Abstract
Betalains are vacuolar pigments present in tubers, flowers or fruits. Their use in the food industry is significant because they are considered bioactive completely safe to consume. However, betalains are susceptible to temperature which affects their stability. The most of the available methods that determine stability involve high costs, are destructive and generate waste. In this work was evaluated the thermal degradation of betalain at 75 °C for several intervals of time, by using different techniques. Colorimetry showed a change in the tone angle (h°) from 359.76° to 20.54° after the heat-treatment, suggesting thermal degradation by changing the color from violet to red–orange. High-pressure liquid chromatography, shows the decrease of the concentration of betanin in addition to the formation of neobetanin, the main degradation product in betalains. UV–visible spectrophotometry suggest also thermal degradation of betanin, by the decrease of the absorption at 538 nm caused by the heat treatment. Finally, Fourier transform infrared spectroscopy (FTIR) showed a decrease in the intensity of two absorption bands at 1243 and 879 cm−1, corresponding to the C–O and C–C vibrations of the carboxylic acid respectively after heat treatment. These results suggest that the main route of degradation corresponds to decarboxylation. We propose the use of FTIR spectroscopy as a practical alternative for the analysis of the degradation of natural dyes during storage, making evident the possible use of this methodology for industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Color is one of the main characteristics that consumers consider to accept or reject a food since it is synonymous with quality and freshness (Ravichandran et al. 2013). This fact makes the colorants market a very profitable business, according to market research companies, the global food color market size will be of USD 2.97 billion by 2025, with an estimated USD 1.79 billion in 2016 (Viera et al. 2019).
Synthetic food colorants reported to associate with behavioral problems such as hyperactivity in children and other health problems (McCann et al. 2007). Thus, consumers diverted towards natural food colors, especially those extracted from plants. In recent years, food industry increasingly focusing on the use of natural colors from red beet, berries or pepper as alternative sources to their synthetic counterparts in order to overcome negative health effects (Esatbeyoglu et al. 2015). Besides adding color to food, natural colorants are also associated with beneficial health effects, being considered as antioxidants (Butera et al. 2002), and with anticancer properties (Kapadia et al. 1996).
Food and Drug Administration (FDA) approved the use of Carmine (E120), anthocyanins (E163) and betanin (betanidin 5-Ο-β-d-glucoside) (E162) as natural red colour additive for food, dairy, beverage, cosmetic and pharmaceutical products (FDA 2013). The advantage of betanins, compared with anthocyanins, is that they are stable in the pH range of 3 and 7 and are thus suitable for coloring from acidic to neutral foods (Herbach et al. 2006b).
Betalains are classified into two groups: the yellow betaxanthins and the red-violet betacyanins (Strack et al. 2003). Betalains accumulate in flowers, roots, seeds, leaves, stems, fruits, petiole and bract in the plant kingdom particularly order Caryophyllales, except anthocyanins producing families Molluginaceae and Caryophyllaceae and some variety of fungus belongs to Basidiomycetes (e.g. Hygrocybe conica, Amanita muscaria) (Terradas and Wyler 1991; Gasztonyi et al. 2001; Cai et al. 2005). Around 75 types of betalains have been identified (Khan and Giridhar 2015). Betacyanins are divided into four groups: amaranthin-type, betanin-type, 2-descarboxybetanins and gomphrenin-type (Esatbeyoglu et al. 2015). The most well-known betacyanin is the red betanin, that is obtained primarily from root of red beet (beta vulgaris spp. vulgaris). Red beet consists approximately 300–600 mg/kg of betanin and has been used for the industrial production of this natural colorant (Kanner et al. 2001). The other source of betanin is cactus pear (genus Opuntia) (Castellar et al. 2006; Castellanos-Santiago and Yahia 2008) and amaranth (Amaranthus) (Cai et al. 1998, 2005).
The color and physical properties of betanins are sensitive to external factors like high temperature, light, high water activity and changes in pH less than 3 or greater than 7 (Herbach et al. 2006b). Heating process was involved in both extraction and production of concentrated pigments and their use in preparation of food. Upon heating the red color of betanin changes into orange-yellow due to hydrolysis, decarboxylation and autooxidation process (Herbach et al. 2006a).
To evaluate the thermal degradation process and identify the main degradation products, high pressure liquid chromatography analysis (HPLC) (Herbach et al. 2006b), HPLC–MS (Herbach et al. 2004a, b; Wybraniec 2005; Kumorkiewicz 2017), UV–visible light spectrophotometry (Castellar et al. 2006), and colorimetry (Herbach et al. 2004a) are commonly used. In addition to that, 1H NMR spectroscopy has been used for investigation of main heat-degradation products of betanin (Esteves et al. 2018). 1H NMR and HPLC are a very accurate techniques of analysis which allow evaluate thermal degradation of betalain and the formed subproducts (neobetanin). However despite being accurate, procedure requires specialized personal and also requires reagents to perform the compound separations, generating waste. On the other hand, Fourier transform infrared spectroscopy (FTIR) is a technique that not requires any reagent to obtain measurement, and although is not as sensible as HPLC/NMR, the sensibility could be enough to evaluate the thermal degradation of betalain, in a rapid and practical form. The use of FTIR spectroscopy has been reported previously in the analysis of betacyanins in Amaranthus species (Cai et al. 1998) and betanin in Bougainvillea glabra (Patil et al. 2014).
The objective of this work was evaluate the thermal degradation of betanin from beetroot, as well as analyze the formation of betanin degradation products, during thermal treatment by HPLC, UV–vis spectrophotometry, and colorimetry and compare these results with the obtained with FTIR spectroscopy, to propose this, as an alternative technique non-destructive with short analysis time, without generating chemical residues.
Materials and methods
Sources
Raw beetroot was used as a source of material, which was purchased in a local market, rinsed with running water and stored at 4 °C until use.
Preparation, extraction, and purification of betalains
The extraction and purification process of the betalains was carried out in the absence of white light to avoid pigment degradation. Beetroot was cut into small pieces and extraction was done with a juice extractor (Turmix 450 W). To separate the substantial part of the extract, as fragments of pulp, pectin or mucilage, ethanol was added at 96% (v/v) in a ratio of 1:1 (v/v) ethanol:extract. After 10 min of contact, the precipitate was separated by centrifugation at 5000 rpm (0.39 g) for 10 min, to complete the separation of the solids, the supernatant was filtered with filter paper (WHA10404026), and immediately after that, the ethanol was evaporated in vacuum at 40 °C. The purification process was carried out at room temperature using a 40 cm × 1.5 cm glass column packed with amberlite resin IRA958 Cl (Rohm & Haas, Darmstadt, Germany). The resin was activated with 10% NaOH (w/v), five washes were made with deionized water to eliminate the excess of alkali. The sugars present were eluted with acidified water pH 3. The betalains were eluted in a single stage with acidified water pH 1, and the fraction with more color was collected and immediately adjusted to pH 4 with a solution 7 M of NaOH. The eluate was concentrated in a rotary evaporator at 60 °C, the concentrated fraction was lyophilized and stored under vacuum at 25 °C until its use.
Preparation of the stock solution of betanin
A stock solution of betanin was prepared to avoid concentration variations in the analyzes, from which all the analyzes were carried out. 500 mg of lyophilized material was weighed and made up in 50 ml of distilled water; the solution was stored under refrigeration until its use.
Heat treatment
5 ml aliquots of betanin stock solution were taken, placed in amber bottles and sealed. They were incubated in a water bath at 75 °C, during different periods 20, 40, 60, 80, 100 and 120 min. The time 0 was considered as the sample without heat treatment. After each heating period, the samples were placed in water at 20 °C for 5 min. Subsequently, the samples were analyzed.
UV–visible spectrophotometry
The spectrophotometric analyzes were performed with a Jenway Genova Plus spectrophotometer (Madrid). The spectra were obtained in a wavelength range of 400–600 nm. The value of the half-life and the speed constant were calculated by quantifying the percentage of color remaining after the heat treatment according to Von-Elbe et al. (1974) and Fernández-López et al. (2013). Equation 1.
Where % R = remaining color percentage of betanin.
The percentage of remaining color was graphed against the heating period, where the straight line corresponds to the constant kinetic K. The value of the half-life was calculated from constant kinetic K (Herbach et al. 2006b), Eq. 2. All measurements were made in triplicate.
Coordinates colour
Colorimetric analyzes were performed with a Minolta colorimeter (Konica Minolta, USA). After the heat treatment, all the betanin samples were diluted with distilled water until reaching maximum absorbance values of 1 ± 0.1, they were incubated for 15 min and immediately after the readings were made, the results were expressed in units CIELab, from which the chroma values [C = (a*2 + b*2) 0.5] and pitch angle [h° = (arctan b*/a*)] were calculated. All measurements were made in triplicate.
High performance liquid chromatography (HPLC) analyses
HPLC analyzes were performed in an Agilent system (Agilent, Santa Clara, CA, USA), consisting of a manual sampler G1328C, diode array detector (DAD) G4212B, quaternary pump G1330B, visible wavelength detector (VWD) G1314F. Separation was achieved on a zorbax SB-C18 column, (25 cm × 4.6 mm i.d) with a particle size of 5 μm (Agilent, Santa Clara, CA, USA). The elution of the compounds was carried out according to the methodology proposed by (Herbach et al. 2004a), modified in gradient time. The solvent system used was 0.2% (v/v) formic acid in water (phase A) and acetonitrile (phase B), at a flow rate of 1 ml/min, the gradient used for the elution of the compounds was: isocratic 100% A for 7 min, 100% A to 93% A in 3 min, 93% A to 90% A in 17 min, 90% A to 80% A in 10 min, 80% A to 0% A in 5 min, isocratic 0% A 10 min. The wavelengths analyzed were 538 and 476 nm for betanin and vulgaxanthin I respectively, and 505, 453, 410, 405 and 280 nm for the degradation products.
Infrared spectroscopy (FTIR) analyses
The infrared spectroscopy analyzes were performed on a Bruker Vertex 70 spectrometer (USA), in attenuated total reflectance sampling (ATR) mode. After subjecting the betanin samples to thermal treatment and conditioning them at room temperature, the infrared spectrum was measured in the mid-infrared region (4000–400 cm−1). The position and intensity of the absorption bands in the FTIR spectra were used to analyze the functional groups according to libraries and bibliography; while the change in intensity was used to evaluate the thermal degradation of betanin.
Results and discussion
Effect of temperature on the stability of betanin monitored by UV–visible spectrophotometry
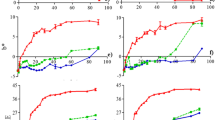
It has been reported that the wavelength of the maximum of absorption of betalains is in the wavelength interval from 536 to 540 nm (Cai et al. 1998; Butera et al. 2002). In this study, the maximum wavelength of absorption for betanin was observed at 538 nm, from which were evaluated the changes of absorbance due to thermal treatment. Figure 1 shows the UV–visible absorption spectra of betanin in the interval 400–600 nm during different heating times.
The absorption band centered at 538 nm is associated with the compound betanin and the intensity decreases homogeneously with the heating time at 75 °C, showing an initial absorbance of 0.85, however after 120 min of heating it decreased to 0.446. The results suggest a decrease in the betanin concentration due to the thermal degradation of this molecule, as seen in Fig. 1. Likewise, a hypsochromic displacement of 9 nm in the maximum of the absorption (538–529 nm) arises after 120 min of heating. This shift is due to the delocalization of the electrons π of the betanin molecule as result of the heat treatment (Herbach et al. 2004a), which was reflected in a change in coloration of the sample from violet-red to red–orange.
In addition to that, other absorption band centered between 490 and 460 nm was also observed in the UV–visible spectra; which is related to the dehydrogenation of the betanin molecule (Herbach et al. 2006b). Therefore, the formation of compounds with shorter wavelengths of absorption or less color agree with the results reported by Fernández-López et al. (2013), who demonstrated that the effect of temperature on extracts of beet, prickly pear, and cabbage presented structural alterations that affect the spectrum of absorption and color properties.
Degradation kinetics
The change in coloration of the betanin samples during the heat treatment was analyzed by quantifying the percentage of remaining color. The percentage of color retained decreases when the heating time increases, after 20 min of heating the color remains at 98%, however, after 120 min, only 55% remains.
Betanin presents degradation kinetics of first-order as seen inset of Fig. 1, with a half-life (t 1/2) of 2.3 h. Although it is difficult to establish a reaction mechanism and obtain a kinetic model that describes browning, generally the color change of natural dyes shows kinetics of first-order degradation (Attoe and Von-Elbe 1981; Herbach et al. 2004b). The t 1/2 value of 2.3 h obtained in this study agrees with the results in previous studies t 1/2 of 2.34 h at 70 °C, pH 5.5 and t 1/2 of 2 h at 85 °C, pH 4.5 (Herbach et al. 2004b; Fernández-López et al. 2013).
Coordinates color
The color change was monitored immediately after the heat treatment, the values of L*, a*, b*, C* and h° obtained were used to evaluate the color changes in the samples, the values are presented in Table 1.
During the heat treatment, the values of L* (brightness) gradually increased as shown increase in temperature (Herbach et al. 2005). According to the study conducted by Fernández-López et al. (2013). The increase of L* is normal during thermal processing of thermosensitive natural extracts and is accompanied by a gradual decrease in the values of a*. In this case, the values of a* (positive for the red color and negative for the green) were positive, however, they decreased gradually concerning the warm-up time. The values of b* (positive for the yellow color and negative for the blue) did not show any trend; data were obtained in the range − 0.34 in the sample without heating to 5.18 in samples heated for 120 min. The value of chroma C*, which represents the purity or concentration of the color, gradually decreased, indicating lower purity of the color, where values lower than C* are due to the generation of different compounds during heating, which absorbs at a shorter wavelength than that absorbed by betanin. In this study, chroma values decreased slightly during the first 100 min. This result indicates that betanin is stable at 75 °C for approximately 90 min and that the denaturation of the molecule occurred after 100 min, indicating that betanin is stable in these conditions.
On the other hand, h° indicates the hue of the color; changed from 359.76 (red–purple tone in the sample without heating) to 20.54. (red–orange tone in samples heated for 120 min at 75 °C), this change is related to the formation of less colorful degradation products derived from the thermal process. Studies carried out by Herbach and his associates (2004a, 2006b) show results similar to those obtained here, where the value of h° changed from 358 in untreated beet samples to 62 after 8 h of heating to 75 °C.
Analysis of the thermal degradation of betanin by Fourier transform infrared spectroscopy (FTIR)
Infrared spectroscopy analyzes (FTIR) were performed to determine the vibration frequencies characteristic of betanin functional groups, as well as to determine the structural changes of this molecule as a result of thermal treatment. The assignment of functional groups and vibration modes was performed according to the literature.
Figure 2a shows the FTIR spectrum of a betanin sample in the region of 4000–400 cm−1 without heating. Different absorption bands characteristic of functional groups of betalains were observed. The band at 3359 cm−1 was attributed to the stretching vibration of the –OH bond (Kumar et al. 2017), on the other hand, the band located at 1624 cm−1 was attributed to the stretching vibration of the C = N bond (Cai et al. 1998; Molina et al. 2014). The next band at 1378 cm−1 was assigned to the extension stretching vibration of the C–H bond, while the band centered at 1243 cm−1 correspond to the stretching vibration of the C–O bond of the carboxylic acid (Molina et al. 2014; Cai et al. 1998). Another band at 1073 cm−1 was attributed to the symmetric stretching vibration of the C–O–C link (Sengupta et al. 2015), the band at 945 cm−1 corresponds to the deformation of the C–H bond and finally the band at 879 cm−1 corresponds to the stretching vibrations of the C–COOH bond (Dong et al. 1997).
Figure 2b shows the FTIR spectra of betanin samples during the thermal treatment at 75 °C during several heating times in the region of 2000–400 cm−1. The main vibrational features and also the changes in intensity of some absorption bands associated with carbonyl compounds characteristic of the betanin molecule are found in this spectral region.
Since betalains are not thermostable compounds, when increase the temperature they lose their stability, causing structural changes in the molecule. For this reason, significant changes were observed in some absorption bands in the FTIR spectra, such as the changes located at 1243 cm−1 and 879 cm−1 which suffers decrease in their intensity with heat time, as observed in Figs. 2b and 3.
The decrease in the intensity of absorption of the band centered at 1243 cm−1, corresponding to the stretching vibration of the C–O bond of the carboxylic acid of betanin is related to the breakdown of the bond, which indicates the degradation of the initial molecular structure that is induced by the increase in temperature. The breakdown of this link is related to the process of decarboxylation that the betanin molecule suffers when exposed to high temperature. Wybraniec (2005); Herbach et al. (2006a) reported the breaking down of the carboxyl group of the betanin molecule by increasing the temperature where the betanin molecule is susceptible to decarboxylation at the C2, C15, and C17 position Fig. 4. On the other hand, it was also determined that the initial decarboxylation site might depend on the type of solvent. However, after a prolonged heating process, the decarboxylation pattern does not differ (Wybraniec 2005).
The signal observed at 879 cm−1 which was attributed to the stretching of the C–COOH bond (Dong et al. 1997), also showed a lower absorbance intensity with respect to the warm-up time, this confirms that the main route of degradation suffered by the betanin molecule when increasing temperature is the decarboxylation process. It can be considered that the loss of COOH radicals is considered an intermediate product that destabilizes the molecule so that the rearrangement of the electrons is done forming the double bond between the C14 and C15 which gives rise to the formation of neobetanin (Herbach et al. 2006a).
The signal at 1624 cm−1 associated with the substantial extension of the aldimine bond did not suffer changes in spectral position or absorption intensity, which indicates that the aldimine bond that binds the betalamic acid with the dopa cycle does not change. This result agrees with studies carried out where they indicate that the hydrolysis of the bond is carried out more frequently under conditions of high pH (Saguy et al. 1978) or in the presence of ethanolic or methanolic solutions (Simon et al. 1993). Otherwise, the band at 1073 cm−1 linkage characteristic associated with polysaccharides does not undergo significant change, which indicates that the betanin molecule is not deglycosylated easily.
HPLC chromatograms of betanin with different thermal treatment times
The HPLC analysis allowed to evaluate the changes of betanin molecule exposed to thermic treatment. The results showed the loss of betanin and the formation of other compounds.
Figure 5 shows the chromatograms of betanin samples without heating and after 20, 80 and 120 min of heating at 75 °C. In betanin samples without heating, Fig. 5a, b, the peaks were assigned according to the retention time, maximum absorption length and previous studies (Schwartz and von Elbe 1980; Kujala 2001; Herbach et al. 2004a, 2005; Mikołajczyk-Bator and Pawlak 2016). Peak (1) was assigned to vulgaxanthin I, which is the yellow pigment present in higher proportion in betalains (Kujala et al. 2001), peaks (2) and (3) were assigned to betanin and isobetanin respectively. Additionally, peak (4) was assigned to neobetanin (14,15-dehydrobetanin), due to its longer retention time compared to betanin and its maximum absorption length (Wybraniec 2005; Kumorkiewicz 2017). Although neobetanin is considered the main thermal degradation product of betanin (Herbach et al. 2004b), there are also reports that it is a natural compound of the extract (Herbach et al. 2004a; Kujala et al. 2001). In this case, the presence of neobetanin in samples without heating indicates that it is a genuine part of the pigment.
On the other hand, the chromatographic profile during the heat treatment at 75 °C in time intervals of 20, 80 and 120 min was analyzed through the changes in intensity/area presented by the peaks. Comparing the area without heating and after thermal treatment, significant changes were observed in the chromatograms; some peaks appeared, decreased or disappeared as a result of the changes suffered by the molecule during the thermal process.
According to the chromatograms shown in Fig. 5, all the graphs of the left side correspond to the absorption at 538 nm, which are associated to the presence of betanin (peak 2) and isobetanin (peak 3) at 0, 20, 80 and 120 min of heating. The intensity/area of the peak 2 decreases with the time of treatment, suggesting directly the thermal degradation of betanin. Isobetanin content (peak 3) also decreased, this result does not agree with the studies carried out by Herbach et al. (2004a) and Schwartz and Elbe (1983), where the content of isobetanine increases due to isomerization caused by heating. In this case, it can be concluded that the formation of isobetanin is carried out during the sample preparation process and decreases during the heating process. For other hand, all the graphs of the right side (Fig. 5) correspond to the absorption at 476 nm, where is observed the presence of vulgaxanthin I (peak 1), betanin (peak 2), isobetanin (peak 3) and neobetanin (peak 4). Vulgaxanthin I was no detectable with the heat treatment; according to previous studies Herbach et al. (2004a); Giménez et al. (2015), vulgaxanthin I is three times more sensible at high temperature, so the lower content recorded in this study is the expected. Finally, neobetanin content (peak 4) increased 100% (after 120 min) with respect to the initial value, indicating that it is a formed product during the heating process. That is, neobetanin is the primary degradation product recorded in HPLC analysis, this result agree with (Herbach et al. 2004a).
Conclusion
In this work, a vibrational (FTIR), spectrophotometric (UV–visible), colorimetric and chromatographic (HPLC) analysis was performed to analyze the thermal degradation of betanin at 75 °C during different heating times. The colorimetric analysis of the betanin samples during treatment allowed to observe a change in color from red violet to red–orange due to the degradation of this molecule. For other hand, HPLC analysis indicated not only the decrease in betanin concentration but also recorded the formation of new compounds generated during heat treatment, among which is mainly neobetanin. The above is consistent with that found by UV–visible spectrophotometry, where two absorption bands (538 and 480 nm) were observed. The absorption band observed at 538 nm is associated with the presence of betanin, and the decrease in its intensity demonstrates the thermal degradation of this molecule in time due to treatment at 75 °C. Finally, the vibrational analysis (FTIR) shows that the molecule of betanin suffers decarboxylation, which is demostrated by the decrease in intensity of the infrared absorption band centered at 1243 cm−1 that correspond to the CO bond of the carboxylic acid; and by the decrease of the band centered at 879 cm −1 corresponding to the carboxylic acid C–C bond. According to HPLC analysis, neobetanin is the main degradation product, although in FTIR analysis, a signal corresponding to the formation of the double bond that originates the formation of neobetanin is not observed. However, it is clear that the thermal degradation process of betanin analyzed by FTIR can be monitored by means of the decrease in intensity corresponding to the carboxylic acid bonds.
Due to the above, the possibility of using the FTIR vibrational technique is proposed as an inexpensive, non-destructive and fast method that would allow monitoring the stability of the dyes or their deterioration in useful life during storage or in the evaluation of color in thermally processed foods.
References
Attoe EL, Von-Elbe JH (1981) Photochemial degradation of betanine and selected anthocyanins. J Food Sci 46:1934–1937. https://doi.org/10.1111/j.1365-2621.1981.tb04522.x
Butera D, Tesoriere L, Di Gaudio F et al (2002) Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: betanin and indicaxanthin. J Agric Food Chem 50:6895–6901. https://doi.org/10.1021/jf025696p
Cai Y, Sun M, Wu H et al (1998) Characterization and quantification of betacyanin pigments from diverse Amaranthus species. J Agric Food Chem 46:2063–2070. https://doi.org/10.1021/jf9709966
Cai Y-Z, Sun M, Corke H (2005) Characterization and application of betalain pigments from plants of the amaranthaceae. Trends Food Sci Technol 16:370–376. https://doi.org/10.1016/j.tifs.2005.03.020
Castellanos-Santiago E, Yahia EM (2008) Identification and quantification of betalains from the fruits of 10 mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J Agric Food Chem 56:5758–5764. https://doi.org/10.1021/jf800362t
Castellar MR, Obón JM, Fernández-López JA (2006) The isolation and properties of a concentrated red-purple betacyanin food colourant from Opuntia Stricta fruits. J Sci Food Agric 86:122–128. https://doi.org/10.1002/jsfa.2285
Dong J, Ozaki Y, Nakashima K (1997) Infrared, Raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid). Macromolecules 30:1111–1117. https://doi.org/10.1021/ma960693x
Esatbeyoglu T, Wagner AE, Schini-Kerth VB, Rimbach G (2015) Betanin-a food colorant with biological activity. Mol Nutr Food Res 59:36–47. https://doi.org/10.1002/mnfr.201400484
Esteves LC, Pinheiro AC, Pioli RM et al (2018) Revisiting the mechanism of hydrolysis of betanin. Photochem Photobiol 94:853–864. https://doi.org/10.1111/php.12897
Fernández-López JA, Angosto JM, Giménez PJ, León G (2013) Thermal stability of selected natural red extracts used as food colorants. Plant Foods Hum Nutr 68:11–17. https://doi.org/10.1007/s11130-013-0337-1
Food and Drug Administration (2013) Listing of color aditives exempt from certification. Publishing Physics Web. https://www.accessdata.fda.gov/scripts/cdrh/cfdoscs/cfcfr/CFRSearch.cfm?fr=7340. Accessed 01 April 2019
Gasztonyi MN, Daood H, Hájos MT, Biacs P (2001) Comparison of red beet (Beta vulgaris var conditiva) varieties on the basis of their pigment components. J Sci Food Agric 81:932–933. https://doi.org/10.1002/jsfa.899
Giménez PJ, Fernández-López JA, Angosto JM, Obón JM (2015) Comparative thermal degradation patterns of natural yellow colorants used in foods. Plant Foods Hum Nutr 70:380–387. https://doi.org/10.1007/s11130-015-0499-0
Herbach KM, Stinzing FC, Carle R (2004a) Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J Food Sci 69:491–498. https://doi.org/10.1111/j.1365-2621.2004.tb10994.x
Herbach MK, Stintzing FC, Carle R (2004b) Thermal degradation of betacyanins in juices from purple pitaya [Hylocereus polyrhizus (Weber) Brittonv & Rose] monitored by high-performance liquid chromatography-tandem mass spectometric analyses. Eur Food Res Technol 219:377–385. https://doi.org/10.1007/s00217-004-0948-8
Herbach KM, Stintzing FC, Carle R (2005) Identification of heat-induced degradation products from purified betanin, phyllocactin and hylocerenin by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 19:2603–2616. https://doi.org/10.1002/rcm.2103
Herbach KM, Stintzing FC, Carle R (2006a) Betalain stability and degradation—structural and chromatic aspects. J Food Sci 71:41–50. https://doi.org/10.1111/j.1750-3841.2006.00022.x
Herbach KM, Stintzing FC, Carle R (2006b) Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. J Agric Food Chem 54:390–398. https://doi.org/10.1021/jf051854b
Kanner J, Harel S, Granit R (2001) Betalains—a new class of dietary cationized antioxidants. J Agric Food Chem 49:5178–5185. https://doi.org/10.1021/jf010456f
Kapadia GJ, Tokuda H, Konoshima T, Nishino H (1996) Chemoprevention of lung and skin cancer by Beta vulgaris (Beet) root extract. Cancer Lett 100:211–214. https://doi.org/10.1016/0304-3835(95)04087-0
Khan MI, Giridhar P (2015) Plant betalains: chemistry and biochemistry. Phytochemistry 117:267–295. https://doi.org/10.1016/j.phytochem.2015.06.008
Kumar SNA, Ritesh SK, Sharmila G, Muthukumaran C (2017) Extraction optimization and characterization of water soluble red purple pigment from floral bracts of Bougainvillea glabra. Arab J Chem 10:S2145–S2150. https://doi.org/10.1016/j.arabjc.2013.07.047
Kumorkiewicz A (2017) Thermal degradation of major gomphrenin pigments in the fruit juice of Basella alba L. (Malabar Spinach). J Agric Food Chem 65:7500–7508. https://doi.org/10.1021/acs.jafc.7b02357
McCann D, Barrett A, Cooper A et al (2007) Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 370:1560–1567. https://doi.org/10.1016/S0140-6736(07)61306-3
Mikołajczyk-Bator K, Pawlak S (2016) The effect of thermal treatment on antioxidant capacity and pigment contents in separated betalain fractions. Acta Sci Pol Technol Aliment 15:257–265. https://doi.org/10.17306/J.AFS.2016.3.25
Molina GA, Hernández-Martínez AR, Cortez-Valadez M et al (2014) Effects of tetraethyl orthosilicate (teos) on the light and temperature stability of a pigment from beta vulgaris and its potential food industry applications. Molecules 19:17985–18002. https://doi.org/10.3390/molecules191117985
Patil PD, Rao CR, Wasif AI et al (2014) Mass transfer enhancement through optimized extraction of a natural dye from Bougainvillea glabra Juss. bracts. Indian J Nat Prod Resour 5:332–337
Ravichandran K, Saw NMMT, Mohdaly A et al (2013) Impact of processing of red beet on betalain content and antioxidant activity. Food Res Int 50:670–675. https://doi.org/10.1016/j.foodres.2011.07.002
Saguy I, Kopelman IJ, Mizrahi S (1978) Thermal kinetic degradation of betanin and betalamic acid. J Agric Food Chem 26:360–362. https://doi.org/10.1021/jf60216a052
Schwartz SJ, Elbe JH (1983) Identification of betanin degradation products. Zeitschrift fur Leb und -forsch 176:448–453. https://doi.org/10.1007/BF01042560
Schwartz SJ, von Elbe JH (1980) Quantitative determination of individual betacyanin pigments by high-performance liquid chromatography. J Agric Food Chem 28:540–543. https://doi.org/10.1021/jf60229a032
Sengupta D, Mondal B, Mukherjee K (2015) Visible light absorption and photo-sensitizing properties of spinach leaves and beetroot extracted natural dyes. Spectrochim Acta A Mol Biomol Spectrosc 148:85–92. https://doi.org/10.1016/j.saa.2015.03.120
Simon P, Drdak M, Altamirano R (1993) Influence of water activity on the stability of betanin in various water/alcohol model systems. Food Chem 46:155–158. https://doi.org/10.1016/0308-8146(93)90029-F
Strack D, Vogt T, Schliemann W (2003) Recent advances in betalain research. Phytochemistry 62:247–269. https://doi.org/10.1016/S0031-9422(02)00564-2
Terradas F, Wyler H (1991) The secodopas, natural pigments in Hygrocybe Conica and Amanita Muscaria. Phytochemistry 30:3251–3253. https://doi.org/10.1016/0031-9422(91)83187
Viera I, Pérez-Gálvez A, Roca M (2019) Green natural colorants. Molecules 24:154. https://doi.org/10.3390/molecules24010154
Von-Elbe JH, Maing JY, Amundson CH (1974) Color stability of betanine. J Food Sci 39:334–337. https://doi.org/10.1111/j.1365-2621.1974.tb02888.x
Wybraniec S (2005) Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS/MS. J Agric Food Chem 53:3483–3487. https://doi.org/10.1021/jf048088d
Acknowledgements
We acknowledge the economical support from National Council of Science and Technology (CONACYT), Instituto Politécnico Nacional, ALTECSA S.A de C.V and Estherly M. Solis Rodriguez for English support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aztatzi-Rugerio, L., Granados-Balbuena, S.Y., Zainos-Cuapio, Y. et al. Analysis of the degradation of betanin obtained from beetroot using Fourier transform infrared spectroscopy. J Food Sci Technol 56, 3677–3686 (2019). https://doi.org/10.1007/s13197-019-03826-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03826-2