Abstract
Alphaproteobacteria of the genus Wolbachia are common intracellular endosymbionts of a variety of insects. Their successful spread over a vast range of host taxa is often attributed to selective advantages conferred by the bacteria to infected individuals. Among the known diversity of Wolbachia pipientis infecting Drosophila melanogaster, a single genotype, wMel, within the wMel strain has been found to dominate over other genotypes world-wide. Genotyping of D. melanogaster wild populations from Ukraine reveals a relatively high frequency of the wMel genotype, although 31 % flies from an Uman’ population are infected with the rare genotype wMelCS. We demonstrate that wMelCS-infected females have lower fecundity compared to wMel-infected flies, which might be the cause of wMel prevalence in D. melanogaster populations. We report no difference in the bacterial transmission rate between these two bacterial genotypes. However, we observed an association between transmission fidelity of Wolbachia and genotype of D. melanogaster indicating that Wolbachia-host relationships in this case are more complex. Furthermore our study reveals fluctuations in Wolbachia infection rates in wMel-infected populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Alphaproteobacteria of the genus Wolbachia are wide-spread maternally-inherited intracellular endosymbionts of a wide range of arthropods (O’Neill et al. 1997; Duron et al. 2008). In particular, they are known to infect up to two thirds of all known insect taxa (Hilgenboecker et al. 2008). This tremendous success is generally explained by a number of phenotypic effects in the host conferred by the bacteria, the most notable of which being various reproductive manipulations conferring a reproductive advantage to infected individuals (Werren et al. 2008). Other selective advantages of being infected with Wolbachia have been shown in various host species, such as elevated resistance to insecticides (Duron et al. 2006), enhanced tolerance to viral infections (Hedges et al. 2008; Teixeira et al. 2008), and increased survival (Fry and Rand 2002) to number a few. From this perspective, Wolbachia might to be a valuable asset in the evolution of their hosts. Yet, while these effects are well known in general, their manifestation appears to significantly vary among different host species, Wolbachia strains, and host genotypes (Fry et al. 2004).
This variation is particularly evident among Drosophila species infected with Wolbachia pipientis which strains are host species-specific. Among the reproductive manipulations, infected D. simulans demonstrate notable cytoplasmic incompatibility with uninfected individuals, resulting in higher overall production of offspring by infected individuals (Turelli and Hoffmann 1995). In D. bifasciata and D. innibula the infection leads to male killing (Hurst et al. 2000; Dyer and Jaenike 2004). Meanwhile, no clear effects on reproduction have been reported from D. santomea, D. yakuba, D. teissieri, D. mauritiana (Hurst and Jiggins 2005). The infection frequencies also vary, ranging from 90–100 % in D. simulans (Ballard 2004) to 30 % and 33 % in D. innibula (Unckless and Jaenike 2011) and D. bifasciata (Hurst et al. 2000), respectively. Even within one species, such as D. melanogaster, infected with one bacterial strain (wMel), the proportion of infected individuals may range from as high as 40–60 % to as low as 8 % in populations from different regions (Solignac et al. 1994; Verspoor and Haddrill 2011). Meanwhile, strongly manifested reproductive effects of Wolbachia are considerably less frequent in D. melanogaster (Belousov and Kozeretskaia 2011; but see Yamada et al. 2007; Reynolds and Hoffmann 2002; Hoffmann et al. 1994, 1998). Therefore the wide variance of the infection rates across populations calls into question possible selective benefits conferred by the bacteria beyond reproductive manipulations. A number of possible mechanisms behind the selective benefits conferred by the bacteria infection have been reported (Fry et al. 2004; Harcombe and Hoffmann 2004), however these mechanisms differed across drosophila strains. Understanding of the mechanisms governing the infection success of Wolbachia is partially impeded by the scarcity of field data on infection frequencies in natural populations (Serga and Kozeretskaya 2014). A number of studies on D. melanogaster populations have revealed significantly dissimilar infection rates across populations with apparently no clear-cut patterns (Solignac et al. 1994; Hoffmann et al. 1998; Ilinsky and Zakharov 2007; Verspoor and Haddrill 2011).
One approach to address the variation in infection rates in the case of D. melanogaster is to look at the bacterial diversity within the strain wMel, supposing that some variants of this strain may be more advantageous to the host compared to the others. Genotyping of the strain wMel by polymorphic markers has revealed two distinct groups of genotypes—wMel proper and wMelCS (Riegler et al. 2005). While the genotype wMelCS is reported to be dominant in isofemale fly lines established in the first half of the 20th century, wMel seems to have replaced it in most parts of the world in a matter of decades during the early second half of the century. Whole genome sequencing (Richardson et al. 2012; Early and Clark, 2013) from different natural populations of D. melanogaster, however, suggests that this replacement occurred much earlier, approximately several thousand years ago, and as yet is not complete in many D. melanogaster populations (Richardson et al. 2012; Ilinsky 2013). Within the “recent replacement” model, this global expansion of the wMel genotype is believed to have coincided with a similar global expansion of one D. melanogaster mtDNA haplotype (haplotype 2) which is associated with the wMel genotype (Nunes et al. 2008). One explanation for such frequent co-occurrence could be that the wMel genotype of Wolbachia confers some selective advantage to the infected host thus promoting the expansion of the latter (Nunes et al. 2008). Yet this hypothesis has not been confirmed.
Still, there is a tangible lack of infection frequency studies for particular genotypes, as studies concentrate around infection frequencies or genotype analysis but not both.
In the present study, we combined these two approaches and analyzed the infection rates for each studied genotype in natural populations of D. melanogaster from Ukraine. We were primarily interested in estimating the frequencies of different Wolbachia genotypes and known Drosophila mtDNA haplotypes in the studied populations to ask if there was any association between the two, as well as in testing if different bacteria genotypes had different transmission efficiencies and fitness impacts on the host.
We report high and stable Wolbachia infection rates in several geographically separated Ukrainian populations of D. melanogaster. Our study reveals a vast predominance of the genotype wMel over the only other minor genotype found, wMelCS, and domination of the mtDNA haplotypes 2 and 10, both observations being consistent with reports published from other regions. Our results suggest that different Wolbachia genotypes may confer differential fecundity in the infected host females, which we hypothesize to be one of factors contributing to differential dispersal of the bacteria in D. melanogaster populations worldwide.
2 Materials and methods
2.1 Flies and Wolbachia detection
Flies were collected at fruit processing facilities and in apple orchards in late August to early September in 2006 through 2012 at 14 locations in Ukraine: Lubny (50° 0’58.89”N–32°59’59.88”E), Uman’ (48°45’45.26”N–30°14’38.97”E), Varva (50°29’33.30”N–32°42’50.93”E), Yalta (44°29’36.91”N–34°9’19.06”E), Chornobyl (51°16’13.73”N–30°13’19.63”E), Kyiv (50°21’9.06”N–30°28’57.70”E), Kharkiv (49°59’24.30”N–36°13’50.44”E), Drogobych (49°21’0.00”N–23°30’0.00”E), Odesa (46°29’13.91”N–30°43’51.59”E), Pyriatyn (50°19’35.40”N–32°29’35.62”E), Poliske (51°14’9.49”N–29°23’51.60”E), Chornobyl Nuclear Power Plant (NPP) (51°22’30.01”N–30°8’21.33”E), Chornobyl Red Forest (51°23’19.66”N–30°4’33.88”E), Motovylivka (50°10’25.07”N–30°5’53.12”E). At each location, flies were collected in the same place each year. In Uman’, three collection points (Uman’, Uman’1 and Uman’2) were designed each placed 5–10 km from the rest for a more detailed genotyping of Wolbachia from this region. In Kyiv and Odesa locations flies were collected every 1–2 months throughout the drosophila activity season (June-September). Sample sizes in Odesa: June 31 isofemale lines, August 23, September 23; Kyiv: July 31; August 28; September 18. Sample sizes through 6 consecutive years from Kyiv location were 2007-10, 2009-30, 2011-20 isofemale lines.
Wolbachia infection was detected by PCR of the pooled DNA from 25 first generation progeny of 25 wild-caught flies using primers specific to the 16S rRNA (O’Neill et al. 1992) and wolbachia surface protein (wsp) genes (Zhou et al. 1998). Identity of the obtained amplicons was confirmed by sequencing. For infection rate analysis DNA was extracted from F1 progeny of each wild female separately. The infection rate was assessed by the proportion of infected females in natural population. The infection rates were compared by Clopper-Pearson’s method and Fischer’s exact test.
2.2 Identification of Wolbachia genotypes and Drosophila mtDNA haplotypes
Wolbachia genotyping was done by PCR based on the number of the minisatellite repeats VNTR-141, VNTR-105 and the presence of the insertion sequence IS5 in the loci WD0516/7 and WD1310 of the Wolbachia genome as described in Riegler et al. (2005).
Fly mtDNA haplotypes were determined by sequencing the cytochrome oxidase subunit I (COI) gene as described in Nunes et al. (2008). We determined the mtDNA haplotypes of 56 isofemale lines from Uman’, 4 from Kharkiv, Poliske, Yalta, and Chornobyl; 6 from the NNP site. Three isofemale lines were taken from each of the rest collection sites.
2.3 Transmission fidelity
Wolbachia transmission fidelity from one fly generation to the next was tested as the proportion of infected F1 females obtained from a single parental female. To score level of Wolbachia transmission three fertilized females from isofemale lines of Uman’ 59, Uman’ 16 (that infected with wMel) and Uman’ 22, Uman’ 26 (that infected with wMelCS) sampled in 2012 were placed in separate tubes. All females (n = 15–20) of the first generation were placed in fresh tubes to obtain progeny, which were analyzed by PCR for the presence of Wolbachia. Statistical analysis was conducted using the two-way ANOVA.
2.4 Fecundity
We tested fecundity of 20 females from Uman’ population infected with each bacteria genotype by keeping them individually on colored with red beet juice media for 22 h and counting the number of eggs laid by each female as described elsewhere. In this experiment we used mass-cultured females derived from the natural population of Uman’ (collected in 2012) and analyzed three cohorts: non-infected, wMel-infected, and wMelCS-infected cultures. To generate non-infected controls genetically identical to the infected groups we kept the infected cultures on a medium with pre-added 0.25 mg/ml tetracycline for two generations, after which the flies were transferred to a normal medium and were cultivated for 2 more generations on it (Poinsot and Mercot 1997; Dobson et al. 2002). Non-infected flies served as control for a possible effect of tetracycline. All the females from the corresponding lines were collected and aged at 25 °C for 5 days. Statistical analysis was conducted using the t-criterion.
3 Results
3.1 Wolbachia in natural populations of D. melanogaster from Ukraine
We found Wolbachia in most of the studied populations, except for the location near the Chornobyl NPP from which the infection was absent during the first three years of study (2006–2008) and then appeared in 2009. Most of the populations demonstrated a stable presence of the infection throughout the study period.
The obtained 16S rRNA and wsp gene fragments were sequenced and found to be identical in all of the studied populations (GenBank accession numbers: HM627277–HM627283 and HM775086–HM775092). Based on the sequence of the wsp gene (as described in Zhou et al. 1998), we determined that all of the populations were infected with the Wolbachia strain wMel (GenBank accession: FJ403330).
We monitored the temporal dynamics of infection rates in Odesa and Kyiv populations throughout the reproductive season. Also we analyzed infection rates in the Kyiv population through 6 consecutive years. We did not observe any significant changes neither throughout the breeding season in Odesa (June-58 ± 9 % (SE of proportion); August-57 ± 10 %; September-43.5 ± 10 %) (Fischer’s exact test p = 0.5731) and Kyiv (July-81 ± 7 %; August-75 ± 8 %; September-78 ± 10 %) (Fischer’s exact test p = 0.8838) nor over 6 consecutive years in Kyiv (2007-80 ± 13 %; 2009-67 ± 5 %; 2011-72 ± 10 %; 2012-78 ± 10 %) (Fischer’s exact test p = 0.8406).
Table 1 demonstrates the infection rates at other locations analyzed, revealing relatively high infection frequencies, ranging from 43 to 78 %, in all of the studied populations. Yet, we did detect significant differences in infection rates in some populations between two successive years of collection using the Fischer’s exact test, such as in Uman’ (F = 3.97, p < 0.05) and Varva (F = 2.98, p < 0.05). Therefore, the infection rates in the studied populations are high and mostly stable both throughout the breeding season and from year to year, despite the fact that the populations undergo a diapause period during the winter and their maximum population size is only reached by late summer.
3.2 Wolbachia genotypes and Drosophila mtDNA haplotypes
Based on our genotyping results, Fig. 1 demonstrates a vast predominance of the genotype wMel in Ukrainian populations. Only Uman’ and Varva populations were found to also harbor another genotype, wMelCS. Notably, these were the two populations which infection rates oscillated from year to year. In one Uman’ population sampled in 2011, we observed an extremely high percentage of the rare genotype wMelCS with higher total infection rate, whereas 2012 samples demonstrate both lower rates and lower proportion of wMelCS, raising a suspicion that this genotype might be more prone to yearly oscillation. Alternatively, the detected oscillation could be due to the relatively small sample size used. Two other populations sampled from Uman’, however, lacked wMelCS and were only infected with wMel.
Distribution of Wolbachia genotypes and D. melanogaster mtDNA haplotypes across studied populations in 2011 (a) and 2012 (b). The relative frequencies of the two studied genotypes of Wolbachia pipientis, wMel and wMelCS are shown as piecharts alongside with numbers indicating D. melanogaster mtDNA haplotypes found in each population (haplotypes are numbered according to Nunes et al. 2008)
We determined the mtDNA haplotype of a total of 102 isofemales across the studied populations (Table 2) and found haplotypes 2 (31.37 %), 10 (57.84 %) and 1 (9.8 %). Due to the limited number of isofemales tested per population, we were not able to estimate haplotype frequencies in each population. However, given the vast dominance of haplotypes 2 and 10 in the total sample, these two haplotypes are likely to prevail in Ukrainian populations of D. melanogaster, which is consistent with other reports from European populations (Nunes et al. 2008). Interestingly, one population from Uman’ which we studied in more detail demonstrated relatively substantial presence of haplotype 1 (35.7 %). This population has been previously reported to contain this haplotype (Ilinsky and Zakharov 2007), and this population might be assumed to represent a minority of locations where this haplotype has yet not been completely displaced by the presumably invasive haplotypes 2 and 10. However this question needs further investigation.
In line with the Nunes et al. (2008) study, Wolbachia infection was not randomly distributed among haplotypes (homogeneity test, P < 0.05), with haplotype 1 flies being more frequently infected with wMelCS.
4 Transmission fidelity
Differential transmission fidelity could potentially stay behind the unequal spread of different Wolbachia genotypes and partial association of some genotypes with D. melanogaster mtDNA haplotypes. We measured the transmission fidelity in 12 infected females, yielding the overall loss of bacteria around 3.97 % (n = 227 progeny; 95 % CI = 1.91–7.73 %), which is consistent with previous records by Hoffmann et al. (1998) from Australian natural populations (2.9 %; 95 % CI: 0.8–5.9 %). We did not detect any differences in transmission rates between the bacterial wMel and wMelCS genotypes (ANOVA, p = 0.66), however Drosophila genotypes seemed to differ in transmission fidelity significantly (ANOVA, p = 0.034).
5 Fecundity
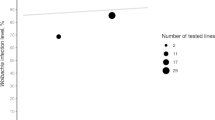
We further asked if flies infected with different Wolbachia genotypes had different fecundity, which is another hypothetic factor that might have contributed to the unequal population success of the genotypes. Flies infected with wMel demonstrated a significantly higher number of produced eggs (17.59 ± 1.96) per female compared with those infected with the genotype wMelCS (8.65 ± 1.24; t = 8.94, p < 0.05; Fig. 2), which suggests that wMel can confer some fitness advantage to its infected host by elevating the host’s fecundity. Interestingly, after antibiotic treatment flies infected with wMel laid significantly fewer eggs (10.83 ± 1.4; t = 2.81, p < 0.05), while those infected with wMelCS (11.92 ± 1.5; t = 1.67, p > 0.05) did not show any significant response to the treatment. In the control non-infected fly line, the number of the eggs laid after antibiotic treatment (12.5 ± 1.3) did not differ from values before treatment (11.9 ± 1.55), indicating no effect of antibiotic therapy on this trait.
Mean number of laid eggs per female by flies infected with wMel, wMelCS, and non-infected controls. All the three studied groups are compared against tetracycline treated controls to eliminate infection in the infected groups and control for possible effects of tetracycline using the uninfected group. Error bars represent SE
6 Discussion
Consistent with previous reports from other locations (Solignac et al. 1994; Riegler et al. 2005; Nunes et al. 2008; Verspoor and Haddrill 2011), we found Wolbachia to be rather widespread in Ukrainian populations of D. melanogaster, with high and mostly stable infection rates both from year to year and throughout the breeding season. This result, still, contrasts with records from Australian populations, which do not undergo the period of diapause, with highly variable infection rates throughout the season (Hoffmann et al. 1998). Therefore, diapause, by significantly reducing population numbers, might act to stabilize the infection rate at least throughout the following season. However, this hypothesis has not been confirmed.
The domination of the wMel genotype we found in Ukrainian populations is also consistent with reports from other parts of the globe and agrees with Riegler et al. (2005) hypothesis of successive displacement of genotypes, such as wMelCS and wMelCS2, by wMel. Still, remnants of more ancient genotypes, such as wMelCS or perhaps others we did not detect, are still present in Ukraine. wMelCS2 has also been detected in Uman’ populations (Ilinsky and Zakharov 2007) 10 km away from one of our collection sites, but its frequency was very low. Simultaneous presence of wMelCS and/or wMelCS2 and/or wMel in one population has been reported to frequently happen in populations from Altay and Asia (Ilinsky and Zakharov 2007). The unexpectedly high frequency of this genotype in Uman’ populations is not typical for Europe, and possibly reflects a peculiar feature of this population, being either an isolated island harboring this genotype or representing some eastern border of the wMel domination range (Riegler et al. 2005).
The two bacterial genotypes we detected apparently do not differ by population infection rates, even when a population harbors a mix of the two, which is consistent with reports from Altay populations (Ilinsky and Zakharov 2007). However, populations hosting both genotypes were the only in our study to demonstrate yearly fluctuations of the infection rates.
The vast prevalence of two hypothetically invasive D. melanogaster mtDNA haplotypes, 2 and 10 (Nunes et al. 2008), along with the dominance of wMel, indicates that Ukraine has recently been invaded by non-aboriginal flies, presumably from western Europe, which brought these new haplotypes and bacterial genotypes and outcompeted the local populations. Rare islands of haplotype 1 are likely to be the remnants of those earlier populations. Some gradient, therefore, should be expected in the frequencies of these haplotypes across Ukraine with a decline in haplotype 2 east/southwards, as this haplotype is supposed to have spread before haplotype 10 based on data by Nunes et al. (2008). Indeed, in the westernmost population from Drogobych we sampled, only haplotype 2 was found, while one population from Odesa was only represented by haplotype 10 (Fig. 1). However, this hypothetical gradient requires more thorough sampling to be confirmed.
The Wolbachia genotype wMel appears to be equally represented in both haplotypes, which is also consistent with data by Nunes et al. (2008), while the presumably the more ancient haplotype 1 tends to be found in flies infected with wMelCS, supporting thus the Nunes et al. (2008) hypothesis that mtDNA haplotypes and Wolbachia genotypes are non-randomly distributed among D. melanogaster populations, putatively due to non-neutral effects of bacterial genotypes on the host flies or vice versa. Our results suggest that Wolbachia of particular genotype are capable of conferring some fitness advantage to the infected hosts by increasing the host’s fecundity. However, the relationship determining the success of particular genotypes is perhaps more complicated, as we found fly genotype to be associated with different transmission fidelities of the bacteria, which may suggest that successful drosophila strains in nature could promote the success of bacterial genotypes that have happened to infect them.
A number of studies have addressed Wolbachia fitness effects on its D. melanogaster hosts (e.g. Hoffmann et al. 1994, 1998; Fry et al. 2004) without bacteria genotyping, but the results are contradicting. One clue to the reasons of this contradiction might lie in genotype-specific effects, such as the differential fecundity we observed.
References
Ballard JWO (2004) Sequential evolution of a symbiont inferred from the host: Wolbachia and Drosophila simulans. Mol Biol Evol 21(3):428–442
Belousov AO, Kozeretskaia IA (2011) Symbiotic bacteria, which modify reproduction processes of Drosophila melanogaster. Mikrobiol Z 73(2):43–52
Dobson SL, Fox CW, Jiggins FM (2002) The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc Biol Sci 269:437–445. doi:10.1098/rspb.2001.1876
Duron O, Labbe P, Berticat C, Rousset F, Guillot S et al (2006) High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60:303–314
Duron O, Bouchon D, Boutin S et al (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6:27. doi:10.1186/1741-7007-6-27
Dyer KA, Jaenike J (2004) Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: molecular evidence from the host and parasite genomes. Genetics 168(3):1443–1455
Early AM, Clark AG (2013) Monophyly of Wolbachia pipientis genomes within Drosophila melanogaster: geographic structuring, titre variation and host effects across five populations. Mol Ecol 22(23):5765–5778
Fry AJ, Rand DM (2002) Wolbachia interactions that determine Drosophila melanogaster survival. Evolution 56:1976–1981
Fry AJ, Palmer MR, Rand DM (2004) Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 93:379–389
Harcombe W, Hoffmann AA (2004) Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J Invertebr Pathol 87:45–50
Hedges LM, Brownlie JC, O’Neill SL, Johnson KN (2008) Wolbachia and virus protection ininsects. Science 322:702
Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol Lett 281:215–220
Hoffmann AA, Clancy DJ, Merton E (1994) Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136:993–999
Hoffmann AA, Hercus M, Dagher H (1998) Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148:221
Hurst GD, Jiggins FM (2005) Problems with mitochondrial DNA as a marker in population inherited symbionts phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc R Soc Lond Ser B 272:1525–1534
Hurst GDD, Johnson AP, Schulenburg JHG, Fuyama Y (2000) Male-killing Wolbachia in Drosophila: a temperature sensitive trait with a threshold bacterial density. Genetics 156:699–709
Ilinsky Y (2013) Coevolution of Drosophila melanogaster mtDNA and Wolbachia genotypes. PLoS ONE 8:e54373. doi:10.1371/journal.pone.0054373
Ilinsky YY, Zakharov IK (2007) The endosymbiont Wolbachia in Eurasian populations of Drosophila melanogaster. Russ J Genet 43(7):748–756
Nunes M, Nolte V, Schlötterer C (2008) Non-random Wolbachia infection status of Drosophila melanogaster strains with different mtDNA haplotypes. Mol Biol Evol 25(11):2493–2498
O’Neill SL, Giordano R, Colbert AME et al (1992) 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA 89:2699–2702
O’Neill SL, Hoffmann AA, Werren JH (1997) Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford
Poinsot D, Mercot H (1997) Wolbachia infection in Drosophila simulans: does the female host bear a physiological cost? Evolution 51:180–186. doi:10.2307/2410971
Reynolds KT, Hoffmann AA (2002) Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet Res 80(2):79–87
Richardson MF, Weinert LA, Welch JJ, Linheiro RS, Magwire MM et al (2012) Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLoS Genet 8(12):e1003129
Riegler M, Sidhu M, Miller WJ, O’Neill SL (2005) Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol 15:1428–1433
Serga SV, Kozeretskaya IA (2014) The puzzle of Wolbachia spreading out through natural populations of Drosophila melanogaster. Biol Bull Rev 4(1):15–24
Solignac M, Vautrin D, Rousset F (1994) Widespread occurrence of the proteobacteria Wolbachia and partial cytoplasmic incompatibility in Drosophila melanogaster. C R Acad Sci 317:461–470
Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e2
Turelli M, Hoffmann AA (1995) Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140:1319–1338
Unckless RL, Jaenike J (2011) Maintenance of a male-killing Wolbachia in Drosophila innubila by male-killing dependent and male-killing independent mechanisms. Evolution 66:678–689
Verspoor RL, Haddrill PR (2011) Genetic diversity, population structure and Wolbachia infection status in a worldwide sample of Drosophila melanogaster and D. simulans populations. PLoS ONE 6(10):e26318
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nature reviews. Microbiol 6:741–751
Yamada R, Floate KD, Riegler M, O’Neill SL (2007) Male development time influences the strength of Wolbachia-induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics 177:801–808
Zhou W, Rousset F, O’Neill SL (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B Biol Sci 265:509–515
Acknowledgments
Authors thank Dr G. Milinevsky, the staff of Biology Department of Mechnikov National University of Odesa, and the staff of the National Institute of Viniculture and Wine Industry UAAS for their valuable help in material collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serga, S., Maistrenko, O., Rozhok, A. et al. Fecundity as one of possible factors contributing to the dominance of the wMel genotype of Wolbachia in natural populations of Drosophila melanogaster . Symbiosis 63, 11–17 (2014). https://doi.org/10.1007/s13199-014-0283-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-014-0283-1