Abstract
Ion conducting thin film polymer electrolytes based on polyethylene oxide (PEO) complexed with NaHCO3 salt has been prepared using solution-cast technique. The complexation of NaHCO3 salt with PEO is confirmed by XRD and IR studies. DC conductivity in the temperature range 303–368 K has been evaluated. The conductivity is found to increase in the PEO complex with the NaHCO3 salt and also with an increase in temperature. Using this polymer electrolyte, an electrochemical cell with the configuration Na/(PEO + NaHCO3)/(I2 + C + electrolyte) has been fabricated and its discharge characteristics studied. Open Circuit Voltage (OCV) and Short Circuit Current (SCC) are found to be 2.69 V and 1.28 mA, respectively. Other parameters associated with the cell are evaluated and presented in this paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ion conducting polymer electrolytes have recently become an area of wide spread interest in solid-state ionics because of their potential application in solid electrochemical devices such as energy conversion systems batteries/fuel cells, electro chromic display devices/smart windows, photo electro chemical solar cells, etc. [1–6]. Advantageous of polymer electrolytes are ease of fabrication into films of desirable sizes and good electrode–electrolyte contacts in different electrochemical devices. Interest began in this field after the studies of materials based on alkali metal salts complexed with polyethylene oxide (PEO) reported by Wright and coworkers [7, 8]. PEO is an exceptional polymer having ability to solvate ionic salts and forms solid polymer electrolyte [9]. Polymer electrolytes consisting of PEO and alkali metal salts were reported for the purpose of application to several kinds of electrochemical cells [10, 11].
In the past three decades, many polymeric electrolytes, based on various salts dissolved in polyethers, particularly PEO, have been widely investigated because of their potential application in high performance batteries. Such electrolytes have been mainly based on alkali metal salt systems, with particular attention given to lithium. The studies on sodium ion conducting polymers are scanty. Less effort has been made on solid polymer electrolytes based on sodium complex systems. Apart from the scientific interest, the use of sodium has several advantages over lithium. Sodium is much more abundant and lower in price than lithium. The softness of this metal makes it easier to achieve and maintain contact to other components in the electrochemical devices.
In the present investigation, the authors prepared solid polymer electrolytes by complexing poly(ethylene oxide) (PEO) with sodium bicarbonate (NaHCO3). Various properties such as XRD, IR studies and dc electrical conductivity measurements in the temperature range 303–368 K have been carried out to characterize this polymer electrolyte system and the results obtained are presented in this paper. Based on this polymer system a new polymer battery with the configuration Na/(PEO + NaHCO3)/(I2 + C + electrolyte) has been fabricated and its discharge characteristics studied.
Experimental
Films (thickness ≈ 100–150 μm) of pure PEO (Aldrich MW ∼ 6 × 105) and PEO complexed with NaHCO3 salt were prepared in the weight ratios (90:10), (80:20) and (70:30). EO/Na+, monomer to cation ratio is (4.7:1), (2.1:1), and (1.2:1). The films were prepared by the solution cast method with methanol (water free) as solvent. Mixtures of PEO and NaHCO3 were stirred for about 10–12 h. The stirred homogeneous solutions were cast onto polypropylene dishes and evaporated slowly at room temperature. The final product was vacuum dried thoroughly at 10−3 Torr.
The XRD studies of these samples were carried out with the help of PHILIPS PW 3710 X-RAY diffractometer in the range 10°–70°. The infrared (IR) Spectra of these samples have been recorded with the help of JASCO FT-IR-5300 Spectrophotometer in the range 400–4,000 cm−1 with resolution of 2 cm−1.
The dc conductivity measurements of these samples have been made using in house made conductivity setup in the temperature range 303–368 K. The solid-state electrochemical cell has been fabricated with the configuration anode (Na)/(PEO + NaHCO3)/cathode (I2 + C + electrolyte). The construction of the electrochemical cell is shown in Fig. 1 [12]. The discharge characteristics of these cells were monitored under the constant load of 100 kΩ. Sodium metal is used as anode; and iodine (I2), graphite (C), and electrolyte materials are used in the weight ratio of 5:5:1, respectively as cathode. The cathode is made in the form of a thin pellet after proper mixing the constituents. In the cathode material iodine is an active cathodic material, graphite to assure adequate electronic conductivity and electrolyte to favour electrode/electrolyte interfacial contact.
Results and discussion
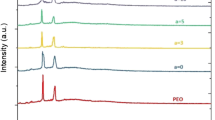
The X-Ray diffraction (XRD) spectra of pure PEO, PEO complexed with NaHCO3 and pure NaHCO3 are shown in Fig. 2. A comparison of X-Ray diffraction spectra shows that the few differences in diffraction patterns are identified between the complexed PEO films and that of pure PEO. The diffraction peaks between 2θ = 15°–30°, clearly apparent in pure PEO [13–17] were found to be less prominent and shifted to higher angle in NaHCO3 complexed PEO films. Accompanying the decrease in intensity with the gradual broadening of the diffraction peaks by increasing of NaHCO3 salt concentration to PEO is apparent. The results affirm both the decrease of degree of crystallinity and the lamellae size with increasing of NaHCO3 salt. However, the additional/new diffraction peaks corresponding to the formation of a stable Na:PEO crystalline complex is apparent between 2θ = 50° and 62°. The X-ray diffraction results evidence of decrease in PEO crystallinity with increasing concentration of NaHCO3 ionic salt and also found Na ion–PEO crystalline stable complex.
IR spectra of pure PEO and PEO complexed with NaHCO3 are shown in Fig. 3. The intensity of the aliphatic C–H stretching vibration band observed around 2,950 cm−1 in PEO is found to increase with increasing the concentration of NaHCO3 salt in the PEO. This spectral change is different from the earlier reported results on various ionic salt complexes to PEO [17–19]. The increase in intensity of aliphatic C–H stretching vibration band in complexed NaHCO3 with PEO maybe due to the physical interaction through hydrogen of (HCO3)− with the PEO chain. The width of the C–O stretching band observed around 1,100 cm−1 in PEO showed a decrease, with an increase of NaHCO3 salt. New peaks around 2,895.41, 1,963.71 and 530.47 cm−1 have been observed in the complexed PEO. If the cations of NaHCO3 get coordinated with the oxygen of PEO, the spectral changes are expected to be in the C–O stretching and deformation ranges. The decrease in the width of 1,100 cm−1 band which is assigned to C–O symmetrical and asymmetrical stretching [18–20] suggested the coordination/complexation of the salt with PEO. The complexations of PEO with alkali metal salts have been studied by using vibration spectroscopic studies [15–20]. The appearance of new peaks along with changes in existing peaks in the IR spectra directly indicates the complexation of PEO with NaHCO3. Thus, the results of XRD and IR data clearly indicate the complexation of NaHCO3 salt with PEO.
Figure 4 shows the variation of conductivity as a function of temperature for pure PEO and different compositions of (PEO + NaHCO3) polymer electrolyte in the temperature range 303–368 K. From Fig. 4 the following have been drawn
-
(a)
In the temperature range of study, the conductivity is found to increase with the temperature in pure PEO and in PEO complexed with NaHCO3 salt films.
-
(b)
The conductivity versus temperature plots indicates two regions (region-I & region-II). In region-I, the conductivity slowly increases with temperature up to melting temperature (T m ∼ 65 °C). At T m there is a sudden increase in conductivity. In the region-II, above T m the conductivity again rapidly increases with temperature. Similar behaviour has been observed in all the composition of (PEO + NaHCO3) polymer electrolyte systems. The temperature, which corresponds to the sudden increase in conductivity indicate that there is a change of phase from semicrystalline to amorphous. The existence of two regions in the conductivity versus temperature plots has been observed in a number of PEO based polymer electrolytes [15, 17–19, 21]. The calculated activation energies (Ea) in two regions (I and II) for pure PEO and PEO complexed with NaHCO3 salt are given in Table 1.
Using the new polymer electrolyte system (PEO + NaHCO3), an electrochemical cell with the configuration Na/(PEO + NaHCO3)/(I2 + C + electrolyte) has been prepared and studied its discharge characteristics is shown in Fig. 5. From the figure it is observed that the initial sharp decrease in the voltage in these cells may be due to polarization effect or formation of a thin-layer of sodium salt at the electrode–electrolyte interface. The Open Circuit Voltage (OCV) and the Short Circuit Current (SCC) are 2.69 V and 1.28 mA, respectively. The various other parameters of the cell evaluated are presented below:
-
Cell weight = 1.46 g
-
Effective area of the cell = 1.34 cm2
-
Thickness of the cell = 150 μm
-
Power density = 2.31 W kg−1
-
Energy density = 183 W h kg−1
-
Plateau region = 65 h
On the basis of present study, a solid state battery with (PEO + NaHCO3) as an electrolyte is promising one. The cell parameters obtained is comparable with the earlier reported sodium based polymer electrolyte systems [12, 13, 22]. Further work is in progress to achieve higher cell parameters.
Conclusions
The complexation of NaHCO3 salt with PEO is confirmed by XRD and IR studies. The conductivity is found to increase in the PEO complexed with the NaHCO3 salt and also with an increase in temperature. An electrochemical cell with the configuration Na/(PEO + NaHCO3)/(I2 + C + electrolyte) has been fabricated and its discharge characteristics studied. Open Circuit Voltage (OCV) and Short Circuit Current (SCC) are 2.69 V and 1.28 mA, respectively. The cell parameters obtained on this polymer electrolyte system is comparable with the earlier reported sodium based polymer electrolyte systems.
References
Mac Callum JR, Vincent CA (eds) (1987) Polymer electrolyte rev. Elsevier, Amsterdam
Armand MB (1986) Mater Sci 16:245
Ratner MA, Shrivar DF (1988) Chem Rev 88:109
Owen JR, Lasker AL, Chandra S (eds) (1989) Superionic solids and solid electrolytes – recent trends. Acadamic Press, New York, p 111
West K, Zachau-Christiansen B, Jacobson T, Atlong I (1985) J Electro Chem Soc 132:306
Munshi MZA, Gilmoor A, Smyl WH, Owens B (1989) J Electro Chem Soc 136:1847
Wright PV (1975) Br Polym J 7:319
Fenton DE, Parker JM, Wright PV (1973) Polymer 14:589
Vincent CA (1987) Progr Solid State Chem 17:347
Armand MB (1983) Solid State Ionics 9–10:745
Berther G, Gorecki W, Miner M (1983) Solid State Ionics 11:91
Jaipal Reddy M, Srinivas Reddy D, Sreepati Rao S, Subba Rao UV (1995) Mater Lett 23:129
Sreepathi Rao S, Jaipal Reddy M, Lakshminarasaiah E, Subba Rao UV (1995) Mater Sci Eng B 33:173–177
Jaipal Reddy M, Sreekanth T, Subba Rao UV (1999) Solid State Ionics 126:55
Yang H, Huq R, Ferington GC (1990) Solid State Ionics 40/41:663
Papke BL, Ratnar MA, Shrivar DF (1982) J Electrochem Soc 129:1434
Maurya KK, Srivastava N, Hashmi SA, Chandra S (1992) J Mater Sci 27:6357
Sreepathi Rao S, Subba Rao UV (1994) J Mater Sci Lett 13:1771
Sreekanth T, Jaipal Reddy M, Subba Rao UV (2000) J Power Sources 1–5:3994
Papke BL, Ratnar MA, Shrivar DF (1982) J Electrochem Soc 129:1434
Jaipal Reddy M, Sreekanth T, Chandrashekar M, Subba Rao UV (2000) J Mater Sci 35:2841
Sreepathi Rao S, Jaipal Reddy M, Narasimha Reddy K, Subba Rao UV (1994) Solid State Ionics 74:225
Acknowledgements
The authors thank the Head, Department of Physics, Osmania University Hyderabad, for his encouragement. One of the authors KVK thank Dr. B.N. Reddy, Chairman, CBIT, D. Kamalakar Reddy, Secretary, CBIT & Prof. I. Ramchandra Reddy, Principal, CBIT, for their help and cooperation. One of the authors MJR thank CSIR, New Delhi for the award of SRA, under Scientist’s Pool scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siva Kumar, J., Vijaya Kumar, K., Subrahmanyam, A.R. et al. Conductivity study of polyethylene oxide (PEO) complexed with sodium bicarbonate. J Mater Sci 42, 5752–5755 (2007). https://doi.org/10.1007/s10853-006-0743-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0743-y