Abstract
Microbial inoculants containing plant-growth-promoting bacteria (PGPB) are low-cost tools to improve crop yield. The prospection of new strains should enable the selection of efficient microbial agents for commercial inoculants. This study aimed to isolate and select PGPB for maize in the Brazilian semiarid region. A field trial using two maize genotypes was implemented, and bacteria were isolated from maize stems and roots. The bacteria were grown in semi-solid BMGM or solid Dyg’s media, and they were characterized in vitro with respect to five plant growth-promoting mechanisms. Twenty-seven strains were assessed for growth promotion using potted maize plants. Nine bacterial strains improved plant biomass and/or N accumulation in shoots and were selected for field assessment and identification by 16S rRNA sequencing. Fourteen and 65 bacterial strains were isolated, respectively, in the semi-solid and solid medium. Auxin production without L-Tryptophan and antagonism against F. verticillioides were found for bacteria isolated on solid medium, and other characteristics were found for the strains from semi-solid medium. These strains were classified as Bacillus (5), Brevibacillus (2), Staphylococcus (1), and Paenibacillus (1). Five strains (Bacillus spp. ESA 593, ESA 597, ESA 599, ESA 600, and Paenibacillus sp. ESA 601) improved maize yield (56–87%) compared with the non-inoculated and non-fertilizated (N) control. In conclusion, the Brazilian drylands maize plants harbor several potential PGPB, and five elite strains were retrieved in the present study. These strains will be used for future network field assays to assure their agronomic performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Brazilian semiarid region covers more than 1.8 million km2, approximately 20% and 89.5% of the whole of Brazilian and Northeast region territories, respectively (IBGE 2018). In the 2018/2019 crop season, the yield of maize (Zea mays) was, in the northeast Brazilian region, 2530 kg ha−1 and 5350 kg ha−1 for the whole country (CONAB 2019). In the northeast region, maize is grown mainly by small farmers in family-based production systems. The development of low-cost and environmentally friendly technologies is urgently needed to benefit these agricultural systems (Alves et al. 2020). The selection of efficient and locally adapted plant-growth promoting bacteria (PGPB) is a promising approach for developing improved microbial inoculants. The elevated temperatures, low rainfall, and high evapotranspiration limit crop growth in the Brazilian drylands. Low-cost technologies are needed to overcome this situation. Efforts have been made to develop microbial inoculants as a promising tool (Bonatelli et al. 2021).
Several research groups built and scrutinized culture collections for PGPB isolated from non-legumes in the tropics in recent years. For example, isolates were examined from sorghum (Sorghum bicolor) (Mareque et al. 2015; da Silva et al. 2018), sunflower (Helianthus annuus) (Ambrosini et al. 2016), forage-grasses (Haiyambo et al. 2015a; Antunes et al. 2019), onion (Allium cepa) (Tinna et al. 2020) rice (Oryza sativa) (Cavite et al. 2020; Ríos-Ruiz et al. 2020), maize (Zea mays) (Hungria et al. 2010; Alves et al. 2016; Bodhankar et al. 2017; Chakra et al. 2019; Bomfim et al. 2020; Cavalcanti et al. 2020; Ikeda et al. 2020), among other crops.
The isolation and selection of bacteria from the semiarid region should lead to the identification of microbes adapted to the local edaphoclimatic conditions and thus be suitable for the formulation of improved inoculants (Kavamura et al. 2013; Fernandes-Júnior et al. 2015; Bodhankar et al. 2017; da Silva et al. 2018; Antunes et al. 2019; Cavalcanti et al. 2020; Bonatelli et al. 2021). Bacterial isolation from plants in the Brazilian drylands revealed that the genera Bacillus, Pantoea, Stenotrophomonas, Rhizobium, Agrobacterium, Enterobacter, and Burkholderia were notable as a result of their abundance and efficiency (Kavamura et al. 2013; Fernandes-Júnior et al. 2015; Lima et al. 2015; da Silva et al. 2018; Antunes et al. 2019; Santana et al. 2020). Surveys showed that the primary “traditional” PGPB groups, such as Azospirillum and Herbaspirillum genera, were absent in the semiarid regions of Brazil (da Silva et al. 2018; Antunes et al. 2019), India (Grover et al. 2014; Kumar et al. 2014), Namibia (Haiyambo et al. 2015a, b), and China (Niu et al. 2018). This fact indicated that other bacteria are locally adapted and probably more efficient than those present in the commercial inoculants.
The standard approach to isolate associative diazotrophic bacteria is the use of N-free semi-solid media. This approach was proposed by Dr. Johanna Döbereiner in the 1960s and has been applied worldwide to obtain efficient diazotrophs in non-legumes (Baldani et al. 2014). The use of other approaches, for example, directly plating plant extracts on a solid medium, has led to a few diazotrophs’ isolation. Fernandes Júnior et al. (2013) isolated 998 bacteria from roots of Oryza glumaepatula in the Brazilian Amazon, of which only 38 were nifH positive. However, this approach may also lead to the isolation of PGPB displaying other mechanisms, such as auxin and siderophore production and phosphate solubilization (Felestrino et al. 2018). Thus, combining the approaches, using N-free semi-solid media and solid media, should enable the assemble of culture collections with more diverse strains at the taxonomic and functional levels.
Information about the taxonomy of maize PGPB and their agronomic efficiency (both obtained in the N-free semi-solid or directly in dishes with solid media) is available for several different climatic regions in Brazil (Oliveira et al. 2009; Pedrinho et al. 2010; Arruda et al. 2013; da Silva Santos et al. 2015). However, to the best of our knowledge, few data are available about the screening of PGPB colonizing maize tissues in the Brazilian semiarid region (Bomfim et al. 2020; Cavalcanti et al. 2020). We hypothesized that maize is colonized by efficient PGPB in the Brazilian semiarid region and that the use of the approaches to bacterial isolation mentioned above would be useful for obtaining efficient PGPB from the field-growing maize in this region.
2 Methods and materials
2.1 Bacterial isolation
One field trial was conducted to obtain the bacterial isolates. The trial was conducted in the Bebedouro Experimental Field in an Ultisol. The experimental field is located at Embrapa Semiárido, Petrolina, Pernambuco state, Brazil (lat. -9.1372; long. -40.3056). Before implementing the study, a composite soil sample was collected and chemically evaluated according to Teixeira et al. (2017). The chemical characteristics of the soils are shown in Table S1. According to the chemical characteristics, we fertilized the soils with 100 kg ha−1 simple-superphosphate (18% of P2O5) and 40 kg of potassium chloride (60% of K2O) to set up the experiment.
The plant samples used to isolate bacteria in the present study were those used by Cavalcanti et al. (2020). In summary, The study was implemented in January of 2015 using the short cycle commercial maize (Zea mays) genotypes BRS Gorutuba and BRS Caatingueiro. For irrigation, a drip irrigation system with 0.5 m between drippers and an irrigation depth of 1.6 L h−1 was implemented. Ten 20 m long lines (1 m between lines and 0.25 m between seedbeds) were sown. The plants were grown for 60 days before being harvested for bacterial isolation. The core plants from each alternate line (15 plants in total) were collected and mixed in the harvest. The plants were transported to the laboratory and kept at 10 °C until isolation of endophytic bacteria.
The fine roots and shoots (around 0.25 m above the soil) were separately washed with running tap water and surface disinfected with ethanol 96% (v v−1) for 30 s and 2.5% NaClO (v v−1) for 10 min, followed by ten washes in sterile distilled water (SDW) (Döbereiner et al. 1995). Aliquots of the last portion of the water used to wash the roots were inoculated in Nutrient-Agar dishes to ensure disinfection efficiency. Fifty grams of the tissues (fine roots and shoots) were crushed in 450 mL of NaCl 0.85% (w v−1) in a blender. Afterward, 100 μL of the macerates were inoculated in Petri dishes containing solid Dyg’s medium [glucose 2 g L−1; malic acid 2 g L−1, peptone 1.5 g L−1yeast extract 2 g L−1, K2HPO4 500 mg L−1, MgSO4 500 mg L−1, glutamic acid 1.5 g L−1, and agar 15 g L−1. pH 6.5 (Rodrigues Neto et al. 1986)] and spread with a Drigalsky loop. One hundred μL-aliquots of the macerates were used to inoculate assay tubes containing 10 mL of BMGM semi-solid medium (Estrada-De Los Santos et al. 2001). The media were incubated at room temperature and checked daily.
The typical microaerophilic pellicle (MP) characteristic of non-symbiotic N2-fixation was checked every day to the semi-solid medium. The positive tubes were used for re-inoculation in the same semi-solid medium, and the presence of MPs was observed after the same incubation period found in the first inoculation. The positive tubes were inoculated in solid Dyg’s medium, and the cultures were purified and reinoculated in the semi-solid medium to assure the diazotrophic capacity. The colonies in the solid medium were purified in the same medium.
All pure cultures were evaluated in the next stages of bacterial characterization. The bacterial collection was stored at −80 °C in the Culture Collection of Micro-organisms with Agricultural Interests of Embrapa Semiárido (CMISA).
2.2 In vitro assessment of plant growth-promoting traits
The bacteria were grown in the liquid Dyg’s medium, and the DNA was extracted using the alkaline lysis method, according to Wang et al. (1993). For amplification of the nifH gene, the primers PolF (TGCGAYCCSAARGCBGACTC) and PolR (ATSGCCATCATYTCRCCGGA) (Poly et al. 2001) were used. The PCR products were visualized in a UV chamber after horizontal electrophoresis in 1% agarose gel (w v−1), stained with GelRed (Biotium, USA). In all PCR rounds, Azospirillum brasilense Ab-V5 was used as a positive control.
The in vitro production of auxin was evaluated by the colorimetric method described by Sarwar and Kremer (1995) with modifications. Bacterial isolates were grown in liquid Dyg’s medium. After 72 h, an aliquot of 1 mL of each broth was centrifuged (6000 x g for 5 min), the supernatant was discarded, the pellet was resuspended in 1 mL of SDW, and the OD540 was adjusted to 0.5 spectrophotometrically (Multiskan GO, Thermo Scientific, Germany).
Aliquots of 150 μL of the resuspended broth were inoculated in 5 mL of liquid Dyg’s medium supplemented or not with 168 mg L−1 of L-tryptophan (L-Try) (Sigma-Aldrich, USA) and stirred at room temperature for 72 h. The cultures were adjusted to OD540 = 0.5 with SDW and centrifuged (6000 x g for 5 min). The supernatant (100 μL) was mixed with 150 μL of Salkowski solution in 96-well ELISA microplates, incubated in the dark for 30 min, and read spectrophotometrically at 530 nm. Auxin concentration was estimated using a standard curve previously prepared with a range of 0 to 500 μg L−1 of synthetic indole-3-acetic acid (Sigma-Aldrich, USA).
The isolates were evaluated for calcium phosphate solubilization in solid GL medium (Sylvester-Bradley et al. 1982). The bacteria were grown in liquid Dyg’s medium, centrifuged, and resuspended as described above. Aliquots of 10 μL were dropped on GL medium and incubated at 28 °C for five days. After the incubation period, the diameter of the colonies and the translucent halo surrounding the colonies were measured in millimeters (mm), and the Solubilization Index (SI) was calculated (Berraquero et al. 1976).
The siderophore production was qualitatively evaluated by adapting the CAS-plate method described by Ribeiro and Cardoso (2012) with few modifications. The bacterial isolates were grown in Dyg’s liquid medium and centrifuged as described above. An aliquot of 100 μL of the supernatant was placed in 96-well ELISA microplates with 100 μL of the CAS reagent (Sigma-Aldrich, USA) (Schwyn and Neilands 1987) and kept in the dark for 1 h. The change from a blue to a yellow-orange color indicates positive reaction for the siderophore production.
Antagonism against the soil-borne pathogen Fusarium verticillioides (Sacc.) Nirenberg was also assessed. The fungal strain LBF-MIL01 P was isolated in the Plant Pathology Laboratory of Embrapa Semiárido, Petrolina, PE, Brazil. The strain was derived from field-grown maize with symptoms of root rot disease in a commercial field. The pathogenic behavior of F. verticillioides LBF-MIL01 P was proven by fulfilling Koch’s postulate.
Bacterial strains were grown in liquid medium, centrifuged, and resuspended in SDW as described above. The fungal pathogen was grown in Potato-Dextrose-Agar (PDA) plates at 28 °C for three days; then, a disc of 50 mm was inoculated in the center Petri dishes with Dyg’s medium. In the same plate, the bacteria were inoculated by spreading a lane near the dish’s border. The plates were incubated at 28 °C for seven days, and the antagonism against the pathogen was assessed by the inhibition of mycelial growth compared to the control, where the fungal isolate grew alone (Dias et al. 2014).
2.3 Plant growth promotion experiment in greenhouse conditions
From the results on plant growth-promoting traits, 44 strains (26 and 18 strains obtained from direct plating and semi-solid medium, respectively) were evaluated with respect to their ability to promote maize growth in pot experiment, which were carried out in 5-L pots filled with a sample of an Ultisol surface layer. A composite soil sample was used for soil chemical analysis, and the results are shown in Table S1.
Before sowing, the seeds of maize BRS Gorutuba were surface disinfected as described above for the maize tissues. For inoculation, the bacteria were grown in liquid Dyg’s medium with constant stirring (120 rpm) for 72 h. The culture was spectrophotometrically adjusted to OD540 = 0.6 The inoculation was carried out applying 2 mL of the OD540 = 0.6 adjusted culture broth on each seed. A positive inoculated control was used with the Azospirillum brasilense Ab-V5 strain (Hungria et al. 2010). Two non-inoculated treatments, one with 1.2 g of urea pot−1 (equivalent to fertilization of 90 kg N ha−1) and another without N supplementation, were also assessed. The parameters evaluated were the dry weight of shoots (g plant−1), dry weight of roots (g plant−1), the nitrogen concentration in the shoots (mg N g plant−1), and the total nitrogen accumulated in the shoots (mg N plant−1). To evaluate the nitrogen accumulation in the shoots, they were milled and sieved (2 mm), and the nitrogen content was assessed by the dry combustion method in a TruSpec CN elemental analyzer (LECO, USA). The results were used to calculate the nitrogen accumulated in the shoots content by multiplicating nitrogen content by the shoot dry weight.
2.4 16S rRNA gene sequence analysis
The 16S rRNA gene was amplified using the universal primers 27F (GAGTTTGATCCTGGCTCAG) and 1492R (GGTTACCTTGTTACGACTT) (Weisburg et al. 1991) and purified with the commercial kit Wizard® SV Gel and PCR Clean-up System (Promega, USA). The purified amplicons were sequenced by Macrogen (Seoul, South Korea) in an ABI 3037 xl genetic analyzer (Applied Biosystems, USA). The quality of the sequences was verified by Sequence Scanner Software v. 2.0 (Applied Biosystems, USA), and the good-quality sequences (QV > 20 in 700 bases of continuous read) were used to assemble the almost complete 16S rRNA gene sequence. The assembled fragments were compared to those of type strains available in the GenBank database using the BLASTn tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequences were deposited in the GenBank database of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/genbank/) under the accession numbers MT498070 to MT498078.
2.5 Agronomic efficiency of the selected strains
The agronomic efficiency of nine selected isolates was assessed in a field study. This assay was implemented in the Mandacaru Experimental Field at Embrapa Semiárido, Juazeiro, Bahia state, Brazil (lat. -9.3925; long. -40.4175) between February and May of 2017. Plowing and harrowing were used for soil preparation. A composite sample was collected for the fertility analysis, and the results are shown in Table S1. According to the technical recommendations, fertilization with 20 kg ha−1 of P2O5 (simple superphosphate) and 20 kg ha−1 of K2O (potassium chloride) was applied in-furrow.
The treatments evaluated in this assay were the response to the inoculation of nine new bacterial isolates (single inoculation), the inoculation of the reference strain A. brasilense Ab-V5 and non-inoculated treatments, which consisted of one negative control (without N fertilization), one treatment with 45 kg of N-urea ha−1 and another treatment with 90 kg of N-urea ha−1, totaling 16 experimental treatments. The assay was implemented in a completely randomized block design with four replications (blocks).
For both N-fertilized treatments, urea application was split into two half-rate applications (at sowing and 35 DAE). The plots had five 4 m rows spaced 1 m apart, with 5 plants m−1. The useful area was the central 3 m in the central 3 rows of each plot. The assay was irrigated by drip irrigation, as mentioned above.
Peat-based inoculants were prepared by mixing 15 mL of the bacterial broth grown in the Dyg’s liquid medium with 50 g of powdered and autoclaved plastic bags containing powdered peat (from Rio Grande do Sul state, Brazil, 84% of organic matter, pH adjusted to 6.5 with CaCO3). The mixture was homogenized and stored at 10 °C until the experiment set up. The maize seeds cv. BRS Gorutuba were inoculated by mixing the peat inoculants and seeds at a 250 g of inoculant for 10 kg of maize seeds. A saturated sucrose solution (50 mL 1 kg seeds−1) was added to the mixture to increase the inoculant’s adherence to the seeds.
At 60 DAE, ten plants were randomly harvested from each plot’s second row in the first harvest. The shoots were placed in paper bags, dried and weighed. The third fully opened leaf of each plant (from top to down) were ground to determine N concentration as above mentioned. In this harvest, the parameters evaluated were the shoot dry weight and the nitrogen content in the third leaf. The second harvest was conducted at 95 DAE when the grain yield was evaluated. Weeds were controlled by hand, and pest control was conducted as technically recommended for the region.
2.6 Statistical analysis
All quantitative data were evaluated by variance analysis (ANOVA) after evaluating the normal distribution by the Shapiro-Wilk test. The data were transformed to (X + 1)0.5 before the ANOVA for the plant growth promotion and field experiments. The statistical analysis package Sisvar v 5.0 (Ferreira 2011) was used.
The principal component analysis (PCA) for the plant growth promotion traits was conducted using the correlation matrixes in the PaSt 4.02 software (Hammer et al. 2011). Due to the low contribution of calcium phosphate solubilizing bacteria, the analysis of this data as quantitative should cause a biased analysis. In this case, this feature was analyzed as binary.
3 Results
3.1 Several potential plant growth-promoting bacteria were isolated
Seventy-nine bacterial strains were isolated in the isolation approaches from the cultivars and plant compartments (Table 1). Fourteen strains were obtained from semi-solid BMGM and 65 from solid Dyg’s medium. Forty-two bacterial strains were obtained from the maize cultivars’ roots, and 37 strains came from maize stems. BRS Caatingueiro was the host of 45 bacterial strains, and BRS Gorutuba hosted 34 strains.
Detectable amounts of auxins in liquid medium supplied with L-Trp were quantified from all strains. Strain ESA 599 stood out, producing more than 413.20 μg mL−1, higher than all the other strains. Thirty-nine strains (49.4%), along with the reference bacterium Azospirillum brasilense Ab-V5, produced between 250.10 and 99.14 μg mL−1 and were ranked in the second-highest cluster of the Scott-Knot average range test. Two other clusters were ranked almost 51% of the bacterial strains as lower auxin producers (Fig. S1 and Table S2).
Without L-Trp supply, the strain G57 produced 588.10 μg mL−1 of auxin and was the highest auxin producer under the conditions used. Sixteen bacterial strains (20.3%) produced between 372.56 and 220.90 μg mL−1 of auxin in liquid Dyg’s medium. In the third statistical cluster ranked by the Scott-Knot average range test (p < 0.05), 19 (24.1%) bacteria produced between 209.90 and 106.00 μg mL−1 of auxin in the culture medium. Twenty-seven maize strains (34.2%) and A. brasilense Ab-V5 producing between 90.82 and 34.84 μg mL−1 were ranked in the fourth statistical cluster. Nineteen bacterial strains (24.1%) produced less than 30 μg mL−1 of auxin in the culture medium and were ranked in the lowest statistical cluster.
A few bacterial strains were calcium phosphate solubilizers and siderophore producers. Thirteen strains (16.5%) were calcium phosphate solubilizers. Three strains (3.8%) were the highest calcium phosphate solubilizers (enzymatic index ranging between 4.13 to 3.90). Six new maize strains (7.6%) and A. brasilense Ab-V5 were considered intermediate solubilizers, showing enzymatic indexes between 2.82 and 1.67. Four strains (5.1%) solubilized low calcium phosphate. Sixty-three strains (79.7%) grew in GL culture medium but did not solubilize the inorganic phosphate. The bacterium ESA 599, the best siderophore producer, produced the equivalent to 6.18 mmol L−1 of EDTA. Three other strains (3.8%) also produced siderophores but in lower amounts than ESA 599.
Seventy-five maize strains (94.9%) and A. brasilense Ab-V5 did not produce detectable amounts of siderophores. In the assay with the soil-borne pathogen Fusarium verticillioides, a total of 36 new maize strains (45.6%) were antagonists against the fungus. Seven (8.9%) and 29 (36.7%) bacterial strains were isolated in BMGM semi-solid and solid Dyg’s medium, respectively. Regarding nifH amplification, all strains isolated via the semi-solid medium approach allowed amplification of the target gene. Two (3.1%) out of 65 strains isolated in solid medium were positive for nifH in the PCRs. The complete analysis of the plant growth promotion traits is shown in Table S2.
In the PCA, the two principal components made up 51.35% of the total variance. The bacteria were separated by the approach used to isolate them (Fig. 1). The bacteria obtained in BMGM semi-solid medium were related to the nifH amplification, production of siderophores, auxins with tryptophan, and calcium phosphate solubilization. The bacteria obtained from the solid Dyg’s medium were related to auxin production in absence of L-tryptophan and antagonism against Fusarium verticillioides.
Biplot of principal component analysis of the plant growth promotion traits of 79 bacteria obtained from roots and stems of maize applying different methodological approaches. PC1 and PC2 are the principal components 1 and 2, respectively. Colors: red symbols – semi-solid BMGM medium, black symbols – solid Dyg’s medium; Symbols: circles – roots, squares: stems: Filling: filled symbols – BRS Caatingueiro, open symbols: BRS Gorutuba
3.2 Selected strains promoted the growth of maize in pots
Inoculation using the bacterial isolates promoted the growth of maize in the pot experiment. Inoculation with nine new maize bacteria (six and three obtained in BMGM semi-solid and Dyg’s solid media, respectively) and the inoculation with Azospirillum brasilense Ab-V5, increased shoot dry mass in maize. These treatments led to a larger increase in shoot dry weight than the inoculation with 18 other bacterial strains (Table 2). Compared with the controls, inoculation with ten new maize isolates (seven from semi-solid and three from solid media), and Ab-V5, improved maize root development, which was comparable to that in the N fertilization treatment.
No differences were observed to the N content in the shoots (mg N g plant−1) between inoculated and control maize plants in the pot experiment. However, the total accumulation of N in the shoots (mg N plant−1) was improved by the inoculation of efficient bacteria and N fertilization. The inoculation of semi-solid borne bacteria ESA 599, ESA 601, ESA 599, and ESA 598, and the solid medium isolates ESA 593 and ESA 594, and Ab-V5 accumulated the total amount of N between 101.50 and 190.00 mg N plant−1, higher than the absolute control and the other 22 inoculation treatment. The N fertilized control treatment resulted in 311.20 mg N plant−1, the highest success for the total N accumulation variable.
Regarding shoot and root dry mass, and total N accumulation in the shoots, the inoculation of bacteria obtained in the semi-solid medium was more beneficial than isolates from solid medium (Fig. 2). Compared to the negative control treatment, the inoculation of maize with the selected bacterial isolates from this study and with Ab-V5 resulted in improved plant performance for all features assessed.
Box-plot/whiskers for the shoot and the root dry mass, N content and accumulation in the shoots of potted maize inoculated with the plant growth-promoting bacteria obtained in semi-solid BMGM medium (SS BMGM, n = 44), solid Dyg’s medium (S Dyg’s, n = 64), Azospirillum brasilense Ab-V5 (Ab-V5, n = 4), non-inoculated and fertilized with urea (N Control, n = 4) and non-inoculated and non-fertilized (Abs Control, n = 4). Upper and lower boxes are the third and first quartiles, respectively. The upper and lower whiskers are 1.5 interquartile ranges. The horizontal solid line is the data median
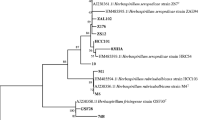
3.3 The outstanding strains belong to the phylum Firmicutes
Based on their 16S rRNA gene sequences, the nine strain were classified in the phylum Firmicutes (Fig. 3). Strains ESA 599 and ESA 600 isolated in semi-solid BMGM medium were related to Bacillus pumilus ATCC 7061T. In the same genetic cluster, strain ESA 593, obtained in solid Dyg’s medium, was closely related to Bacillus zhangzhouensis DW5-4T. Strain ESA 597 from semi-solid BMGM medium was genetically related to Bacillus nealsonii DSM 15011T. ESA 595 from solid medium was related to Bacillus aryabhatthai B8W22T.
Neighbor-Joining phylogenetic tree (Jukes-Cantor model) based on partial sequences 16S rRNA gene (1306 nucleotides) of 9 maize plant-growth-promoting bacteria and related type strains. Numbers in the nodes of branches are the bootstrap values (%) with 1000 pseudoreplication (values <60% are not shown)
The strain ESA 594 was classified as Staphylococcus, close related to S. edaphicus P5085T. Two strains were classified in the Brevibacillus genus. Strain ESA 596 was close to Brevibacillus formosus DSM 9885T, and ESA 598 was related to Brevibacillus gelatini PDF4T. ESA 601, also derived from BMGM semi-solid medium, was close to Paenibacillus massiliensis 2301065T.
3.4 Four Bacillus and a Paenibacillus boosted maize grain yield
The field assay showed some selected strains’ ability to promote maize yield. Plants inoculated with the strains ESA 593, ESA 599, ESA 597, ESA 600 (Bacillus spp.), ESA 601 (Paenibacillus sp.), and Ab-V5 (Azospirillum brasilense) had grain yields ranging from 4601 to 5532 kg ha−1, higher than that observed in the plants with 45 kg N ha−1 (12–35%) and in the absolute control treatment (56–87%) (Table 3). The inoculation with these bacteria also increased grain productivity compared to the non-inoculated treatment and the other four new maize strains. The fertilization with 90 kg N ha−1 was the best treatment and led to a grain yield of 7810 kg ha−1.
4 Discussion
The isolation of bacteria using two different approaches resulted in a culture collection containing strains displaying different features and occupying different ecological niches. Most of the strains obtained in this study were isolated in solid Dyg’s medium. The N-free semi-solid media approach aimed at obtaining putative N2-fixing bacteria and has been successfully applied worldwide (Baldani et al. 2014). On the other hand, isolation of bacteria in the non-selective solid medium yields heterotrophic bacteria, which may or may not display plant-growth promoting abilities. For example, Fernandes Júnior et al. (2013) isolated 998 heterotrophic bacteria from the roots of Oryza glumaepatula in Dyg’s medium. Among those bacteria, only 38 were diazotrophic and displayed other mechanisms of plant growth promotion. The value of combining two or more approaches to obtain PGPB was confirmed by the results observed in this study.
Variability was observed in the present study for the plant growth-promoting mechanisms among the 79 strains. Detectable amounts of auxin were produced by all bacterial strains in the culture medium supplied with L-Trp, highlighting the ability of those putative diazotrophs (those from semi-solid medium) to produce plant hormones in culture media as previously reported (Kuss et al. 2007; Silva et al. 2013; Antunes et al. 2019). In the absence of L-Trp, the bacterial strains also produced detectable amounts of auxin. However, under these conditions, those obtained in Dyg’s medium were the best auxin producers, as indicated by the PCA analysis. Altogether, several strains produced auxin, both with and without L-Trp supply. L-Trp is the primary precursor of the auxins, but several other metabolic pathways are known (Costacurta and Vanderleyden 1995).
Since L-Trp is not abundant in the apoplastic space, producing auxins within plant tissues and promoting plant growth without L-Trp is a desirable trait in PGPB. Poaceae-associated bacteria from the Brazilian semiarid region produced auxins both with and without L-Trp supply in the medium and showed potential to promote plant growth (da Silva et al. 2018; Antunes et al. 2019). These findings reinforce the notion that the production of auxin in vitro via different metabolic pathways is related to plant growth promotion in vivo. It is important to realize that Dyg’s is a rich medium (1.5 g L−1 of peptone). Therefore, an amount of L-trp should be available after autoclaving. However, the large difference between the average amount of auxin produced by several strains in the culture media with and without L-Trp, support the notion that different metabolic pathways were being used in the media with and without the amino acid.
All bacteria obtained in the BMGM semi-solid medium were positive in nifH amplification. This approach is the principal method used to isolate putative diazotrophic bacteria worldwide (Baldani et al. 2014). The absence of the nifH gene in almost all bacteria obtained from Dyg’s solid medium corroborates the previous results where few bacteria obtained in solid media were diazotrophs (Stoltzfus et al. 1997; Fernandes Júnior et al. 2013). Most of the bacteria showing antagonism against the maize soil-borne pathogen Fusarium verticillioides were isolated in solid Dyg’s medium. The in vitro antagonism against Fusarium verticillioides is an essential trait for selecting the efficient biocontrol agents against Fusarium wilt disease (Cavaglieri et al. 2005; Figueroa-López et al. 2016). As observed in the case of auxin production, applying different approaches for maize-associated bacteria isolation is a promising strategy for accessing microbes with different ecological niches and plant growth-promoting abilities.
Few bacteria were positive to calcium phosphate solubilization and siderophore production, in contrast to sorghum (da Silva et al. 2018; Antunes et al. 2019) buffel grass (Cenchrus ciliaris), and Urochloa spp. (Antunes et al. 2019) which were carried out in the same region of the present study. These findings demonstrate the host’s role in selecting their associated bacteria that have an actual or potential associative role. Cowpeas were growing in the same experimental field as used in the present study and cowpea-rhizobia were found infecting maize tissues. Those cowpea isolates from maize were also low calcium phosphate solubilizers and siderophore producers (Cavalcanti et al. 2020).
Siderophore-producing bacteria do not colonize much the inner tissues of maize’s shoots and roots, but these bacteria are abundant in the rhizosphere (Singh et al. 2015). Phosphorus solubilizing bacteria are also more abundant in the rhizosphere than in the inner tissues (Oliveira et al. 2009). The present study results indicate that maize endophytic bacteria are efficient in direct plant growth promotion via hormone biosynthesis. Indirect plant growth-promoting mechanisms, such as nutrient release, are rare in maize endophytes, however they are commonly found in rhizospheric bacteria (Compant et al. 2010).
Among the bacterial strains selected for the potted-maize assay, ESA 599 showed several promising metabolic features. This strain isolated in semi-solid medium from BRS Caatingueiro maize stems was the best auxin producer in medium with L-Trp and produced high amounts of auxin in L-Trp free medium. This bacterium also was a phosphate solubilizer, the best siderophore producer, and nifH positive. Bacteria displaying several mechanisms that promote plant growth are sought for prospective PGPB, as they increase the probability of promoting plant growth in vivo (de Souza et al. 2015; Gopalakrishnan et al. 2015; Grover et al. 2021).
The bacterial strains obtained in the semi-solid medium were more efficient than those isolated in Dyg’s solid medium at promoting plant growth in potted maize plants. However, the findings reinforce the value of using more than one approach for isolating effective PGPB for use in drylands (Gopalakrishnan et al. 2015; Antunes et al. 2019). All selected strains were very efficient for two or more parameters (plant dry mass and nitrogen accumulation in the shoots, for example) and had their 16S rRNA gene sequenced and were assessed in the field trial.
The nine initially seleced nine strains were classified within the phylum Firmicutes, order Bacillales. This group encompasses several plant growth promoters that have been isolated from different dryland sites worldwide (Grover et al. 2011; Kavamura et al. 2013; Fernandes-Júnior et al. 2015; Gopalakrishnan et al. 2015; Aeron et al. 2020; Bonatelli et al. 2021; Grover et al. 2021). Three diazotrophic strains were classified within the Paenibacillaceae family, two strains were identified as Brevibacillus, and one as Paenibacillus. In the screening for diazotrophic PGPB in several crops, such as sugarcane (Saccharum spp.) (Ratón et al. 2012), sorghum (Sorghum bicolor) (Mareque et al. 2015), and maize (Chakra et al. 2019), Brevibacillus and Paenibacillus are commonly isolated. However, few strains of these two genera appear to have the same potential of plant-growth promotion as those observed by Paenibacillus sp. ESA 601 and Brevibacillus sp. ESA 596, isolated in the present study.
Paenibacillus sp. ESA 601 showed multiple plant growth promotion traits in vitro and was efficient in promoting maize growth. These features are shared by other elite Panibacillus strains (Grady et al. 2016; Liu et al. 2018, 2019), indicating that this genus contains strong candidates for the application as maize inoculants. The strains ESA 598 and ESA 596 were classified in the Brevibacillus genus and related to Brevibacillus formosus/brevis and Brevibacilus gelatini, respectively. Efficient PGPB related to Brevibacillus brevis has also been isolated in several other screening projects (Mareque et al. 2015; Nehra et al. 2016; Chakra et al. 2019). However, strains related to Brevibacillus gelatini are not usually isolated in the same survey, indicating new ecological niches for the strains isolated from maize in the present study.
Bacillus and Staphylococcus were two other genera isolated in the present study. Both members of the Bacillaceae were previously reported as plant-associated bacteria. Bacillus strains are usually obtained in isolation projects in drylands, probably due to their ability to form endospores and resist various environmental conditions (Ratón et al. 2012). Staphylococcus is an etiological agent of several human and animal diseases. Plant-associated Staphylococcus strains have been isolated using solid and semi-solid media (Mareque et al. 2015; Akram et al. 2016). Our Staphylococcus sp. ESA 594 was related to Staphylococcus edaphicus P5085T. This soil bacterium was isolated in Antarctica, showing its ability to adapt to extreme environments (Pantůček et al. 2018). The genetic relationships and the plant growth promotion results observed in the present study indicate that Staphylococcus sp. ESA 594 is a PGPB highly resistant to harsh environmental conditions.
In the Brazilian drylands, Bacillus spp. have already been isolated from several crops (Andrade et al. 2014; Antunes et al. 2019) and native species (Kavamura et al. 2013; Fernandes-Júnior et al. 2015; Santos et al. 2020). Besides promoting plant growth, some of these strains have already proven to act as drought-stress alleviating agents (Kavamura et al. 2013; Santana et al. 2020; Santos et al. 2020), corroborating the biotechnological potential of the strains isolated in this study.
Under field conditions, five out of the nine strains acted as efficient inoculants for promoting maize yield. Paenibacillus sp. ESA 601, Bacillus spp. ESA 599, ESA 600, ESA 597, and ESA 593 were as efficient as the commercial maize inoculant containing Azospirillm brasilense Ab-V5. The inoculation with these five elite strains resulted in grain yield higher than those observed in the experimental treatment with 50% of the N application in the field, indicating that these isolates can contribute more than 50% of the plant N demand, this is an excellent performance for Poaceae inoculants (Santi et al. 2013).
Bacillus spp. can promote the growth and yield of crops using several of the mechanisms assessed in this study (Ríos-Ruiz et al. 2020), but Paenibacillus, despite results of pot experiments (Marra et al. 2012; Mareque et al. 2015; Ambrosini et al. 2016), has not been tested under field conditions (Grady et al. 2016). The present study reinforces the value of Bacillus as an inoculant and indicates the potential of Paenibacillus strains from soils of the Brazilian semiarid region as inoculants for maize fields.
The commercial inoculant Azospirillum brasilense Ab-V5 is a very efficient plant growth promoter with positive results for maize and other Poaceae in the whole of Brazil (Hungria et al. 2010, 2016; da Costa Leite et al. 2019). However, under our field assay conditions, the five elite strains from the present study performed as well as the reference strain Ab-V5. The field performance of these five new elite maize strains could be considered excellent, and due to their adaptability to the dry conditions of our region, they should perform better than Ab-V5 in, for example, the semiarid rain-fed conditions of the northeastern region of Brazil. New network trials should now be conducted in different parts of this region to ensure that our selected strains Paenibacillus sp. ESA 601, and the Bacillus spp. ESA 599, ESA 600, ESA 597, and ESA 593 have viability as maize inoculants and have multi-field efficiency.
References
Aeron A, Khare E, Kumar C et al (2020) Revisiting the plant growth - promoting rhizobacteria : lessons from the past and objectives for the future. Arch Microbiol 202:665–676. https://doi.org/10.1007/s00203-019-01779-w
Akram MS, Shahid M, Tariq M, Azeem M, Javed MT, Saleem S, Riaz S (2016) Deciphering Staphylococcus sciuri SAT-17 mediated anti-oxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.). Front Microbiol 7:1–14. https://doi.org/10.3389/fmicb.2016.00867
Alves GC, de Matos Macedo AV, dos Reis FB et al (2016) Plant growth promotion by four species of the genus Burkhoderia. Plant Soil 399:373–387. https://doi.org/10.1007/s11104-015-2701-4
Alves GC, Sobral LF, Reis VM (2020) Grain yield of maize inoculated with diazotrophic bacteria with the applicationof nitrogen fertilizer. Rev Caatinga 33:644–652. https://doi.org/10.1590/1983-21252020v33n307rc
Ambrosini A, Stefanski T, Lisboa BB, Beneduzi A, Vargas LK, Passaglia LMP (2016) Diazotrophic bacilli isolated from the sunflower rhizosphere and the potential of Bacillus mycoides B38V as biofertiliser. Ann Appl Biol 168:93–110. https://doi.org/10.1111/aab.12245
Andrade LF, de Souza GLOD, Nietsche S, Xavier AA, Costa MR, Cardoso AMS, Pereira MCT, Pereira DFGS (2014) Analysis of the abilities of endophytic bacteria associated with banana tree roots to promote plant growth. J Microbiol 52:27–34. https://doi.org/10.1007/s12275-014-3019-2
Antunes GR, Santana SRA, Escobar IEC et al (2019) Associative diazotrophic bacteria from forage grasses in the Brazilian semiarid region are effective plant growth promoters. Crop Pasture Sci 70:899–907. https://doi.org/10.1071/CP19076
Arruda L, Beneduzi A, Martins A, Lisboa B, Lopes C, Bertolo F, Passaglia LMP, Vargas LK (2013) Screening of rhizobacteria isolated from maize (Zea mays L.) in Rio Grande do Sul state (South Brazil) and analysis of their potential to improve plant growth. Appl Soil Ecol 63:15–22. https://doi.org/10.1016/j.apsoil.2012.09.001
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Berraquero FR, Baya B, Cormenzana AR (1976) Establecimiento de índices para el estudio de la solubilización de fosfatos por bacterias del suelo. Ars Pharm 17:399–406
Bodhankar S, Grover M, Hemanth S et al (2017) Maize seed endophytic bacteria: dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 biotech 7:232. https://doi.org/10.1007/s13205-017-0860-0
Bomfim CSG, da Silva VB, Cursino LHS, Mattos WS, Santos JCS, de Souza LSB, Dantas BF, de Freitas ADS, Fernandes-Júnior PI (2020) Endophytic bacteria naturally inhabiting commercial maize seeds occupy different niches and are efficient plant-growth promoting agents. Symbiosis 83:255–269. https://doi.org/10.1007/s13199-020-00701-z
Bonatelli ML, Lacerda Júnior GV, dos Reis Junior FB et al (2021) Beneficial plant-associated microorganisms from semiarid and seasonally dry environments: a review. Front Microbiol 12:553223. https://doi.org/10.3389/fmicb.2020.55322
Cavaglieri L, Orlando J, Rodríguez MI, Chulze S, Etcheverry M (2005) Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res Microbiol 156:748–754. https://doi.org/10.1016/j.resmic.2005.03.001
Cavalcanti MIP, Nascimento R de C, Rodrigues DR et al (2020) Maize growth and yield promoting endophytes isolated into a legume root nodule by a cross-over approach. Rhizosphere 15:100211. https://doi.org/10.1016/j.rhisph.2020.100211
Cavite HJM, Mactal AG, Evangelista EV, Cruz JA (2020) Growth and yield response of upland rice to application of plant growth-promoting rhizobacteria. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10114-3
Chakra PS, Kumar PGV, Swamy CT (2019) Isolation and biochemical characterization of plant growth promoting bacteria from a maize crop field. Int J Curr Microbiol Appl Sci 8:1415–1422
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. https://doi.org/10.1016/j.soilbio.2009.11.024
CONAB (2019) Séries históricas. https://www.conab.gov.br/conteudos.php?a=1252&t=&Pagina_objcmsconteudos=3#A_objcmsconteudos. Accessed 9 Jun 2017
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol 21:1–18. https://doi.org/10.3109/10408419509113531
da Costa Leite R, dos Santos JGD, Silva EL et al (2019) Productivity increase, reduction of nitrogen fertiliser use and drought-stress mitigation by inoculation of Marandu grass (Urochloa brizantha) with Azospirillum brasilense. Crop Pasture Sci 70:61–67
da Silva Santos J, de Oliveira Viana T, de Jesus CM et al (2015) Inoculation and isolation of plant growth-promoting bacteria in maize grown in Vitória da Conquista, Bahia, Brazil. Rev Bras Cienc do Solo 39:78–85. https://doi.org/10.1590/01000683rbcs20150725
da Silva JF, da Silva TR, Escobar IEC, Fraiz ACR, dos Santos JWM, do Nascimento TR, dos Santos JMR, Peters SJW, de Melo RF, Signor D, Fernandes-Júnior PI (2018) Screening of plant growth promotion ability among bacteria isolated from field-grown sorghum under different managements in Brazilian drylands. World J Microbiol Biotechnol 34:186. https://doi.org/10.1007/s11274-018-2568-7
de Souza R, Ambrosini A, Passaglia LMP (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38:401–419. https://doi.org/10.1590/S1415-475738420150053
Dias A, Pacheco RS, dos Santos SG et al (2014) Screening of fluorescent rhizobacteria for the biocontrol of soilborne plant pathogenic fungi. Caatinga 27:1–9
Döbereiner J, Baldani VLD, Baldani JI (1995) Como isolar e identificar bactérias diazotróficas de plantas não-leguminosas. Embrapa Agrobiologia, Seropédica
Estrada-De Los Santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl Environ Microbiol 67:2790–2798. https://doi.org/10.1128/AEM.67.6.2790-2798.2001
Felestrino ÉB, Vieira IT, Caneschi WL, Cordeiro IF, Assis RAB, Lemes CGC, Fonseca NP, Sanchez AB, Cepeda JCC, Ferro JA, Garcia CCM, do Carmo FF, Kamino LHY, Moreira LM (2018) Biotechnological potential of plant growth-promoting bacteria from the roots and rhizospheres of endemic plants in ironstone vegetation in southeastern Brazil. World J Microbiol Biotechnol 34:156. https://doi.org/10.1007/s11274-018-2538-0
Fernandes Júnior PI, Pereira GMD, Perin L et al (2013) Diazotrophic bacteria isolated from wild rice Oryza glumaepatula (Poaceae) in the Brazilian Amazon. Rev Biol Trop 61:991–999
Fernandes-Júnior PI, de Tarso Aidar S, Morgante CV et al (2015) The resurrection plant Tripogon spicatus (Poaceae) harbors a diversity of plant growth promoting bacteria in northeastern Brazilian Caatinga. Rev Bras Cienc do Solo 39:993–1002. https://doi.org/10.1590/01000683rbcs20140646
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Cienc e Agrotecnologia 35:1039–1042
Figueroa-López AM, Cordero-Ramírez JD, Martínez-Álvarez JC, López-Meyer M, Lizárraga-Sánchez GJ, Félix-Gastélum R, Castro-Martínez C, Maldonado-Mendoza IE (2016) Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. Springerplus 5:330. https://doi.org/10.1186/s40064-016-1780-x
Gopalakrishnan S, Sathya A, Vijayabharathi R, et al (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5
Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC (2016) Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Factories 15:1–18. https://doi.org/10.1186/s12934-016-0603-7
Grover M, Ali SZ, Sandhya V, Rasul A, Venkateswarlu B (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol 27:1231–1240. https://doi.org/10.1007/s11274-010-0572-7
Grover M, Madhubala R, Ali SZ, Yadav SK, Venkateswarlu B (2014) Influence of Bacillus spp. strains on seedling growth and physiological parameters of sorghum under moisture stress conditions. J Basic Microbiol 54:951–961. https://doi.org/10.1002/jobm.201300250
Grover M, Bodhankar S, Sharma A et al (2021) PGPR mediated alterations in root traits: way toward sustainable prop production. Frontiers in Sustainable Food Systems 4:1–28. https://doi.org/10.3389/fsufs.2020.618230
Haiyambo DH, Chimwamurombe PM, Reinhold-Hurek B (2015a) Isolation and screening of rhizosphere bacteria from grasses in East Kavango region of Namibia for plant growth promoting characteristics. Curr Microbiol 71:566–571. https://doi.org/10.1007/s00284-015-0886-7
Haiyambo DH, Reinhold-Hurek B, Chimwamurombe PM (2015b) Effects of plant growth promoting bacterial isolates from Kavango on the vegetative growth of Sorghum bicolor. African J Microbiol Res 9:725–729. https://doi.org/10.5897/AJMR2014.7205
Hammer Ø, Harper DAT, Ryan PD et al (2011) PaSt: paleontological statistics software package for education and data analysis. Palaentologia Electron 4:5–7. https://doi.org/10.1016/j.bcp.2008.05.025
Hungria M, Campo RJ, Souza EM, Pedrosa FO (2010) Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 331:413–425. https://doi.org/10.1007/s11104-009-0262-0
Hungria M, Nogueira MA, Araujo RS (2016) Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: an environment-friendly component in the reclamation of degraded pastures in the tropics. Agric Ecosyst Environ 221:125–131. https://doi.org/10.1016/j.agee.2016.01.024
IBGE (2018) Brazilian Semi-Arid Region. In: Reg. Maps. https://www.ibge.gov.br/en/geosciences/maps/regional-maps/19380-brazilian-semi-arid.html?=&t=o-que-e. Accessed 27 Apr 2020
Ikeda AC, Savi DC, Hungria M, Kava V (2020) Bioprospecting of elite plant growth-promoting bacteria for the maize crop. Acta Sci - Agron 42:e44364. https://doi.org/10.4025/actasciagron.v42i1.44364
Kavamura VN, Santos SN, da Silva JL et al (2013) Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol Res 168:183–191. https://doi.org/10.1016/j.micres.2012.12.002
Kumar V, Kayasth M, Chaudhary V, Gera R (2014) Diversity of diazotrophs in arid and semi-arid regions of Haryana and evaluation of their plant growth promoting potential on Bt-cotton and pearl millet. Ann Microbiol 64:1301–1313. https://doi.org/10.1007/s13213-013-0774-y
Kuss AV, Kuss VV, Lovato T, Flôres L (2007) Fixação de nitrogênio e produção de ácido indolacético in vitro por bactérias diazotróficas endofíticas. Pesqui Agropecu Bras 42:1459–1465
Lima JVL, Weber OB, Correia D, Soares MA, Senabio JA (2015) Endophytic bacteria in cacti native to a Brazilian semi-arid region. Plant Soil 389:25–33. https://doi.org/10.1007/s11104-014-2344-x
Liu K, He L, Li S, Tian F, Sun Z, Li C (2018) Draft genome sequence of Paenibacillus strain LK1, a phytohormone producing bacterium. 3. Biotech 8:9–11. https://doi.org/10.1007/s13205-017-1042-9
Liu X, Li Q, Li Y, Guan G, Chen S (2019) Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. PeerJ 2019:e7445. https://doi.org/10.7717/peerj.7445
Mareque C, Taulé C, Beracochea M, Battistoni F (2015) Isolation, characterization and plant growth promotion effects of putative bacterial endophytes associated with sweet sorghum (Sorghum bicolor (L) Moench). Ann Microbiol 65:1057–1067. https://doi.org/10.1007/s13213-014-0951-7
Marra LM, Soares CRSF, Oliveira SM et al (2012) Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant Soil 357:289–307. https://doi.org/10.1007/s11104-012-1157-z
Nehra V, Saharan BS, Choudhary M (2016) Evaluation of Brevibacillus brevis as a potential plant growth promoting rhizobacteria for cotton (Gossypium hirsutum) crop. Springerplus 5:948. https://doi.org/10.1186/s40064-016-2584-8
Niu X, Song L, Xiao Y, Ge W (2018) Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid and their potential in alleviating drought stress. Front Microbiol 8:1–11. https://doi.org/10.3389/fmicb.2017.02580
Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, Carneiro NP, Guimarães CT, Schaffert RE, Sá NMH (2009) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado biome. Soil Biol Biochem 41:1782–1787. https://doi.org/10.1016/j.soilbio.2008.01.012
Pantůček R, Sedláček I, Indráková A, Vrbovská V, Mašlaňová I, Kovařovic V, Švec P, Králová S, Krištofová L, Kekláková J, Petráš P, Doškař J (2018) Staphylococcus edaphicus sp. nov., isolated in Antarctica, harbors the mecC gene and genomic islands with a suspected role in adaptation to extreme environments. Appl Environ Microbiol 84:e01746–e01717. https://doi.org/10.1128/AEM.01746-17
Pedrinho EAN, Galdiano RF, Campanharo JC et al (2010) Identificação E Avaliação De Rizobactérias Isoladas De Raízes De Milho. Bragantia 69:905–912. https://doi.org/10.1590/s0006-87052010000400017
Poly F, Monrozier LJ, Bally R (2001) Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol 152:95–103. https://doi.org/10.1016/S0923-2508(00)01172-4
Ratón T de los MO, Yano R, Gámez OR et al (2012) Isolation and characterisation of aerobic endospore forming bacilli from sugarcane rhizosphere for the selection of strains with agriculture potentialities. World J Microbiol Biotechnol 28:1593–1603. https://doi.org/10.1007/s11274-011-0965-2
Ribeiro CM, Cardoso EJBN (2012) Isolation, selection and characterization of root-associated growth promoting bacteria in Brazil pine (Araucaria angustifolia). Microbiol Res 167:69–78. https://doi.org/10.1016/j.micres.2011.03.003
Ríos-Ruiz WF, Torres-Chávez EE, Torres-Delgado J et al (2020) Inoculation of bacterial consortium increases rice yield (Oryza sativa L.) reducing applications of nitrogen fertilizer in San Martin region, Peru. Rhizosphere 14:100200. https://doi.org/10.1016/j.rhisph.2020.100200
Rodrigues Neto J, Malavolta VA Jr, Victor O (1986) Meio simples para o isolamento e cultivo de Xanthomonas campestris pv. citri tipo B. Summa Phytopathol 12:32
Santana SRA, Voltolini TV, Antunes G dos R, et al (2020) Inoculation of plant growth-promoting bacteria attenuates the negative effects of drought on sorghum. Arch Microbiol 202:1015–1024. https://doi.org/10.1007/s00203-020-01810-5
Santi C, Bogusz D, Franche C (2013) Biological nitrogen fixation in non-legume plants. Ann Bot 111:743–767
Santos AFJ, Morais JS, Miranda JS et al (2020) Cacti-associated rhizobacteria from Brazilian Caatinga biome induce maize growth promotion and alleviate abiotic stress. Revista Brasileira de Ciências Agrárias - Brazilian Journal of Agricultural Sciences 15(3):15:e8221. https://doi.org/10.5039/agraria.v15i3a8221
Sarwar M, Kremer RJ (1995) Determination of bacterially derived auxins using a microplate method. Lett Appl Microbiol 20:282–285. https://doi.org/10.1111/j.1472-765X.1995.tb00446.x
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Silva MCP, Figueiredo AF, Andreote FD, Cardoso EJBN (2013) Plant growth promoting bacteria in Brachiaria brizantha. World J Microbiol Biotechnol 29:163–171. https://doi.org/10.1007/s11274-012-1169-0
Singh NP, Singh RK, Meena VS, Meena RK (2015) Can we use maize (Zea mays) rhizobacteria as plant growth promoter? Vegetos 28:86–99. https://doi.org/10.5958/2229-4473.2015.00012.9
Stoltzfus JR, So R, Malarvithi PP, Ladha JK, de Bruijn FJ (1997) Isolation of endophytic bacteria from rice and assessment of their potential for supplying rice with biologically fixed nitrogen. Plant Soil 194:25–36. https://doi.org/10.1023/A:1004298921641
Sylvester-Bradley R, Asakawa N, La Torraca S et al (1982) Levantamento quantitativo de microrganismos solubilizadores de fosfatos na rizosfera de gramíneas e leguminosas forrageiras na Amazônia. Acta Amaz 12:15–22
Teixeira PC, Donagemma GK, Fontana A, Teixeira WG (eds) (2017) Manual de métodos de análise de solo, 3rd edn. Brasilia, Empresa Brasileira de Pesquisa Agropecuária
Tinna D, Garg N, Sharma S, Pandove G, Chawla N (2020) Utilization of plant growth promoting rhizobacteria as root dipping of seedlings for improving bulb yield and curtailing mineral fertilizer use in onion under field conditions. Sci Hortic (Amsterdam) 109432:109432. https://doi.org/10.1016/j.scienta.2020.109432
Wang H, Qi M, Cutler AJ (1993) A simple method of preparing plant samples for PCR. Nucleic Acids Res 21:4153–4154
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
Acknowledgments
We thank the Brazilian Council for Scientific and Technological Development (CNPq 485168/2013–8), INCT—Plant Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq/Fundação Araucária STI/CAPES INCT-MPCPAgro 465133/2014-4) and the Brazilian Agricultural Research Corporation (Embrapa 23.13.08.003.00.00) for financial support. We also thank the Coordination of Improvement of Higher Education Personnel (CAPES) for awarding scholarships to the first four authors (Code 001). The sixth and last authors are research fellows of CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts/competing interest
The authors declare that they have no conflict of interest. The funding agencies did not influence the data acquisition and its analyses.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nascimento, R.d., Cavalcanti, M.I.P., Correia, A.d. et al. Maize-associated bacteria from the Brazilian semiarid region boost plant growth and grain yield. Symbiosis 83, 347–359 (2021). https://doi.org/10.1007/s13199-021-00755-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-021-00755-7