolyte sample under study was sandwiched between the pair of silver electrodes configured as Ag/polymer electrolyte/Ag in which the complex impedance was measured and then the cell was allowed to polarize with DC polarization by an applied potential across the electrodes, while the resulting current was monitored with an aid of a Keithley model 2400 Source Meter for the accurate measurement of current as a function of time. Subsequently, silver ionic transference numbers tAg+ of the polymer electrolyte films were obtained qualitatively using the relationship (Suthanthiraraj et al. 2009),
where ΔV denotes a constant polarization potential of 100 mV, I0 the initial current, Is steady-state current, R0 initial interfacial resistance and Rs is the steady-state interfacial resistance. Whereas I0, and Is were noted from DC polarization the values of R0 and Rs were extracted from the observed complex impedance plot.
Results and discussion
X-ray diffraction analysis
Figure 1 shows the normalized XRD patterns obtained for a variety of compositions of (PEO)50AgCF3SO3:2 wt% SnO2 + x wt% PC (where x = 10, 20, 30 and 40, respectively) nanocomposite polymer electrolyte system. It was evident from our recent work that those diffraction peaks characteristic of crystalline PEO are noticeable at 2θ ~19° and 23.5° in each composition of the (PEO)50AgCF3SO3:2 wt% SnO2 + EC system (Suthanthiraraj and Vadivel 2011a). In the present investigation, dispersion of nano-size filler namely, SnO2 into the polymer–salt complex appears to modify the intensity of diffraction peaks as a result of probable Lewis acid–base type of interactions occurring between the ether oxygen of PEO and Lewis acid sites of SnO2. It is now obvious from Fig. 1 that while introducing the plasticizer namely propylene carbonate (PC) into the nanocomposite system, the above said diffraction peaks tend to show variations in intensity as well as broadening in the case of all the plasticized systems containing 10, 20, 30 and 40 wt% PC. It is interesting to note from Fig. 1 that as the content of the plasticizer (PC) is increased from 10 to 40 wt%, the value of full-width half maximum (FWHM) corresponding to 2θ = 19° decreases from 0.15 to 0.13 thus revealing the effect of PC in reducing the crystallinity as is evident from the typical XRD peak obtained at 23.5° wherein the lowest FWHM of 0.2 has been realized. These changes may be attributed to probable destruction of ordered arrangement of polymer chains and hence enhancement in the amorphous phase (Kuila et al. 2007). Finally, it is also clear that the system containing 30 wt% PC has a reduced degree of crystallinity as compared to other chosen systems, thus leading to the occurrence of a high ionic conductivity. The changes in the relative degree of crystallinity have been quantified from the present XRD patterns by means of Origin Pro 8 software which tend to depict that the relative degree of crystallinity (χc) is decreased with increasing plasticizer concentration i.e., the value of (χc) may be calculated as follows (Woo et al. 2011)
where Ic, is the total crystalline integrated intensity ~19.2° and 23.5°, Ia is the amorphous integrated intensity of halo centered around 2θ ~19.5°. From this quantification, the estimated value of relative degree of crystallinity has been found to decrease as 32, 31 and 29 % in the case of 10, 20 and 30 wt% PC-added samples, respectively, and this observed reduction in the degree of crystallinity trend is found to be in good agreement with the present DSC results too. The above elucidation therefore appears to suggest that the enhancement in the degree of disorder i.e., reduced degree of crystallinity (enhanced amorphicity) of the polymer electrolyte film generally increases as a function of plasticizer concentration (Pradhan et al. 2005) in good agreement with the present DSC analysis discussed in a later section.
Surface morphological analysis
Figure 2a and b depicts the typical SEM images observed for thin films of pure PEO and (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC, respectively, under the same magnification of ×500. It is clear from Fig. 2a that pure PEO has a rough surface consisting of spherulites of an almost equal size with several crystalline domains as noticed in the case of (PEO)50AgCF3SO3:2 wt% SnO2 nanocomposite polymer electrolyte during the course of our earlier investigation (Suthanthiraraj and Vadivel 2011a). In the present work involving addition of 30 wt% PC plasticizer within the (PEO)50AgCF3SO3:2 wt% SnO2 nanocomposite system, it is interesting to note that smoothening of the surface associated with complete disappearance of the spherulite texture occurs as seen in Fig. 2b. These changes may be attributed to the favorable influence of the chosen plasticizer viz., PC on the reduction of crystallinity of the host polymer (i.e., polyethylene oxide) and subsequent enhancement in the overall amorphous fraction within the composite polymeric material (Chu and Reddy 2003). The above feature of reduction of crystallinity is found to be in good agreement with the present XRD, DSC and conductivity results too.
Differential scanning calorimetry (DSC) analysis
Differential scanning calorimetry traces for various compositions of (PEO)50AgCF3SO3:2 wt% SnO2 + x wt% PC-plasticized nanocomposite polymer electrolyte system viz., x = 10, 20, 30 and 40, respectively, corresponding to the melting enthalpy (Tm) and glass transition (Tg) temperature region are shown in Fig. 3a and b, respectively. It is obviously seen from Table 1 that the observed Tg and calculated degree of crystallinity (χc) values decreased with respect to increase in the plasticizer PC content from 10 to 40 wt% added into the nanocomposite polymer electrolyte. The glass transition temperature (Tg) of 202 K and the corresponding degree of crystallinity value of 41.5 were observed for the best conducting composition of (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC. The reduction noticed in the degree of crystallinity suggests that the structural reformation of polymer host PEO would have occurred as a result of addition of PC into the filler-added polymer electrolyte system. This structural reformation is likely to facilitate the mobility of ions viz., segmental motion through the polymer host PEO, and result in an enhanced conductivity observed in the case of PC-plasticized polymer electrolyte system. From Table 1, it is interesting to note that the value of degree of crystallinity of 40 wt% PC is less than that of the 30 wt% PC-incorporated system. However, the fact that the conductivity value is greater for the 30 wt% PC specimen than 40 wt% PC system may be due to the rich plasticization effect i.e., there may be a competition occurring between the PC and PEO molecules to react with available conducting species thus affecting the ionic motion. This disordered structure within the polymer host PEO occurring due to the addition of plasticizer may result in segmental flexibility of polymer chains as revealed from the present XRD results too.
Fourier transform infrared (FTIR) spectroscopic analysis
Figure 4 shows the FTIR spectra observed in the case of (PEO)50AgCF3SO3:2 wt% SnO2 + x wt% PC (where x = 10, 20, 30 and 40, respectively) nanocomposite polymer electrolyte samples. The appearance of strong absorption bands of pure PEO corresponding to CH2 stretching, CH2 wagging, couple of CH2 twisting modes and C–O–C stretching vibrations in Fig. 4 is evident from those bands observed at 2,888, 1,346, 1,280 and 1,242, 1,150 cm−1, respectively, as reported in our earlier work on PEO–AgCF3SO3–Al2O3 complexes (Suthanthiraraj and Sheeba 2007). The pair of absorption peaks appearing at 965 and 843 cm−1 may be assigned to the CH2 rocking configuration. An additional spectral feature observed at 1,032 cm−1 on addition of silver triflate into the polymer host, may be assigned to symmetric SO3 stretching of free triflate ion which emerges to be sensitive for the dissociation of the silver salt due to the feeble ion pairing between the cation (Ag+) and anion (CF3SO3−) within the metal salt. Further, the set of vibrational bands observed at 638 and 573 cm−1 may be attributed to δs (SO3) and δas (CF3) modes of free CF3SO3−, respectively (Rhodes and Frech 2000). It is interesting to note that as a result of incorporation of the plasticizer namely, propylene carbonate (PC) there is an interaction occurring between PC and AgCF3SO3 which could be observed in the form of the symmetric ring deformation mode (C=O bending) of PC which appears at 712 cm−1 (Roger and Sangamithra 1996). Whereas a gradual reduction in the intensity of those peaks obtained at 1,360 and 1,343 cm−1 corresponding to the crystalline phase of PEO is seen to occur with increasing amount of the plasticizer, the broadening of the peak at 1,360 cm−1 and complete disappearance of the typical peak at 1,343 cm−1 has been noticed in the case of the 30 wt% PC-incorporated sample as shown in Fig. 4. This may be due to the reduction in the degree of crystallinity of PEO as revealed by the present XRD and DSC results. As a consequence, the above spectral changes appear to reveal the presence of substantial interactions occurring between the nanocomposite polymer electrolyte system and PC, and thus an appreciable complexation of the plasticized nanocomposite polymer electrolyte systems.
Electrical conductivity analysis
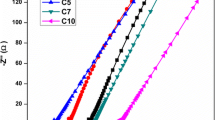
Complex impedance plots obtained for the plasticized nanocomposite polymer electrolyte having the general formula (PEO)50AgCF3SO3:2 wt% SnO2 + x wt% propylene carbonate (PC) viz., 10, 20, 30 and 40 are shown in Fig. 5. In Fig. 5, the observed intercept of each of these plots in the high frequency semicircular region may be attributed to the bulk resistance (Rb), whereas the low frequency semicircle implies the interfacial resistance (Rf). From Fig. 5, it is also apparent that the semicircular arc tends to move toward the origin while increasing the amount of the chosen plasticizer and that the diameter of the first semicircle decreases whereas the domination of the second semicircle increases, thus illustrating that the total conductivity is mainly due to the contribution of ions (Jacob et al. 1997). Subsequent addition of 30 wt% PC as the plasticizer into the nanocomposite polymer electrolyte system (PEO)50AgCF3SO3:2 wt% SnO2 further reduced the crystallinity and resulted in the maximum electrical conductivity (σ) value of 5.9 × 10−5 S cm−1 at 298 K, as shown in Table 2. The electrical conductivity value is thus enhanced ascribable to possible interactions between the plasticizer namely PC and Ag+ ions in the nanocomposite polymer matrix which in turn favor the mobility of Ag+ ions in the polymer matrix. The fact that σ value becomes 1.6 × 10−6 S cm−1 for 40 wt% PC-incorporated sample shows that this interaction may be weakening while increasing the plasticizer content beyond 30 wt%. On the other hand, the present XRD, DSC and FTIR results depict the reduction in the degree of crystallinity and therefore the maximum conductivity value of 5.9 × 10−5 S cm−1 is attained in the plasticized nanocomposite polymer electrolyte having 30 wt% propylene carbonate (PC).
Transference number data
In view of the fact that the mobility of the ionic species plays a considerable role in the electrical conduction of polymer electrolytes in general (Ferry et al. 1998) and that ionic transference number analysis gives an additional information in order to substantiate the conductivity results, the measured values of Ag+ ionic transference number (tAg+) in the case of four different samples of (PEO)50AgCF3SO3:2 wt% SnO2 + x wt% PC, viz., x = 10, 20, 30 and 40, respectively, are given in Table 2. Table 2 suggests that the observed conductivity and tAg+ values are quite comparable to those obtained in the case of PVdF–AgCF3SO3-based plasticized polymer electrolyte system (Suthanthiraraj et al. 2009). From Table 2, it is apparent that the estimated value of tAg+ is enhanced from 0.44 to 0.52 when the amount of PC in the case of the system (PEO)50AgCF3SO3:2 wt% SnO2 is increased from 10 to 30 wt% PC due to favorable Ag+ ionic migration within the nanocomposite polymer system and possible lack of disordered nature due to the rich plasticization effect which may affect the conducting species and thus reduce ionic migration in a PC-rich specimen, i.e., 40 wt% PC in conformity with the present characterization data.
Discharge characteristics of solid-state cell
Figure 6 shows the discharge characteristics observed at room temperature for all solid-state cell fabricated based on the nanocomposite polymer electrolyte system (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC with the cell configuration, Ag| (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC|(C + I2 + electrolyte) for a constant load of 1 MΩ. From Fig. 6, it is clear that a voltage drop has occurred at the initial stage which may be due to the polarization of the cell, which arises from the formation of the silver salt at the electrode/electrolyte interface. The open circuit voltage of 676 mV and short circuit current of 192 μA have been noticed for this cell and the various cell parameters are presented below:
-
Weight of the cell = 0.55 g
-
Area of the cell = 0.5024 cm2
-
Current density = 382.2 μA/cm2
-
Power density = 235.9 mW/Kg
-
Time of plateau region = 30 h
-
Discharge capacity = 5.7 mAh
-
Energy density = 7.07 Wh/Kg
Similar batteries involving PEO-based polymer electrolytes complexed with different salts and combined with a variety of electrodes were investigated by several groups (Hashmi et al. 1992; Rao et al. 1995; Reddy 1998; Kumar et al. 2007; Bhide and Hariharan 2007). On the basis of the present investigation, it is demonstrated that PC-plasticized nanocomposite polymer electrolyte has exhibited a good electrochemical stability and hence suitable for application in ambient temperature solid-state batteries too.
Conclusion
Propylene carbonate–plasticized nanocomposite polymer electrolytes based on (PEO)50AgCF3SO3:SnO2 have been successfully prepared by solution casting technique and the complexation of all the prepared specimens was confirmed by Fourier transform infrared analysis, whereas the reduced degree of crystallinity is revealed by XRD and SEM also quantified by DSC analysis. Enhanced Ag+ ionic conductivity value of 5.9 × 10−5 S cm−1 has been attained in the plasticized nanocomposite polymer electrolyte containing 30 wt% propylene carbonate having the silver ionic transference number of 0.52, whereas an electrochemical cell based on the (PEO)50AgCF3SO3:2 wt% SnO2 + 30 wt% PC solid electrolyte system has also been fabricated and tested.
References
Bhide A, Hariharan K (2007) Ionic transport studies on (PEO)6:NaPO3 polymer electrolyte plasticized with PEG400. Eur Polym J 43:4253–4270
Bruce PG, Gray FM (1997) Polymer electrolytes II: physical principles. In: Bruce PG (ed) Solid state electrochemistry. Cambridge University Press, Cambridge, pp 119–160
Bruce PG, Vincent CA (1987) Steady state current flow in solid binary electrolyte cells. J Electroanal Chem 225:1–17
Chu PP, Reddy MJ (2003) Sm2O3 composite PEO solid polymer electrolyte. J Power Sources 115:288–294
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28:2324–2328
Ferry A, Doeff MM, DeJonghe LC (1998) Transport property measurements of polymer electrolytes. Electrochim Acta 43:1387–1393
Hashmi SA, Chandra A, Chandra S (1992) Ion transport studies in PEO-NaPF6 polymer electrolyte. In: Chowdri BVR, Chandra S, Singh S, Srivastava PC (eds) Solid state ionics: materials and applications. World scientific, Singapore, pp 567–571
Huchang Z, Jianji W, Honghe Z, Kelei Z, Yang Z (2005) Interactions of nanosized Al2O3 and ZnO with poly(ethylene oxide)-NaSCN polymer electrolytes. J Phys Chem B 109:2610–2616
Isebella N, Guiseppe AR, Mario T, Chadwick AV, Webster MI (2002) A study of stability of plasticized PEO electrolytes. Solid State Ionics 146:143–150
Jacob MME, Prabaharan SRS, Radhakrishna S (1997) Effect of PEO addition on the electrolytic and thermal properties of PVDF–LiClO4 polymer electrolytes. Solid State Ionics 104:267–276
Jayathilaka PARD, Disanayake MAKL, Albinson I, Mellander BE (2002) Effect of nano-porous Al2O3 on thermal, dielectric and transport properties of the (PEO)9LiTFSI polymer electrolyte system. Electrochim Acta 47:3257–3268
Kuila T, Acharya H, Srivatsava SK, Samantardy BK, Kureti S (2007) Enhancing the ionic conductivity of PEO based plasticized composite polymer electrolyte by LaMnO3 nanofiller. Mater Sci Eng B 137:217–224
Kumar JS, Kumar KV, Subrahmanyam AR, Reddy MJ (2007) Conductivity study of polyethylene oxide (PEO) complexed with sodium bicarbonate. J Mater Sci 42:5752–5755
Kwang-Sun J, Hee-Soo M, Jong-Wook K, Jong-Wan P (2003) Role of functional nano-sized inorganic fillers in poly(ethylene)oxide-based polymer electrolytes. J Power Sources 117:124–130
MacCallum JR, Vincent CA (1987) Polymer electrolyte reviews-2. Elsevier, London
Pitawala HMJC, Dissanayake MAKL, Seneviratne VA (2007) Combined effect of Al2O3 nano-fillers and EC plasticizer on ionic conductivity enhancement in the solid polymer electrolyte (PEO)9LiTf. Solid State Ionics 178:885–888
Pitawala HMJC, Dissanayake MAKL, Seneviratre VA, Mellander BE, Albinson I (2008) Effect of plasticizers (EC or PC) on the ionic conductivity and thermal properties of the (PEO)9LiTf:Al2O3 nanocomposite polymer electrolyte system. J Solid State Electrochem 12:783–789
Pradhan DK, Samantaray BK, Choudhary RNP, Thakur AK (2005) Effect of plasticizer on microstructure and electrical properties of a sodium ion conducting composite polymer electrolyte. Ionics 11:95–102
Pradhan DK, Choudhary RNP, Samantaray BK (2008) Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int J Electrochem Sci 3:597–608
Rao SS, Reddy MJ, Narsaiah EL, Rao UVS (1995) Development of electrochemical cells based on (PEO + NaYF4) and (PEO + KYF4) polymer electrolytes. Mater Sci Eng, B 33:173–177
Reddy MJ, Sreekanth T, Rao UVS (1998) Conductivity and parametric studies of a (PEO + (glass) (15Na2O–15NaF–70B2O3)) cell. J Power Sources 76:30–35
Rhodes CP, Frech R (2000) A symmetry-based analysis of Raman and infrared spectra of the compounds (poly (ethylene oxide))3 LiCF3SO3 and (poly(ethylene oxide))NaCF3SO3. Solid State Ionics 136:1131–1137
Roger F, Sangamithra C (1996) Effect of propylene carbonate as a plasticizer in high molecular weight PEO-LiCF3SO3electrolytes. Solid State Ionics 85:61–66
Scrosati B (ed) (1993) Applications of electroactive polymers. Chapman and Hall, London
Scrosati B, Croce F, Persi L (2000) Impedance spectroscopy study of PEO-based nanocomposite polymer electrolytes. J Electrochem Soc 147:1718–1721
Suthanthiraraj SA, Sheeba JD (2007) Structural investigation on PEO-based polymer electrolytes dispersed with Al2O3 nanoparticles. Ionics 13:447–450
Suthanthiraraj SA, Vadivel MK (2011a) Electrical and structural studies on (PEO)50–AgCF3SO3: SnO2 nanocomposite gel polymer electrolyte materials. Trans Indian Inst Met 64:149–153
Suthanthiraraj SA, Vadivel MK (2011b) Electrical and structural properties of poly (ethylene oxide)/silver triflate polymer electrolyte system dispersed with MgO nanofillers. Ionics. doi:10.1007/s11581-011-0637-0
Suthanthiraraj SA, Sheeba DJ, Paul BJ (2009) Impact of ethylene carbonate on ion transport characteristics of PVdF–AgCF3SO3 polymer electrolyte system. Mater Res Bull 44:1534–1539
Wang YJ, Pan Y, Wang L, Pang MJ, Chen L (2005) Conductivity studies of plasticized PEO–lithium chlorate–FIC filler composite polymer electrolytes. Mater Lett 59:3021–3026
Woo HJ, Majid SR, Arof AK (2011) Transference number and structural analysis of proton conducting polymer electrolyte based on poly(ε-caprolactone). Mater Res Innov 15:49–54
Acknowledgments
The authors are grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi for the financial assistance received. The authors wish to thank the National Center for Nanoscience and Nanotechnology (NCNSNT), University of Madras for providing necessary SEM facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Suthanthiraraj, S.A., Vadivel, M.K. Effect of propylene carbonate as a plasticizer on (PEO)50AgCF3SO3:SnO2 nanocomposite polymer electrolyte. Appl Nanosci 2, 239–246 (2012). https://doi.org/10.1007/s13204-012-0099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0099-3