Abstract
Wolbachia are intracellular symbionts of many species of animals, mostly arthropods. Vertical transmission of Wolbachia is exclusively maternal and this endobacterium promotes reproductive manipulations of its hosts, increasing the fitness of infected females. Moreover, Wolbachia provides its hosts with a wide range of adaptive features ranging from protection against viral infections to dietary niche occupancy. Therefore, Wolbachia can potentially contribute to the evolutionary processes of sexual selection and speciation. The horizontal transmission of Wolbachia is strongly suggested by the non-concordant phylogeny of this endosymbiont and that of its hosts. However, the ecological mechanism(s) responsible for endosymbiont transmission between different hosts is still largely unknown. In the present study, we look at ingestion as a possible natural form of Wolbachia horizontal transmission. To this aim, we tested cannibalism between infected and uninfected Drosophila hosts, under different conditions of nutrition and gut integrity. Although ingestion represents a general and incontestable portal of entry for microorganisms, we did not find infection by Wolbachia in the progeny of cannibal individuals fed on infected flies. Our study suggests that if ingestion is a vehicle for horizontal transmission of Wolbachia in nature, either it happens very rarely or it requires other factors or conditions to be effective. We discuss the likeliness of this mechanism with respect to the likelihood of each step necessary for horizontal transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The α-proteobacteria of the Genus Wolbachia live intracellularly in a variety of animals, including arthropods and nematodes (Werren 1997; Harris et al. 2010). In arthropods, Wolbachia is typically transmitted vertically from mother to offspring. It causes a wide range of reproductive manipulations in different host species whereby increasing the fitness of infected females and, consequently, also increasing its own transmission rate (Charlat et al. 2003). These mechanisms include: (i) the induction of cytoplasmic incompatibility between individuals that do not share infection status, (ii) the induction of parthenogenesis in diploid females and (iii) the feminization or death of infected males (for revision see Werren et al. (2008)). Additionally, recent studies have shown that in Drosophila melanogaster, Wolbachia infection may also confer an advantage to its host through an increased resistance to RNA virus infection (Hedges et al. 2008; Teixeira et al. 2008).
It is estimated that Wolbachia infects 20–80 % of insect species (Jeyaprakash and Hoy 2000) possibly making it the most recurrent endosymbiont on the planet. The wide distribution of these bacteria is attributed to the high efficacy of vertical transmission. This efficacy may rely on Wolbachia using the host’s cytoskeleton and intracellular transport system to migrate towards the germline precursors and ensure its presence inside future embryos (Ferree et al. 2005; Serbus and Sullivan 2007). In addition to the colonization of the germline during embryogenesis, Wolbachia remaining inside the embryo are internalized in progenitor cells of the somatic tissue (Frydman et al. 2006; Goto et al. 2006), with potential physiological and evolutionary consequences (Faria and Sucena 2013).
The widespread presence of Wolbachia must also rely on horizontal transmission, which can be attested by the presence of close strains of Wolbachia in phylogenetically distant hosts (Vavre et al. 1999; Baldo et al. 2008). Indeed, unlike mitochondria or obligatory bacterial endosymbionts, the molecular phylogeny of Wolbachia is not always concordant with that of its hosts (Werren and O’Neill 1997; Jiggins et al. 2002). These well-established patterns raise two important questions: i) which ecological conditions and mechanisms mediate horizontal transmission and ii) how does a transient horizontal transfer turn into a stable vertical transmission? Regarding this problem Frydman and colleagues reported that when haemolymph of an infected D. melanogaster fly is microinjected into adult uninfected females, Wolbachia could be transmitted vertically (Frydman et al. 2006). After 15 days upon haemolymph microinjection into uninfected female flies, Wolbachia could be detected in their offspring after preferentially establishing itself in the ovaries somatic stem cell niches. Also, it has been shown that Wolbachia is viable for several days outside the host’s cell, thus allowing for a possible transfer across cells (Rasgon et al. 2006). Together these reports provide a link between horizontal and vertical transmission, indicating that any mechanism capable of introducing Wolbachia into the female’s haemolymph may permit the establishment and perpetuation of Wolbachia in new hosts.
Despite their importance for understanding the epidemiological and evolutionary dynamics of Wolbachia infection, the ecological mechanisms responsible for the transfer of bacteria to new hosts in nature are still largely unknown (Haine et al. 2005). One strong candidate mechanism consists of parasitoid wasps acting as Wolbachia vectors. This is based on different evidence: i) the extensive similarities between Wolbachia strains found in parasitoids and their hosts (Vavre et al. 1999; Li et al. 2013); ii) Wolbachia can be transmitted to a parasitic wasp from its infected host (Heath et al. 1999; Morrow et al. 2014); iii) when infected and uninfected parasitoid wasp larvae share the same host egg, intra- and interspecific horizontal transfer of parthenogenesis-inducing Wolbachia may occur (Schilthuizen and Stouthamer 1997; Huigens et al. 2000; Huigens et al. 2004). Another hypothetical vector for horizontal transmission of Wolbachia are ectoparasitic mites, known to transfer the Drosophila endosymbiont, Spiroplasma poulsonni, from infected D. nebulosa to D. willistoni (Jaenike et al. 2007). Based on our observations of Drosophila larval and adult behaviour in crowded environments, we reasoned that cannibalism or scavenging, often witnessed not only in the laboratory but also in nature, could constitute a route for horizontal Wolbachia transfer. Moreover, occasional horizontal transmission via the oral route has been reported for the pea aphid Bemisia-like symbiont (Darby and Douglas 2003). Indeed, the digestive system is considered to be the major interface between the insect host and the microbial environment, constituting a privileged gateway for microorganism invasion (Douglas and Beard 1996). However, as most ingested bacteria are eliminated by the immune system or by peristalsis, few bacteria can persist in large numbers in the digestive tract of insects (Vallet-Gely et al. 2008). Nonetheless it is important to note that some bacterial species ensure their proliferation in recent hosts by passing through the digestive tract to other organs or cavities (Marsollier et al. 2005; Chiel et al. 2009).
Recent studies have demonstrated that, after predation of infected hosts, previously uninfected isopods, Armadillidium vulgare and Porcellio dilatatus dilatatus, would become infected with Wolbachia (Le Clec’h et al. 2013). Also, in the ant Acromyrmex echinatior, it has been hypothesized that the faecal-oral route could constitute a means for horizontal transmission of Wolbachia (Frost et al. 2014).
In this work, we have tested if upon ingestion Wolbachia could be transmitted stably to the offspring of a Drosophila host. For this, several ingestion experiments were performed using infected and uninfected hosts of D. melanogaster and D. simulans, at different developmental stages. Nutritional variation, dehydration and intestinal injury were used in an attempt to mimic naturally-occurring potentiating factors for the passage of Wolbachia into the body cavity of the fly and the subsequent establishment of a symbiotic relationship with the new host. Through a PCR-based analysis of the offspring we were unable to find any infection by Wolbachia, both in early and late progeny. This result suggests that the ingestion of Wolbachia by a non-infected new host is not sufficient in itself to establish a stable infection horizontally or is too rare to be detected within the limits of our experiment.
2 Materials and methods
2.1 Foundation and maintenance of drosophila outbred populations
Outbred populations of Drosophila melanogaster and Drosophila simulans were established in the laboratory (Martins et al. 2013). Wolbachia-infected D. melanogaster and D. simulans, collected from the southwest of Portugal (Azeitão) were used to establish two laboratory populations (MelO+ and SimO+, respectively). After over 50 generations in the laboratory, MelO+ and SimO+ were replicated for the establishment of four new populations: two infected with Wolbachia as the founding populations (mel+ and sim+) and two treated with tetracycline during four generations for total Wolbachia elimination (mel− and sim−). We confirmed the absence of Spiroplasma in all populations. For the Serratia assays, the D. simulans populations were established using two isofemale lines from the Drosophila Species Stock Centre (UC San Diego, California, US) sim+ (14,021–0251.138) and sim− (14,021–0251.01). All populations were kept in cages with an effective size between 1500 and 2000 individuals with non-overlapping generations, in a day/night cycle of 12 h, constant temperature of 25 °C, standard level of relative humidity (70 %) and fed on standard cornmeal-agar medium. The infection status of populations was monitored regularly through PCR (see below).
2.2 Wolbachia extraction
Wolbachia was extracted by crushing 100 infected adults or approximately 500 embryos of D. melanogaster or D. simulans, previously washed in 70 % ethanol, and transferred to 1 mL of ice-cold PBS (adapted from (Frydman et al. 2006)). For adult co-infected ingestion assays and adapting a protocol described previously (Rasgon et al. 2006), Wolbachia was extracted by smashing approximately 500 infected flies in 10 mL of Schneider’s medium. The confirmation of bacterial viability after extraction was also performed as described in Rasgon et al. (2006). In all cases, the homogenate was used entirely.
2.3 Adult ingestion assay
For ingestion experiments with adults, 4–7 day old females were used from the mel− population. From the regular stock of flies (which were maintained in rich medium), 20 replicates of 20 adult females were used to exclusively ingest 250 μL of a Wolbachia-containing suspension homogenized in PBS (from infected adults of mel+ populations) for a period of 48-h. These experiments were also undertaken with a previous 72-h treatment either with a poor medium (rich medium diluted 1:10 in water) or in a condition of starvation, where the females spent a 48-h period in total absence of nutritional resources until the beginning of the ingestion treatment.
2.4 Larval ingestion assay
For the ingestion experiments with larvae, we used mel− larvae from the three larval stages. Larvae ingested a homogenate, containing adults (or embryos), from mel+ or sim+ populations infected with Wolbachia for a period of 24 h. In each of the experiments, 5 replicates of 50 larvae were fed on 500 μL of homogenate from 40 flies.
2.5 Adult co-infected ingestion assays
For ingestion experiments with adults, 4–7 day old females were used from the mel− population. From the regular stock of flies, 10 adult females were used per replicate to exclusively ingest i) 250 μL of Serratia marcescens (a kind gift from B. Lemaitre) for a period of 24 h, or ii) 250 μL of a Wolbachia-containing suspension for a period of 24 h. The food solution containing Serratia was prepared from an overnight culture grown exponentially at 37 °C and was diluted with a sterile 50-mM sucrose solution to a final OD600 = 15. These experiments were also undertaken either with Wolbachia with a previous 24-h ingestion treatment with LB or with Serratia and posterior treatment with sim− and mel−.
2.6 Diagnostic PCR
In all procedures, tested females gave rise to the adult F1 from which genomic DNA was extracted (in pools of 10 adult females) and screened for Wolbachia infection by PCR through the amplification of a wsp gene fragment using primers wsp81F 5’TGG TCC AAT AAG TGA TGA AGA AAC 3′ and wsp691R 5’AAA AAT TAA ACG CTA CTC CA 3′ (Zhou et al. 1998). Wolbachia strains of D. melanogaster and D. simulans generate PCR amplicons of different sizes, 632 bp and 611 bp, respectively. This diagnostic PCR was further confirmed in 10 % of the samples chosen randomly by sequencing the respective PCR products.
3 Results and discussion
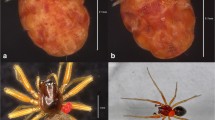
We fed D. melanogaster larvae and adults of the Wolbachia negative outbred population (mel−) with embryo or adult fly homogenates from Wolbachia infected populations of D. melanogaster (mel+) and D. simulans (sim+). As controls we applied the same procedures using homogenates from uninfected populations referred to as mel− and sim−. The status of Wolbachia infection of the populations used in these experiments is shown in Fig. 1A, also illustrating the size difference between wsp gene amplification products of Wolbachia strains from D. melanogaster and D. simulans. Confirmation of the different strains was obtained by sequencing the wsp gene fragment (Fig. 1B). These results validate our procedure for the simultaneous determination of the infection status and Wolbachia strain present in individual or pooled adult flies (as to ascertain instances of intra- or interspecific transmission). We tested the F1 of fed females at two time points: early F1 (8 to 10 days) and in late F1 (more than 15 days), determined by the description of Wolbachia dynamics upon entry into the haemolymph and subsequent stable establishment in the germline (Frydman et al. 2006). A representative gel of the PCR-based screen for Wolbachia infection is presented in Fig. 1C.
Screen for Wolbachia in the initial and tested populations. a Infection status in males and females of initial populations – F0; b Differentiation of Wolbachia strains of D.melanogaster and D.simulans by wsp gene sequencing; c Representative PCR for Wolbachia wsp gene in tested females progeny, indicating Wolbachia absence in F1 (10 replicates + controls)
Larval ingestion could lead to the stable transmission of Wolbachia by one of two ways: i) establishing itself in cells of somatic tissue, surviving the metamorphosis stage of the host and colonizing the ovaries of adult females, or ii) crossing the epithelium of the digestive system and colonizing the stem cells of the future ovary. We fed D. melanogaster larvae of different stages, previously maintained in normal medium, a homogenate of mel+ and sim+ infected embryos or adults for 24 h (Table 1). In a second set of experiments, we placed mel− adult flies on a diet composed of a mel+ adult homogenate for 48 h (Table 2 – A). If ingestion of Wolbachia occurs in the adult stage, it should be enough for a successful transmission that the endosymbiont crosses the midgut and passes to the haemolymph (Frydman et al. 2006). Yet, it should be stressed that it is unclear what is the necessary concentration of haemolymph Wolbachia for the establishment of these bacteria in the ovaries.
Both in the larvae and adult ingestion experiments, the early and late F1 flies tested did not show the presence of Wolbachia (Tables 1 and 2 – A, “Wol F1e and Wol F1l”). This negative result holds true even when varying the Wolbachia source, both D. melanogaster and D. simulans (intra- or interspecific), and the stage at which the Wolbachia homogenate was extracted, embryos or adults. Our findings indicate that if horizontal transmission by ingestion occurs in nature, within or between Drosophila species, it is a rare event.
Another aspect to consider is that our progeny analysis treats the whole putative process of infection as a binary outcome (F1 infected or non-infected) and cannot pinpoint the critical step at which the infection fails to progress. We may consider the absence of Wolbachia in the D. melanogaster F1 flies as the product of low probability events, each one necessary for the occurrence of horizontal transmission. We can formalize this idea through the equation:
where the probability of any horizontal transmission of Wolbachia (P HT ( W ) ) is equal to multiplying the probabilities of all the independent steps required for its occurrence: the environmental interaction between Wolbachia infected and non-infected individuals (PEI), here tested as ingestion; the access of Wolbachia to the haemolymph (PAH); the bacterial survival in the new host (PBS); the colonization of ovaries (POC); and the vertical transmission (PVT). Each of these steps can still be associated with a correction factor (α, β, γ, δ and ε) linked to specific ecological conditions.
Wolbachia ingestion by a non-infected new host is not in itself sufficient to establish a stable infection in Drosophila but specific ecological conditions may favour this process (here, formalized as α, β, γ, δ and ε). Indeed, there is ample evidence that several aspects of host life-history have a significant impact on the transmission of Wolbachia (McGraw and O’Neill 1999; Hurst et al. 2001; Mouton et al. 2007). Thus, we have manipulated some of these factors in order to favour horizontal transmission via ingestion, namely starvation and infection with a known natural bacterial pathogen. Interestingly, under nutritional restriction, the apoptotic region present in the ovaries (region 2a/2b of the germarium) (Drummond-Barbosa and Spradling 2001) overlaps with the region of Wolbachia entrance into the germinal tissue (Frydman et al. 2006), raising the hypothesis that the invasion of the germinal tissue by Wolbachia is opportunistic (δ). Additionally, the absence of nutritional resources in nature could also trigger an increase in cannibalism (α) and in bacterial infections due to the weakening of the host’s tissue barrier by cell death (β). With this aim, we placed mel− adult females, previously maintained in nutritionally poor medium or under starvation, on a diet composed of a mel+ adult homogenate for 48 h (Table 2 – B). Under these conditions we observed a total absence of Wolbachia in F1 tested females. Next, we used an oral infection model by previous infection with Serratia marcescens as an enhancer of secondary infection with ingested Wolbachia (β). Indeed, it has been shown that severe intestinal injury produced by S. marcescens promotes its crossing from the gut to the fly’s body cavity (Nehme et al. 2007). The subsequent ingestion of Wolbachia could follow the same route, increasing the probability of Wolbachia entry into the Drosophila haemolymph. In this experiment, adult females ingested a suspension of the entomobacterium S. marcescens and, subsequently, ingested Wolbachia extracted from infected adults of D. melanogaster and D. simulans (mel+ and sim+) (Table 3). Here, only the late progeny of female flies was analyzed and the percentage of female mortality three days after ingestion of S. marcescens is shown (Table 3 – “F0 Mortality”). Regardless of a previous exposure to injury stress, these females did not give rise to Wolbachia infected F1s, indicating the absence of Wolbachia transmission (Table 3 – “Wol F1l”). Despite the absence of Wolbachia in late progeny of tested females, this co-infection scenario presents itself as an excellent model to study the horizontal transmission of several endosymbionts to different potential new hosts. Indeed, recently it has been proposed that the ingestion of mushrooms could constitute the gateway for Wolbachia transmission between species (Stahlhut et al. 2010).
After an ingestion episode and once inside a potential new host, bacteria must endure the local defence deployed by the digestive system, such as low pH, the production of Reactive Oxygen Species (ROS) and the action of Anti-Microbial Peptides (AMPs). Insect parasitoids, mites or wounding can avoid this immune local challenge by providing a more direct path for bacteria to penetrate the body cavity of the new host. This route is not without danger as invading Wolbachia must survive the host melanization reaction triggered by injury. Finally, for Wolbachia to establish a viable horizontal infection once in the haemolymph (Frydman et al. 2006), it must overcome the systemic action of AMPs and phagocytosis by haemocytes. As a result, it is still unclear if the individual frequencies or efficiencies of each one of these potential mechanisms would be enough to explain all the evidence for horizontal transmission. An additional important element consists on the effects that ecological co-factors (such as those studied here: resource limitation and co-infection) have on Drosophila immune response translating into changes in the success of bacteria to invade and establish (γ) (Schneider 2009).
Thus, the mechanisms governing horizontal transmission of facultative endobacteria, particularly of Wolbachia, remain unknown. As mentioned above, insect parasitoids and parasitic mites may promote some of these symbiotic exchanges; however, other mechanisms that complete the puzzle of the pathways that facultative endobacterial species utilize to accomplish a new invasion, have yet to be explained. Although Wolbachia has been specializing throughout evolution in the vertical transmission strategy, we do not know the true horizontal transmission capacity of this endobacterium, a feature which is an ancestral characteristic of rickettsial bacteria and is still conserved in close related Genera (Anderson and Karr 2001). Therefore, it is essential to continue the study of the mechanisms responsible for horizontal transmission phenomena that, associated with several phenotypic and reproductive manipulations, may play an important role in the generation of the enormous diversity of arthropods (Faria and Sucena 2015).
References
Anderson CL, Karr TL (2001) Wolbachia: evolutionary novelty in a rickettsial bacteria. BMC Evol Biol 1:10
Baldo L, Ayoub NA, Hayashi CY, et al. (2008) Insight into the routes of Wolbachia invasion: high levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol Ecol 17:557–569. doi:10.1111/j.1365-294X.2007.03608.x
Charlat S, Hurst GD, Mercot H (2003) Evolutionary consequences of Wolbachia infections. Trends Genet 19:217–223
Chiel E, Zchori-Fein E, Inbar M, et al. (2009) Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS One 4:e4767
Darby AC, Douglas AE (2003) Elucidation of the transmission patterns of an insect-borne bacterium. Appl Environ Microbiol 69:4403–4407
Douglas AE, Beard CB (1996) Microbial symbiosis in the midgut of insects. Chapman and Hall, London
Drummond-Barbosa D, Spradling AC (2001) Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol 231:265–278. doi:10.1006/dbio.2000.0135
Faria VG, Sucena É (2013) Wolbachia in the malpighian tubules: evolutionary dead-end or adaptation? J Exp Zool B Mol Dev Evol 320:195–199. doi:10.1002/jez.b.22498
Faria VG, Sucena É (2015) Novel endosymbioses as a catalyst of fast speciation. In: Gontier N (ed) Reticulate evolution: symbiogenesis and lateral gene transfer. Springer, Dordrecht, Germany
Ferree PM, Frydman HM, Li JM, et al. (2005) Wolbachia utilizes host microtubules and dynein for anterior localization in the drosophila oocyte. PLoS Pathog 1:e14. doi:10.1371/journal.ppat.0010014
Frost CL, Pollock SW, Smith JE, Hughes WOH (2014) Wolbachia in the flesh: symbiont intensities in germ-line and somatic tissues challenge the conventional view of Wolbachia transmission routes. PLoS One 9:e95122. doi:10.1371/journal.pone.0095122
Frydman HM, Li JM, Robson DN, Wieschaus E (2006) Somatic stem cell niche tropism in Wolbachia. Nature 441:509–512. doi:10.1038/nature04756
Goto S, Anbutsu H, Fukatsu T (2006) Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol 72:4805–4810. doi:10.1128/AEM.00416-06
Haine ER, Pickup NJ, Cook JM (2005) Horizontal transmission of Wolbachia in a Drosophila community. Ecol Entomol 30:464–472
Harris HL, Brennan LJ, Keddie BA, Braig HR (2010) Bacterial symbionts in insects: balancing life and death. Symbiosis 51:37–53. doi:10.1007/s13199-010-0065-3
Heath BD, Butcher RD, Whitfield WG, Hubbard SF (1999) Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr Biol 9:313–316
Hedges LM, Brownlie JC, O’Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322:702
Huigens ME, Luck RF, Klaassen RH, et al. (2000) Infectious parthenogenesis. Nature 405:178–179. doi:10.1038/35012066
Huigens ME, de Almeida RP, Boons PA, et al. (2004) Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc Biol Sci 271:509–515. doi:10.1098/rspb.2003.2640
Hurst GD, Jiggins FM, Robinson SJ (2001) What causes inefficient transmission of male-killing Wolbachia in Drosophila? Heredity (Edinb) 87:220–226
Jaenike J, Polak M, Fiskin A, et al. (2007) Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol Lett 3:23–25
Jeyaprakash A, Hoy MA (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76 % of sixty-three arthropod species. Insect Mol Biol 9:393–405
Jiggins FM, Bentley JK, Majerus ME, Hurst GD (2002) Recent changes in phenotype and patterns of host specialization in Wolbachia bacteria. Mol Ecol 11:1275–1283
Le Clec’h W, Chevalier FD, Genty L, et al. (2013) Cannibalism and predation as paths for horizontal passage of Wolbachia between terrestrial isopods. PLoS One 8:e60232. doi:10.1371/journal.pone.0060232
Li J, Wang Z, Bourguet D, He K (2013) Wolbachia infection in populations of Ostrinia furnacalis: diversity, prevalence, phylogeny and evidence for horizontal transmission. J Integr Agric 12:283–295. doi:10.1016/S2095-3119(13)60227-0
Marsollier L, Aubry J, Coutanceau E, et al. (2005) Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell Microbiol 7:935–943. doi:10.1111/j.1462-5822.2005.00521.x
Martins NE, Faria VG, Teixeira L, et al. (2013) Host adaptation is contingent upon the infection route taken by pathogens. PLoS Pathog 9:e1003601. doi:10.1371/journal.ppat.1003601
McGraw EA, O’Neill SL (1999) Evolution of Wolbachia pipientis transmission dynamics in insects. Trends Microbiol 7:297–302. doi:10.1016/S0966-842X(99)01531-0
Morrow JL, Frommer M, Shearman DCA, Riegler M (2014) Tropical tephritid fruit fly community with high incidence of shared Wolbachia strains as platform for horizontal transmission of endosymbionts: horizontal transmission of Wolbachia. Environ Microbiol 16:3622–3637. doi:10.1111/1462-2920.12382
Mouton L, Henri H, Charif D, et al. (2007) Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett 3:210–213. doi:10.1098/rsbl.2006.0590
Nehme NT, Liegeois S, Kele B, et al. (2007) A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog 3:e173
Rasgon JL, Gamston CE, Ren X (2006) Survival of Wolbachia pipientis in cell-free medium. Appl Environ Microbiol 72:6934–6937
Schilthuizen M, Stouthamer R (1997) Horizontal transmission of parthenogenesis-inducing microbes in Trichogramma wasps. Proc Biol Sci 264:361–366. doi:10.1098/rspb.1997.0052
Schneider DS (2009) Physiological integration of innate immunity. In: Insect infection and immunity: evolution, ecology, and mechanisms. Oxford University Press, Oxford, pp. 106–118
Serbus LR, Sullivan W (2007) A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog 3:e190. doi:10.1371/journal.ppat.0030190
Stahlhut JK, Desjardins CA, Clark ME, et al. (2010) The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol Ecol 19:1940–1952. doi:10.1111/j.1365-294X.2010.04572.x
Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e2. doi:10.1371/journal.pbio.1000002
Vallet-Gely I, Lemaitre B, Boccard F (2008) Bacterial strategies to overcome insect defences. Nat Rev Microbiol 6:302–313
Vavre F, Fleury F, Lepetit D, et al. (1999) Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol Biol Evol 16:1711–1723
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42:587–609. doi:10.1146/annurev.ento.42.1.587
Werren JH, O’Neill SL (1997) The evolution of heritable symbionts. Oxford University Press, Oxford
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751
Zhou W, Rousset F, O’Neil S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci 265:509–515. doi:10.1098/rspb.1998.0324
Acknowledgments
The authors wish to thank Alexandre Leitão, Nelson Martins, Inês Trancoso and Luis Teixeira for discussions and Patrícia Beldade for the critical reading of the manuscript. Fundação para a Ciência e a Tecnologia (FCT), Portugal, supported this work (PPCDT/BIA-BDE/60950/2004) and VGF (#SFRH/BD/ 82299/2011). Tânia F. Paulo is a student of the Masters Programme in Evolutionary and Developmental Biology of Faculdade de Ciências da Universidade de Lisboa.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors have declared that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Faria, V.G., Paulo, T.F. & Sucena, É. Testing cannibalism as a mechanism for horizontal transmission of Wolbachia in Drosophila . Symbiosis 68, 79–85 (2016). https://doi.org/10.1007/s13199-015-0354-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-015-0354-y