Abstract
The use of phosphate solubilizing plant growth-promoting microorganisms as inoculants assists in the hydrolysis of insoluble forms of phosphorus leading to increased plant growth. Pseudomonas putida PCI2 was evaluated for phosphatase activity and solubilization of AlPO4 and FePO4. The effect of different incubation temperatures, concentrations of NaCl and different pH on growth of PCI2 and P solubilization was studied. PCI2 proved to be positive for phosphatase activity, solubilized AlPO4 and hydrolyzed Ca3(PO4)2 even in medium with 5 % NaCl. In addition, PCI2 produced 45 % units of siderophores. The production of IAA by PCI2 was stimulated in vitro by the addition of different concentrations of L-tryptophan to the culture medium. Assays with tomato seedlings showed that the length of the root was reduced as the concentration of IAA increased. On the other hand, inoculation with PCI2 caused a clear growth-promoting effect on shoot growth in the presence of L-tryptophan. P. putida PCI2 is adapted to different environmental conditions and has potential to be developed and used as an inoculant for increasing the growth of tomato plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tomato (Solanum lycopersicon) is one of the most important vegetable crops worldwide. Tomato plants are economically attractive because of their relatively rapid growth and high yields. Tomato fruits are rich in minerals, vitamins, essential amino acids, sugars and fibers (Naika et al. 2005). Fructose and glucose represent nearly 50 % of the dry matter and more than 95 % of the sugars contained in fruits. In addition, tomato fruits are an important source of vitamins A and C and contain high concentrations of iron (Fe) and phosphorus (P). In Argentina, 14,389 ha (6.34 % of the country’s surface) are used for production of tomato plants in the field (Corvo Dolcet 2003).
At the present time, millions of tonnes of chemical fertilizers are applied to soils worldwide to improve the growth of different crops; however, further increases in application of chemical fertilizers are unlikely to be effective at increasing yields (Son et al. 2006). In addition, the chemicalized agriculture subverts ecology, disrupts environments, degrades productivity of soils and mismanages water resources (Ayala and Prakasa Rao 2002).
Bacteria displaying high efficiency in improving plant development and increasing tolerance to pathogenic microorganisms have been designated as “plant growth-promoting rhizobacteria” (PGPR) (Kloepper and Schroth 1978). The use of PGPR offers an attractive means to replace or supplement chemical fertilizers. During the last two decades, the use of PGPR in agriculture has increased in several parts of the world. In fact, significant increases in the growth and yield of agronomically important crops in response to inoculation with PGPR have been repeatedly described (Asghar et al. 2002; Cassán et al. 2009; Chen et al. 1994; Figueiredo et al. 2008; Gray and Smith 2005; Okon and Labandera-Gonzalez 1994; Rosas et al. 2006). PGPR have been reported to directly enhance plant growth by a variety of mechanisms: fixation of atmospheric nitrogen, solubilization of minerals such as P, production of siderophores and synthesis of plant growth hormones such as indole-3-acetic acid (IAA) (Arshad and Frankenberger 1998; Van Loon 2007; Vessey 2003). Pseudomonas spp. are very versatile bacteria, found abundantly and ubiquitously in nature, and well adapted to several ecological niches due to rather simple nutritional requirements. The main saprophytic Pseudomonas species associated with plants include P. fluorescens, P. putida and P. aeruginosa, and certain strains from these species are known to be PGPR (Mercado-Blanco and Bakker 2007; Ramamoorthy et al. 2002). Strains of Pseudomonas have been shown to improve the growth of common bean (Ahmadzadeh and Tehrani 2009), sesame (Kumar et al. 2009), wheat and maize (Rosas et al. 2009), mung bean (Sharma and Johri 2003) and alfalfa (Yanes et al. 2012), among several other crops.
Although soils generally contain a large amount of total P, only a small proportion is immediately available for plant uptake. Most of the soil’s P is in insoluble forms and plants obtain this nutrient as orthophosphate anions (predominantly as HPO4 2− and H2PO4 −) from the soil solution (Richardson et al. 2009). In addition, it has been found that approximately 75–90 % of applied P chemical fertilizer is precipitated by calcium (Ca), Fe and aluminum (Al) metal cations (Banerjee et al. 2010). The phosphate solubilizing microorganisms provide an alternative biotechnological solution to cope with the P requirements of plants (Saharan and Nehra 2011). The phosphate solubilizing microorganisms are common in the rhizosphere and secretion of organic acids and phosphatases constitute common methods to facilitate the conversion of insoluble phosphates into forms available to plants (Kim et al. 1998). In addition, the potential of microorganisms for solubilizing P is directly related to the production of siderophores and phytohormones (Vassilev et al. 2006). Rodriguez and Fraga (1999) reviewed that strains from the genera Pseudomonas, Rhizobium and Bacillus are among the bacteria with the highest potential for converting insoluble compounds of phosphorus into available phosphates. A promoting effect on plant growth has been demonstrated with promising phosphate solubilizing bacteria in tomato (Adesemoye et al. 2009), wheat (Babana and Antoun 2006), barley (Canbolat et al. 2006), maize (Hameedaa et al. 2008) and raspberry (Orhan et al. 2006), among others. To our knowledge, there are no reports on the potential use of a Pseudomonas putida strain with the capacity to use both organic and mineral phosphate substrates as an inoculant for increasing the growth of tomato plants.
In a previous work, we demostrated that Pseudomonas putida PCI2 enhanced growth of tomato root system by over 50 %. In addition, P. putida PCI2 was found to solubilize tricalcium phosphate, to synthesize IAA and to produce siderophores (Pastor et al. 2010, 2012). The objectives of the present work were to determine the capacity of P. putida PCI2 to produce phosphatases and to solubilize AlPO4 and FePO4, to evaluate the effect of different environmental conditions on growth and phosphate solubilizing activity of PCI2, to examine the influence of iron on siderophore production by PCI2, to evaluate the effect of L-tryptophan, as a physiological precursor for biosynthesis of IAA, on IAA production in PCI2 and to test the effect of IAA and L-tryptophan on the growth of tomato seedlings inoculated with PCI2.
2 Materials and methods
2.1 Pseudomonas strains and culture media
Pseudomonas putida PCI2 (GenBank accession number GU004535) is a native strain isolated from tomato rhizosphere. Strain PCI2 exhibited inhibition of the fungal phytopathogen S. rolfsii (Pastor et al. 2010). P. putida SP22 (GenBank accession No. HM014234) belongs to the collection of our research group in the Universidad Nacional de Río Cuarto, Argentina. Strains were routinely grown at 28 °C on King’s B medium (King et al. 1954) and 30 % Tryptic Soy Agar (TSA) medium and stored at −20 °C in Tryptic Soy Broth (TSB) (Britania®) amended with 20 % (v/v) glycerol.
2.2 Assays of phosphatase activity and solubilization of inorganic phosphates
P. putida PCI2 was screened for phosphatase activity by inoculating on Sperber medium containing 50 mg/l 5-bromo-4-chloro-3-indolyl phosphate (BCIP, Sigma). Plates were incubated at 28 °C overnight and observed for the presence of blue-stained colonies. To compare the phosphate solubilization capabilities of strain PCI2 with control strain P. putida SP22, 25 μl of bacterial suspension diluted to 108 CFU/ml were spotted on the center of solid Sperber medium plate containing Ca3(PO4)2. The diameter of the clear zone around each colony was measured after 1, 2 and 3 days, in triplicate. Phosphatase activities in strains were compared based on scoring the intensities of blue-stained colonies after 1, 2 and 7 days in the presence of soluble phosphate (KH2PO4) or insoluble phosphate [Ca3(PO4)2] (Malboobi et al. 2009). Sperber medium without BCIP and replaced with 2.5 g of aluminum phosphate (AlPO4) or iron phosphate (FePO4) as the sole phosphorus source was used to measure AlPO4 and FePO4-solubilizing activities, respectively. Plate assays were performed for 5 days at 28 °C.
2.3 Quantitative estimation of phosphate solubilization
Time-coursed quantitative measurements were carried out according to Malboobi et al. (2009) in 50 ml Erlenmeyer flasks containing 15 ml of Sperber broth medium inoculated with ~104 CFU/ml of P. putida PCI2 and incubated at 30 °C and 120 rpm. The effect of temperature was examined by incubation at 40 °C. For high-salt treatments, an extra amount of NaCl (1, 2.5 and 5 %; w/v) was added to Sperber broth medium. The initial pH of the medium was adjusted by the addition of either KOH or HCl. Sampling was performed at 24 and 48 h and the growth was estimated by counting colonies on solid Sperber medium. In addition, Sperber broth medium replaced with 2.5 g of aluminum phosphate (AlPO4) or iron phosphate (FePO4) as the sole phosphorus source was used to measure AlPO4 and FePO4-solubilizing activities, respectively, at 30 °C. For AlPO4, sampling was performed until 144 h. Then, supernatants were obtained by centrifugation of cultures at 10,000 rpm for 10 min. The phosphorus in supernatants was estimated by the vanado-molybdate-yellow color method. Sterile uninoculated medium was used as control in all cases. To a 5 ml aliquot of the supernatant, 2.5 ml Barton’s reagent were added and volume was made to 50 ml with deionized water. The absorbance of the resultant color was read after 10 min at 430 nm in a spectrophotometer. The total soluble phosphorus was calculated from the regression equation of a standard curve. The values of soluble phosphate released were expressed as mg/L over control (Kumar et al. 2012).
2.4 Siderophore quantification in culture supernatant
Iron free succinate medium (SM) was used to inoculate a 24 h old culture of P. putida PCI2 at the proportion of 1 % (v/v) inoculum. It was incubated for 24 h at 30 °C with constant shaking at 120 rpm. Following incubation, the culture was centrifuged (10,000 rpm at 4 °C for 10 min) and the cell free supernatant was used to estimate the production of siderophores. One ml of culture supernatant was mixed with 1 ml of CAS reagent, and absorbance was measured at 630 nm against a reference consisting of 1 ml of uninoculated broth and 1 ml of CAS reagent. Siderophore content in the aliquot was calculated by using the following formula:
where, Ar = absorbance of reference at 630 nm (CAS reagent) and As = absorbance of sample at 630 nm. In order to determine the effect of iron on siderophore biosynthesis, P. putida PCI2 was grown in SM externally supplemented with 50 and 100 μM of iron (FeCl3 · 6H2O) (Sayyed et al. 2005). Experiments were repeated three times.
2.5 Effect of increasing concentrations of L-tryptophan on IAA production by P. putida PCI2
The production of IAA by P. putida PCI2 was evaluated by LC-ESI/MS-MS. Liquid cultures were prepared in 250 ml erlenmeyer flasks containing 100 ml of Luria Bertani broth medium (LB). The formulation of the culture medium was modified for IAA determination by increasing concentrations of L-tryptophan (Sigma-Aldrich) (0, 100, 200 and 500 μg/ml), from a filter-sterilized stock prepared in warm water. The flasks were inoculated with 104 CFU/ml of P. putida PCI2 and incubated for 72 h at 30 °C, under shaking (80 rpm). After incubation, 20 ml of bacterial culture were taken and centrifuged at 8,000 rpm in the cold (4 °C) for 15 min. Supernatants were acidified at pH 3.0 with acetic acid solution. Then, 100 ng of 2H5-IAA (OlChemIm, Czech Republic) deuterated internal standard were added and the supernatants were kept at 4 °C for 1 h. The samples were partitioned three times with 20 ml ethyl acetate. After the last partition, the ethyl acetate was evaporated to dryness at 35 °C. Then, the samples were resuspended in methanol prior to passing through a pre-purification Sephadex DEAE 25 column. The fractions (eluted IAA) containing the deuterated internal standard were evaporated to dryness and resuspended in vials containing 100 μl methanol. The vials were introduced into the autosampler of an Alliance 2695 liquid chromatographer (Waters Inc, USA) and 10 μl were injected. Chromatographic conditions were constant, with acetonitrile:water (65:35) at a flow rate of 0.2 ml/min, and the samples were further analyzed using a Quattro UltimaTM Mass Spectrometer (Micromass, UK). IAA identification was carried out by comparing the retention times of the samples with the pure standard, and quantification was performed using the MRM (Multiple Reaction Monitoring) function, following the 174/179 molecular masses and 130/135 transition masses, which correspond to endogenous/deuterated, respectively. For quantification, values were obtained from the calibration curve, previously designed using IAA pure standard (Sigma-Aldrich).
2.6 Effect of exogenous IAA on root development of tomato seeds inoculated with P. putida PCI2
The effect of IAA on root development of tomato seeds inoculated with P. putida PCI2 was performed according to Gravel et al. (2007). Tomato seeds were surface sterilized by soaking in 70 % ethanol for 5 min and subsequently in 2 % hypochloric acid for 1 min. Then, seeds were rinsed thoroughly three times with sterile distilled water (Gravel et al. 2007). Seeds were inoculated by soaking for 1 h in a suspension of P. putida PCI2 (4 × 107 CFU/ml) grown in 30 % TSB. Control seeds were soaked in sterile distilled water. Subsequently, four seeds were placed in individual growth pouches containing 20 ml of a sterile solution of 0, 0.01, 0.1, 1 or 10 μg/ml de IAA. Pouches, wrapped in aluminium foil, were placed in a growth chamber at 28 °C for 10 days after which the length of the initial root was measured for each seedling. The experimental design was a complete randomized design with five replicates. The experiment was repeated two times.
2.7 Effect of L-tryptophan on the growth of tomato seeds inoculated with P. putida PCI2
The effect of L-tryptophan on the growth of tomato seeds inoculated with P. putida PCI2 was performed according to Gravel et al. (2007). Tomato seeds were surface sterilized and inoculated with P. putida PCI2 as described above. Control seeds were soaked in sterile distilled water. Subsequently, four seeds were placed in individual growth pouches containing 20 ml of a sterile solution of 0, 0.25, 0.50 or 0.75 mM L-tryptophan. Pouches, wrapped in aluminium foil, were placed in a growth chamber at 28 °C for 15 days after which the following parameters were measured: shoot length, fresh weight of the shoot and fresh weight of the roots. The experimental design was a complete randomized design with five replicates. The experiment was repeated two times.
2.8 Statistical analyses
Results obtained in the experiments of effect of IAA and L-tryptophan on the growth of tomato seeds inoculated with PCI2 were analyzed by using analysis of variance (ANOVA). When ANOVA showed treatment effect, the Fisher’s least significant difference (LSD) test was applied to compare means at P < 0.05. Data from root fresh weight were analyzed for significance after square root transformation. All data were subjected to statistical analysis using Statgraphics plus software for Windows V 4.1 (Statistical Graphics, USA).
3 Results
3.1 Assays of phosphatase activity and solubilization of inorganic phosphates
P. putida PCI2 proved to be positive for phosphatase activity, developing a strong blue color. In the comparative assay, P. putida PCI2 produced a large clear zone (20.8 mm) in minimal medium containing Ca3(PO4)2, after 72 h of incubation. The largest clear zone was observed for control strain SP22 (23.6 mm). However, colonies from SP22 did not develop a blue color after 7 days of incubation (Table 1). On the other hand, neither PCI2 nor SP22 colonies exhibited a clear halo on agar plates supplemented with either AlPO4 or FePO4.
3.2 Quantitative estimation of phosphate solubilization
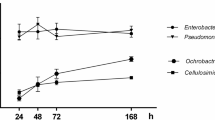
The effect of different growth conditions on survival of P. putida PCI2 and release of bound P from Ca by this strain in the Sperber broth medium were assessed. Growth of P. putida PCI2 was greatly reduced at 40 °C and, consequently, no solubilization of phosphate was observed under these conditions after 48 h of incubation. A high amount of solubilized P (94 mg/L) was recorded after 48 h of incubation in the control treatment (30 °C). Quantitative estimation of bacterial growth and phosphate solubilization in the presence of extra amounts of NaCl demonstrated that addition of 1 % NaCl did not affect the growth of P. putida PCI2. However, as salt concentration in the media increased, the bacterial growth decreased accordingly. On the other hand, the highest values of solubilized P (100 and 102 mg/L) after 24 h of incubation were obtained in media with 1 and 2.5 % added NaCl, respectively, whereas the highest values after 48 h were observed in media with 1, 2.5 and 5 % added NaCl. Results of bacterial growth in the presence of different pH demonstrated that although P. putida PCI2 was able to grow in Sperber broth medium with varying pH, the optimal pH for P. putida PCI2 in this growth medium was from 8 to 10. Phosphate solubilization results showed that soluble P was lowest at pH 5 (53 mg/L after 48 h of incubation) and absent at pH 4 (Fig. 1). Although P. putida PCI2 did not produce halos when cultured on agar plates supplemented with either AlPO4 or FePO4, it solubilized 16 mg/L of P over the appropriate control after 144 h of incubation in Sperber broth medium replaced with AlPO4 (Fig. 2b). Under these conditions, the bacterial concentration remained steady (Fig. 2a).
Effect of different conditions on growth (a, c and e) of P. putida PCI2 and P solubilization (b, d and f). a, b Temperatures (30 and 40 °C); c, d Concentrations of NaCl (0, 1, 2.5 and 5 %); e, f. Different pH (4, 5, 6, 7, 8, 9 and 10). PCI2 was grown in Sperber broth medium containing tricalcium phosphate. The values of soluble phosphate are expressed as mg/L over the appropriate control. All data points are the mean of three replicates. Standard deviations are represented by vertical bars
3.3 Siderophore quantification in culture supernatant
The development of a green coloured pigment in SM medium by P. putida PCI2 after 24 h of incubation indicated production of siderophores. PCI2 produced 45 % units of siderophores in this medium. In order to determine the level of iron at which siderophore production is repressed in P. putida PCI2, the strain was grown in SM amended with different concentrations of iron. As a result, we detected no siderophore production at and above 50 μM of iron in culture supernatant of PCI2.
3.4 Effect of increasing concentrations of L-tryptophan on IAA production by P. putida PCI2
In the absence of a L-tryptophan supplement, P. putida PCI2 produced 1.7 μg/ml of IAA. On the other hand, measurement of IAA by LC-ESI/MS-MS confirmed that, in the presence of 100, 200 and 500 μg/ml of L-tryptophan, PCI2 responded by producing 5, 26 and 39 μg/ml of IAA, respectively, after 72 h of incubation (Fig. 3).
3.5 Effect of exogenous IAA on root development of tomato seeds inoculated with P. putida PCI2
The increase in the concentration of IAA from 0.01 to 10 μg/ml affected seedlings germinated from both inoculated and uninoculated seeds. On the other hand, we observed that, in the absence of exogenous IAA, seedlings inoculated with PCI2 showed an increase of 19 mm in root length (Fig. 4).
Effect of different concentrations of exogenous IAA on the elongation of tomato roots grown in pouches in the absence (control, white square) and in the presence of P. putida PCI2 (4 × 107 CFU/ml, black square). Bars with the same letter are not significantly different according to Fisher’s least significant difference (LSD) test (P < 0.05)
3.6 Effect of L-tryptophan on the growth of tomato seeds inoculated with P. putida PCI2
The effect of P. putida PCI2 on growth of tomato seedlings was evaluated in the presence of different concentrations of L-tryptophan. The supply of L-tryptophan caused an inhibitory effect on seedling growth, decreasing length and fresh weight of the shoot. Inoculation of seeds with P. putida PCI2 positively affected shoot length without and with addition of L-tryptophan. However, the highest increase in shoot length (68 %) was observed without addition of L-tryptophan. In addition, P. putida PCI2 significantly stimulated shoot fresh weight with L-tryptophan at 0.25 (67 %), 0.50 (61 %) and 0.75 mM (85 %), compared to controls. There were no significant differences in shoot fresh weight of inoculated seedlings as the concentration of L-tryptophan increased. P. putida PCI2 significantly stimulated root fresh weight (58 %) without addition of L-tryptophan. On the other hand, no significant differences were observed in root fresh weight of inoculated and control seedlings as the concentration of L-tryptophan increased (Figs. 5 and 6).
Effect of different L-tryptophan concentrations on shoot length (a) and fresh weight of shoot (b) and roots (c) of tomato seedlings grown in pouches for 15 days in the presence of P. putida PCI2 (4 × 107 CFU/ml, black square). Control seedlings were grown in absence of P. putida PCI2 (white square). Bars with the same letter are not significantly different according to Fisher’s least significant difference (LSD) test (P < 0.05)
Effect of different L-tryptophan concentrations on growth of tomato seedlings inoculated or not with P. putida PCI2. a Uninoculated control, without L-Trp; b Inoculated control, without L-Trp; c Uninoculated, with 0.25 mM L-Trp; d Inoculated, with 0.25 mM L-Trp; e Uninoculated, with 0.50 mM L-Trp; f Inoculated, with 0.50 mM L-Trp; g Uninoculated, with 0.75 mM L-Trp; (H) Inoculated, with 0.75 mM L-Trp
4 Discussion
Soil contains a wide range of organic substrates, which can be a source of P for plant growth. To make this form of P available for plant nutrition, it must be hydrolyzed to inorganic P by means of phosphatase enzymes. The major source of phosphatase activity in the rhizosphere is considered to be of microbial origin (Rodriguez and Fraga 1999). In this work, we evaluated P. putida PCI2 for phosphatase activity. PCI2 colonies developed a strong blue color in culture medium containing BCIP, which proves that PCI2 is positive for phosphatase activity.
Drought, high/low temperature and salinity are abiotic stress factors accepted as the main reason for crop productivity losses in a world with growing population and food price increases. The problem of phosphate fertilizers, P plant nutrition, and existing phosphate bearing resources can also be related to the scarcity of rock phosphate. The modern agricultural systems are highly dependent on the existing fertilizer industry based exclusively of this natural, finite, non-renewable resource. Biotechnology offers a number of sustainable solutions that can mitigate these problems by using plant beneficial, including P-solubilizing, microorganisms (Vassilev et al. 2012). Bacterial inoculation has been shown to cause beneficial effects on plant physiology and growth under abiotic stress conditions (Bianco and Defez 2011). Thus, P. putida PCI2 was evaluated for tolerance toward high temperature, different concentrations of NaCl and a wide range of pH. P. putida PCI2 could not grow at 40 °C. Similarly, Behbahani (2010) showed that P. putida PS13 could not withstand a temperature of 42 °C. Phosphate availability is reduced in saline soils not only because of ionic strength effects that reduce the activity of phosphate but also because phosphate concentrations in soil solution are tightly controlled by sorption processes and by the low-solubility of Ca-P minerals (Grattan and Grieve 1999). Since the availability of P in salt affected soils is limited, one appropriate approach to address the problem of plant growth under such conditions is the use of bioinoculants containing bacteria adapted to these environments. P. putida PCI2 showed solubilizing activity in the presence of 1–5 % of NaCl. Indeed, an increase in phosphate solubilization with increasing salt concentration was observed. P. putida PCI2 solubilized 94 and 104 mg/L of P after 48 h of growth in culture medium with 1 and 5 % added NaCl, respectively. By comparison, Malboobi et al. (2009) reported that Pantoea agglomerans strain P5, Microbacterium laevaniformans strain P7 and P. putida strain P13 solubilized 60–100 and ~ 40 mg/L of P after 48 h of growth with 1 and 5 % NaCl, respectively. Mundra et al. (2011) also observed that the amount of phosphate solubilized by the yeast Rhodotorula sp. PS4 gradually decreased with increasing concentration of NaCl. Rhodotorula sp. PS4 solubilized 271 and 211 mg/L of P when the concentration of NaCl was 1 and 5 %, respectively. P. putida PCI2 was also able to grow and produce soluble phosphate at initial pH ranging from 5 to 10. The inorganic phosphate solubilizing activity of PCI2 was highest at pH 7–9. However, this activity was only severely affected at an initial pH of 4. Similar results were observed by Behbahani (2010), whose bacterial strains were capable of solubilizing insoluble inorganic phosphate at a wide range of initial pH. On the other hand, Nautiyal et al. (2000) reported that the phosphate solubilizing activity of three of their bacterial strains decreased markedly at pH 10. In the present study, P. putida PCI2 was tested for solubilization of AlPO4 and FePO4 in solid and liquid culture media. PCI2 showed solubilization of AlPO4 in liquid medium. However, the solubilization of P by PCI2 was higher from Ca3(PO4)2 than from AlPO4.
Several rhizobacteria can solubilize inorganic phosphate from soil and such bacteria improve solubilization of unavailable soil phosphate resulting in a high efficiency of phosphorus use. For instance, a phosphate solubilizing P. fluorescens was reported to enhance growth and to increase yield, N and P contents in shoot and kernels of peanut (Dey et al. 2004). Preliminary studies involving tomato plants inoculated with strain PCI2 showed growth enhancement and an increased P content in leaves at 30 days (10 % as compared to uninoculated controls).
The use of microbial inoculants based on plant growth-promoting rhizobacteria is a biotechnological alternative to improve production of horticultural crops. According to Datta et al. (2011), inoculation of chilli plants (Capsicum annuum L.) with strains of Bacillus sp. that solubilize phosphate and produce IAA increased weight and number of fruits. Organic acids produced by PGPR also increase the availability of micronutrients such as iron (Fe) in the rhizosphere. Iron may be captured by siderophores, organic molecules secreted by these bacteria, and form chelates that can be absorbed by plants (Crowley 2006).
Like other phytohormones, auxins are also endogenously synthesized by plants. However, their hormonal effects have been elucidated by their exogenous applications. There is also ample evidence that several soil microorganisms are actively involved in the synthesis of auxins in pure cultures and in soil (Arshad and Frankenberger 1998). Different plant seedlings respond differentially to variable concentrations of auxins and type of microorganisms. At relatively high concentrations, natural auxins, such as IAA, stimulate shoot elongation while reducing root elongation (Tanimoto 2005). Higher (micromolar) concentrations of exogenous IAA applied to roots have clear-cut negative effects (Kawaguchi and Syono 1996). In a previous study, we reported that tomato plants emerged from seeds inoculated with strain PC12 showed an increase in root dry weight. Additionally, the shoot biomass was slightly greater in inoculated plants. We also demonstrated that PCI2 produces IAA (Pastor et al. 2010). Results from this study showed that addition of increasing concentrations of L-tryptophan to culture medium increases biosynthesis of IAA in PCI2 accordingly. Arshad and Frankenberger (1998) and Asghar et al. (2002) reported that addition of L-tryptophan as an auxin precursor substantially increased auxin production. In order to evaluate the possible effect of IAA on growth of tomato seedlings, we performed assays with addition of IAA and L-tryptophan to growth pouches. Thus, the effect of exogenous IAA on root development of seeds inoculated with strain PCI2 was evaluated. We observed that IAA modified the growth of tomato seedlings. The root development of uninoculated (control) and inoculated seedlings was affected by the addition of IAA. The length of the root was significantly reduced as the concentration of IAA increased from 0.1 to 10 μg/ml.
It is known that tryptophan is naturally secreted in root exudates of tomato plants and most of the auxin found in the rhizosphere is believed to come from the biosynthesis by microorganisms (Kamilova et al. 2006). The application of tryptophan to the rhizosphere can stimulate vegetative growth of maize and this effect has been ascribed to the conversion of tryptophan to IAA by rhizosphere bacteria (Sarwar and Frankenberger 1994). The effect of different concentrations of L-tryptophan on the growth of seeds inoculated with strain PCI2 was evaluated. The evaluated parameters were shoot length and shoot and root fresh weight. Although Bashan and Bashan (2005) concluded that environmental and technical variables, not related to bacterial inoculation, are an important issue and can significantly influence root fresh weight measurements, we did not record dry weight of roots in our assays due to the obtained seedling size after 15 days of growth in pouches. Adding L-tryptophan had a negative impact on shoot length as compared to the respective inoculated or non-inoculated controls without L-tryptophan. The effect of the addition of L-tryptophan was not dependent on the concentration used. Inoculation with PCI2 reverted the observed negative effect of L-tryptophan on shoot fresh weight. Thus, in the presence of L-tryptophan, inoculation with PCI2 caused a clear growth-promoting effect on shoot growth. On the other hand, the increase in root fresh weight was only significant in inoculated seedlings without L-tryptophan. These results proved that plant growth regulators, such as IAA, produced by P. putida PCI2 could also play a critical role in plant growth promotion.
To conclude, P. putida PCI2 proved to be positive for phosphatase activity, solubilizes a high amount of P in Sperber broth medium and is adapted to different environmental conditions. The ability of PCI2 to solubilize inorganic P could be an important trait for improving plant growth under limited P availability in soil. Further research is needed to determine the physiological responses of tomato plants to accumulation of P in the plant tissue and its role in growth promotion. Increasing L-tryptophan concentrations showed inhibitory effect on shoot fresh weight of tomato seedlings but inoculation with PCI2 reverted this negative effect. Thus, P. putida PCI2 has the potential to be developed and used as an inoculant for increasing the growth of tomato plants while decreasing the use of chemical fertilizers.
References
Adesemoye AO, Torbert HA, Kloepper JW (2009) Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb Ecol 58:921–929
Ahmadzadeh M, Tehrani AS (2009) Evaluation of fluorescent pseudomonads for plant growth promotion, antifungal activity against Rhizoctonia solani on common bean, and biocontrol potential. Biol Control 48:101–107
Arshad M, Frankenberger WT (1998) Plant growth-regulating substances in the rhizosphere: microbial production and functions. Adv Agron 62:45–151
Asghar HN, Zahir ZA, Arshad M, Khalig A (2002) Relationship between in vitro production of auxins by rhizobacteria and their growth-promoting activities in Brassica juncea L. Biol Fertil Soils 35:231–237
Ayala S, Prakasa Rao EVS (2002) Perspectives of soil fertility management with a focus on fertilizer use for crop productivity. Curr Sci India 7:797–807
Babana AH, Antoun H (2006) Effect of Tilemsi phosphate rock-solubilizing microorganisms on phosphorus uptake and yield of field-grown wheat (Triticum aestivum L.) in Mali. Plant Soil 287:51–58
Banerjee S, Palit R, Sengupta C, Standing D (2010) Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Aust J Crop Sci 4:378–383
Bashan Y, de-Bashan LE (2005) Fresh-weight measurements of roots provide inaccurate estimates of the effects of plant growth-promoting bacteria on root growth: a critical examination. Soil Biol Biochem 37:1795–1804
Behbahani M (2010) Investigation of biological behavior and colonization ability of Iranian indigenous phosphate solubilizing bacteria. Sci Hortic 124:393–399
Bianco C, Defez R (2011) Soil bacteria support and protect plants against abiotic stresses. In: Shanker A (ed) Abiotic stress in plants, mechanisms and adaptations. Intech, Rijeka, pp 143–170
Canbolat MY, Bilen S, Çakmakçı R, Şahin F, Aydın A (2006) Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol Fertil Soils 42:350–357
Cassán F, Perrig D, Sgroy V, Masciarelli O, Penna C, Luna V (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109 promote seed germination and early seedling growth, independently or co-inoculated in maize (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol 45:28–35
Chen C, Bauske EM, Musson G, Rodriguez-Kabaña R, Kloepper JW (1994) Biological control of Fusarium on cotton by use of endophytic bacteria. Biol Control 5:83–91
Corvo Dolcet S (2003) Zonas de producción del cultivo del tomate en la Argentina. http://www.seedquest.com/News/releases/2005/pdf/13528.pdf. Accessed 9 Oct 2013
Crowley DE (2006) Microbial siderophores in the plant rhizosphere. In: Barton LL, Abadia J (eds) Iron, nutrition in plants and rhizosphere microorganisms. Springer, Riverside, pp 169–198
Datta M, Palit R, Sengupta C, Kumar PM, Banerjee S (2011) Plant growth promoting rhizobacteria enhance growth and yield of chilli (Capsicum annuum L.) under field conditions. Aust J Crop Sci 5:531–536
Dey R, Pal KK, Bhatt DM, Chauhan SM (2004) Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth promoting rhizobacteria. Microbiol Res 159:371–394
Figueiredo MVB, Burity HA, Martinez CR, Chanway CP (2008) Alleviation of water stress effects in common bean (Phaseolus vulgaris L.) by co-inoculation Paenibacillus x Rhizobium tropici. Appl Soil Ecol 40:182–188
Grattan SR, Grieve CM (1999) Salinity-mineral nutrient relations in horticultural crops. Sci Hortic 78:127–157
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem 37:395–412
Hameedaa B, Harinib G, Rupelab OP, Wanib SP, Reddy G (2008) Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163:234–242
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophan in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact 19:250–256
Kawaguchi M, Syono K (1996) The excessive production of indole-3-acetic acid and its significance in studies of the biosynthesis of this regulator of plant growth and development. Plant Cell Physiol 37:1043–1048
Kim KY, Jordan D, McDonald GA (1998) Effect of phosphate solubilizing bacteria and vesicular–arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol Fertil Soils 26:79–87
King EO, Ward MK, Ranney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. In: Station de pathologie végétale et phytobactériologie (ed) Proceedings of the fourth international conference on plant pathogenic bacteria, vol II. Gilbert Clary, Tours, pp 879–882
Kumar S, Pandey P, Maheshwari DK (2009) Reduction in dose of chemical fertilizers and growth enhancement of sesame (Sesamum indicum L.) with application of rhizospheric competent Pseudomonas aeruginosa LES4. Eur J Soil Biol 45:334–340
Kumar A, Kumar A, Devi S, Pati S, Payal C, Negi S (2012) Isolation, screening and characterization of bacteria from rhizospheric soils for different plant growth promotion (PGP) activities: an in vitro study. Recent Res Sci Technol 4:1–5
Malboobi MA, Owlia P, Behbahani M, Sarokhani E, Moradi S, Yakhchali B, Deljou A, Morabbi Heravi K (2009) Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World J Microbiol Biotechnol 25:1471–1477
Mercado-Blanco J, Bakker AHM (2007) Interactions between plants and beneficial Pseudomonas spp.: exploiting bacterial traits for crop protection. Antonie Van Leeuwenhoek 92:367–389
Mundra S, Arora R, Stobdan T (2011) Solubilization of insoluble inorganic phosphates by a novel temperature-, pH-, and salt-tolerant yeast, Rhodotorula sp. PS4, isolated from seabuckthorn rhizosphere, growing in cold desert of Ladakh, India. World J Microbiol Biotechnol 27:2387–2396
Naika S, van Lidt de Jeude J, de Goffau M, Hilmi M, van Dam B (2005) Cultivation of tomato: production, processing and marketing. Agromisa Foundation and CTA, Wageningen, Netherlands. http://journeytoforever.org/farm_library/AD17.pdf. Accessed 9 Oct 2013
Nautiyal CS, Bhadauria S, Kumar P, Lal H, Mondal R, Verma D (2000) Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol Lett 182:291–296
Okon Y, Labandera-Gonzalez CA (1994) Agronomic applications of Azospirillum: An evaluation of 20 years worldwide field inoculation. Soil Biol Biochem 12:1591–1601
Orhan E, Esitken A, Ercisli S, Turan M, Sahin F (2006) Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci Hortic 111:38–43
Pastor NA, Reynoso MM, Tonelli ML, Masciarelli O, Rosas SB, Rovera M (2010) Potential biological control Pseudomonas sp. PCI2 against damping-off of tomato caused by Sclerotium rolfsii. J Plant Pathol 92:737–745
Pastor NA, Carlier E, Andrés J, Rosas SB, Rovera M (2012) Characterization of rhizosphere bacteria for control of phytopathogenic fungi of tomato. J Environ Manag 95:332–337
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Enhancing resistance of tomato and hot pepper to Pythium diseases by seed treatment with fluorescent pseudomonads. Eur J Plant Pathol 108:429–441
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–333
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rosas SB, Andrés JA, Rovera M, Correa NS (2006) Phosphate-solubilizing Pseudomonas putida can influence the rhizobia–legume simbiosis. Soil Biol Biochem 38:3502–3505
Rosas SB, Avanzini G, Carlier E, Pasluosta C, Pastor N, Rovera M (2009) Root colonization and growth promotion of wheat and maize by Pseudomonas aurantiaca SR1. Soil Biol Biochem 41:1802–1806
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Sarwar M, Frankenberger WT (1994) Influence of L-tryptophan and auxins applied to the rhizosphere on the vegetative growth of Zea mays L. Plant Soil 160:97–104
Sayyed RZ, Badgujar MD, Sonawane HM, Mhaske MM, Chincholkar SB (2005) Production of microbial iron chelators (siderophores) by fluorescent Pseudomonads. Indian J Biotechnol 4:484–490
Sharma A, Johri BN (2003) Combat of iron-deprivation through a plant growth promoting fluorescent Pseudomonas strain GRP3A in mung bean (Vigna radiata L. Wilzeck). Microbiol Res 158:77–81
Son HJ, Park GT, Cha MS, Heo MS (2006) Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour Technol 97:204–210
Tanimoto E (2005) Regulation of root growth by plant hormones—roles for auxin and gibberellin. Crit Rev Plant Sci 24:249–265
Van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
Vassilev N, Vassileva M, Nikolaeva I (2006) Simultaneous P-solubilizing and biocontrol activity of microorganisms: potential and future trends. Appl Microbiol Biotechnol 71:137–144
Vassilev N, Eichler-Löbermann B, Vassileva M (2012) Stress-tolerant P-solubilizing microorganisms. Appl Microbiol Biotechnol 95:851–859
Vessey JK (2003) Plant growth-promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Yanes ML, De La Fuente L, Altier N, Arias A (2012) Characterization of native fluorescent Pseudomonas isolates associated with alfalfa roots in Uruguayan agroecosystems. Biol Control 63:287–295
Acknowledgments
This work was supported by grants from Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto (Córdoba, Argentina), Agencia Nacional de Promoción Científica y Tecnológica (Secretaría de Ciencia y Técnica de la Nación) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pastor, N., Rosas, S., Luna, V. et al. Inoculation with Pseudomonas putida PCI2, a phosphate solubilizing rhizobacterium, stimulates the growth of tomato plants. Symbiosis 62, 157–167 (2014). https://doi.org/10.1007/s13199-014-0281-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-014-0281-3