Abstract
Betalains are nitrogen-containing colorants with antioxidant properties that can be found in plant materials such as pitaya peels. However, thermo-stability of these natural colors may vary with different source, yet few study has reported the rate orders of degradation for pitaya-sourced betalains. In this study, accelerated storage test of betalains, namely betacyanin and betaxanthin, extracted from pitaya peel are investigated by heat treatment of the extract at elevated temperatures. The results show that degradation kinetics of betacyanins and betaxanthins can both fit first-order kinetics and Arrhenius equation with activation energies at − 49.2 kJ/mol and − 40.0 kJ/mol, respectively. The result of Student’s t-test indicated that the predicted k values are statistically the same as compared to their corresponding experimental values. LSD estimation also showed that k value variation tendency of the two betalains appears to be the same at 60 °C or below, while betacyanins tend to degrade faster above 80 °C than betaxanthins due to higher coefficient value of k value variation. This result also suggests that the pitaya-sourced betalains tend to degrade gradually even though they are stored under refrigerated condition. However, the betalains showed appreciably lower rate of degradation if they are processed at 60 °C or below.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The two categories of betalains, which include betacyanins and betaxanthins, have traditionally been used as food colorants for red-violet (betacyanins) and yellow-orange (betaxanthins) colors (Pavokovic and Krsnik-Rasol 2011). In recent decades, betalains have gained interest from scientific communities due to its health medicinal values (Zhang et al. 2013; Tesoriere et al. 2014; Khan 2016). Despite the health benefits, the thermal instability and molecular degradability drawbacks of natural colorants demonstrated in other studies (Chandran et al. 2014; Priatni and Pradita 2015; Güneser 2016; Rodriguez-Sanchez et al. 2017) also led to the importance of the investigations on the rate orders for betalain degradation.
Betalains, a group of nitrogen-containing colorants, can be extracted from various plant materials, including Caryophyllales, the flowers of Aizoaceae and Portulacaceae, red beetroots, and Cactaceae fruits such as pitayas (Delgado-Vargas et al. 2000; Slavov et al. 2013). In the past decades, betalains from different sources have been identified. The properties of betalains can vary with different plants in terms of betacyanin/betaxanthin ratio, water solubility, range of spectrum covered, and thermostability (Azeredo 2009). Hence, realizing the stability of betalains derived from each plant material is important while proposing a proper storage condition. In addition, the study reported by Rodríguez-Sánchez et al. (2015) also shows that, among the factors that cause molecular break down of betalains, storage temperature is one of the most critical ones.
In recent years, the properties of betalains extracted from the fruit of Hylocereus cacti, or pitayas, are found to be more promising than those from other sources (Azeredo 2009). Pitayas, or locally called dragon fruit, are cultivated in most tropical regions in the world, especially in Malaysia, Thailand, Vietnam, and Taiwan. Among the different genus of pitayas, white (Hylocereus undatus) and red pitayas (Hylocereus polyrhizus) are the most commonly grown (Merten 2003; Wiset et al. 2012). The peel of pitayas, representing approximately 18–24% of the whole fruit (Chuck-Hernández et al. 2016), can contain up to 150 mg of betacyanin per 100 g fresh sample (Jamilah et al. 2011). In practice, pitaya peels are discarded while the fruit pulps are processed or consumed. Therefore, recycling of pitaya peel for betalain extraction not only can reduce production cost of the health benefit colorants, but also create a higher economic value for the fruit. However, few studies have reported the rate orders for degradation of pitaya peel derived betalains.
Accelerated storage test (AST) has been used widely to estimate and predict shelf-lives of labile products in food, biology, and pharmaceutical industry (Franks 1994; Presa-Owen et al. 1995). Among the methods of AST, Arrhenius equation is most generally used in the valid assumption for deterioration (or degradation) rate of temperature-dependent compounds, components, or overall food products (King et al. 1998; Reyes and Cisneros-Zevallos 2007; Danisman et al. 2015; Yalçinöz and Erçelebi 2015; Ruiz-Gutiérrez et al. 2015; Priatni and Pradita 2015). Hence, by means of Arrhenius relationship, degradation kinetics of betalains extracted from pitaya peels can be predicted (Ruiz-Gutiérrez et al. 2015; Priatni and Pradita 2015).
In this study, AST of betalains extracted from pitaya peels were carried out to investigate the degradation kinetics of betacyanins and betaxanthins under different storage and processing temperatures. While predicting the rate constant (k) of betacyanins and betaxanthins at different storage temperatures, the predicted k values under refrigerated and ambient storage conditions were also compared with their corresponding experimental values.
Materials and methods
The procedures to investigate thermostability of betalains extracted from pitaya peels included handling of raw materials, extract preparation, heat treatment, sample analysis, calculation of betacyanin/betaxanthin content, and degradation kinetic studies of betacyanins and betaxanthins.
Handling of raw materials
Fresh pitaya peels from different sources were cleaned and cut into small pieces with an area of approximately 2 cm × 2 cm. The pitaya peel pieces were well-mixed and stored under − 18 °C for not more than 14 days.
Extract preparation
To prepare 10% pitaya peel extract, 100 g pitaya peel was added into a Waring Blender filled with 900 g deionized water and blended for 30 s. Pitaya peel extract was obtained after passing through a 200 μm filter.
Heat treatment
Twenty grams of the pitaya peel extract was added into a 100 mL blue cap bottle and placed within an oil bath at 70, 80, 90, 100, 110, and 120 °C for 1, 3, 5, 10, and 30 min, respectively. For extracts stored under refrigerated (4 °C) and ambient (25 °C) conditions, samples were taken every 2 h for 8 h in total. The contents of betacyanins and betaxanthins were then analyzed.
Sample analysis
Analysis of betacyanin and betaxanthin content was according to the method reported by Castellar et al. (2003), Stintzing et al. (2005), and Sumaya-Martinez et al. (2011) with some modification. After heat treatment, pitaya peel extract samples were centrifuged at 11,200×g for 2 min to remove the sediments. The samples were analysed by photospectrometer (Hitachi UV–Vis U-2800, Japan) at 535 nm and 480 nm respectively for analysis of betacyanin and betaxanthin content. Absorbance readings for all samples were made against distilled water as a blank.
Calculation of betacyanin/betaxanthin content
According to Beer Lambert’s Law, betacyanin and betaxanthin content can be calculated by using the equation below (Ruiz-Gutiérrez et al. 2015; Priatni and Pradita 2015):
where A is the absorbance at 535 or 480 nm; MW is the molecular weight of betanin (550 g/mol) or indicaxanthin (308 g/mol); DF is the dilution factor of sample; ɛ is the extinction coefficient of betanin (60,000 L/mol cm) or indicaxanthin (48,000 L/mol cm); l is the pathway of light transmittance (1 cm). The contents of betacyanin and betaxanthin are expressed as mg betanin and indicaxanthin equivalent/L extract, respectively.
Degradation kinetic analysis
The degradation kinetics of betacyanin and betaxanthin content were described by fitting first-order kinetic model to the experiment data based on the equation below:
where w0 is the content of betacyanin and betaxanthin before heat treatment; w(t) is the content of betacyanin and betaxanthin at time t (min); k (min−1) is the rate constant at the temperature determined; and t is the time of heat treatment.
Temperature dependence of betacyanin and betaxanthin degradations can be determined by calculating the activation energy (Ea) values based on Arrhenius equation (Chandran et al. 2014):
where k(T) is the k value (min−1) at temperature T (K); A is the pre-exponential factor; R is the universal gas constant [8.314 × 10−3 kJ/(K mol)]; Ea is the activation energy (kJ/mol); and T (K) is the temperature of heat treatment.
Statistical analyses
Duplicate results were obtained for all experiments performed. Arithmetic means, standard deviations, correlation coefficients, Student t-test, and Fisher Least Significant Difference (LSD) were calculated using Microsoft Excel 2010 and Sigma Plot 10.0. Student t-test and Fisher LSD were respectively used to estimate statistical significance of the predicted k values and the correlation coefficients of betalain k values at different temperatures.
Results and discussion
Thermostability of betalains
The overall result in this study shows that betalains extracted from pitaya peels are found to be thermos-unstable. The content of betalains decreased with increasing time of heat treatment, while the degradation rate also enhanced at elevated temperatures. The degradation kinetics of betacyanins and betaxanthins extracted from pitaya peels are shown in Fig. 1.
Data shown in Fig. 1a, b indicated that degradation of pitaya-peel-based betacyanins and betaxanthins fits first-order kinetics during heat treatment from 70 to 120 °C. It was also found in the comparison between the degradation kinetics of betacyanins and betaxanthins that the k values for both components were approximately 0.008 at 70 °C. However, the value for betacyanin degradation increases 10 times (0.08) as the temperature increased to 120 °C. At the same temperature, k value for betaxanthins was 0.04, only 5 times increase. The result in Fig. 1 can therefore imply that thermal instability of betacyanins appeared to be higher than that of betaxanthins at elevated temperatures, namely 70–120 °C. The result can also be observed from the residual yellow color of pitaya peel extract after 30 min heat treatment (Table 1).
k values and activation energies
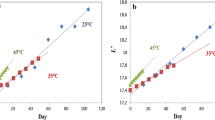
To predict the degradation kinetics of pitaya-peel-based betalains stored under different temperatures, regression lines of k values for betacyanins and betaxanthins found at different temperatures were created based on Arrhenius model, namely Arrhenius regression lines (Fig. 2).
In Fig. 2, the activation energies (Ea) for betacyanin and betaxanthin degradation are found to be − 49.21 kJ/mol (or − 11.76 kcal/mol) and − 38.99 kJ/mol (or − 9.32 kcal/mol), respectively. Ea for betacyanin found in this study appears to be lower as compared to that reported by Priatni and Pradita (2015), − 14.37 kcal/mol, in the investigation for degradation kinetics of betacyanins extracted from red dragon fruit. In addition, the study reported by Chandran et al. (2014) related to heat treatment of beetroot to determine the degradation of visual color also indicated that betaxanthins can show higher stability at elevated temperatures than betacyanin and degradation of both pigments follow first order kinetics and Arrhenius model. In other words, the content of betacyanin and betaxanthin at different storage temperatures can be predicted based on the Arrhenius regression lines.
Predicted and experimental k value comparison
The predicted k values under refrigerated (4 °C) and ambient temperature (25 °C) storage condition of betacyanins and betaxanthins, have been compared with their corresponding experimental values. Statistical significance of these predicted and experimental values is estimated by Student’s t-test (P = 0.95) as shown in Table 2.
Data presented in Table 2 showed that the predicted k values for storage of betacyanins and betaxanthins under refrigerated (4 °C) and ambient temperature (25 °C) condition were within the confidence interval, which indicates that no statistically significant difference as compared with its corresponding experimental k value (t value < t0.05). This can also designate that there was no significant difference between predicted and actual results at the storage temperatures and time intervals tested.
Variation of k values at different temperature
In Fig. 3a, b, regression coefficients of k value variation at different temperatures have been determined for betacyanin and betaxanthin degradation. It can be seen that the changes of first-order coefficient values for both betacyanin and betaxanthin degradation are both found at 60–80 °C. According to the estimation based on Fisher Least Significant Difference (LSD) at P = 0.05, the tendency of the k value variations for both betacyanins and betaxanthins are found to be identical at temperatures below 60 °C. The tendency can also be observed from the identical first-order coefficient (1.00E−04) shown in Fig. 3a, b. This also explains why color change can hardly be observed from the pitaya peel extract samples of which heat treatment were carried out at 70 °C (data not shown). Above 80 °C, however, the rates of k value increase appear to be approximately 7 (for betaxanthin) or 15 (for betacyanins) times higher, while betacyanins show appreciably higher growth than betaxanthins. This finding implies a higher color stability of the two colorants at 60 °C or below, suggesting a temperature limit of their application or process (e.g. drying, extraction, sterilization…etc.).
The tendency of k value variation below 60 °C can be attributed to reversibility of Schiff base reactions, which suggested by both Elbe et al. (1981) and Molina et al. (2014) in the stability study of red-beet derived betalains. Results for both studies indicated that the color maintenance of betalains due to degradation reversibility can mostly occur at temperatures below 66 °C (or below 60 °C found in this study). As for the tendency of k value variation above 80 °C, the study of yellow pitaya peel color stability reported by Cejudo-Bastante et al. (2016) also agreed that betalains can suffer a pronounced degree of degradation at 80 °C or higher. The same tendency can also be observed from the data in Fig. 1, which shows that the k values (or slopes) for betacyanins and betaxanthins appear to be similar at 70 °C, while a more significant difference of k values for the two categories of betalains is found at 80–120 °C.
The main cause of this situation can be due to reversibility and degradability of the broken down molecules of betalains, such as betalmic acid from betacyanins and betaxanthins, cyclo-Dopa from betacyanins, and amines from betaxanthins. These molecules can undergo Schiff base condensation which leads to betanin regeneration under a lower processing temperature. However, subsequent degradation or reaction, such as aldol condensation or Millard reaction, of these broken down molecules can mostly occur at higher processing temperatures (e.g. 80 °C or above found in this study), and hence, leading to loss of color (Woo et al. 2011). The overall finding of the above data also indicates that aqueous solution for both betacyanin and betaxanthin are highly unstable. However, thermos-degradation can be retarded significantly if they are stored under lower temperature.
Conclusion
Betacyanins and betaxanthins are two categories of valuable colorants that can be extracted easily from pitaya peels. Despite the thermos-unstable properties, their degradation fits first order kinetics and Arrhenius model with activation energies of − 49.2 kJ/mol (betacyanin) and − 40.0 kJ/mol (betaxanthin). In the comparison of predicted and experimental k values based on Student’s t-test, no significant difference was found between betacyanins and betaxanthins, indicating that the predicted value can be designated as reliable. The regression coefficients of k value variation for betacyanins and betaxanthins showed that a transition was found at 60–80 °C. According to the result of LSD estimation, the tendency of the k value variations for the two colorants is found to be identical at temperatures below 60 °C. Above 80 °C, the regression coefficients appeared to increase significantly, while betacyanins is expected to be higher than that of betaxanthin, which implies that the colorants can be more stable and increased rate of degradation can be avoided if they are processed below 60 °C. Data presented in this study shows that both pitaya-sourced aqueous betacyanins and betaxanthins can degrade gradually even though they are stored under refrigerated conditions, yet the appreciably reduced rate constant (k value) of degradation below 60 °C also suggests a tolerance limit of their processing temperature. To improve the stability of betalains, some countermeasures are to be investigated in future studies.
References
Azeredo HMC (2009) Betalains: properties, sources, applications, and stability—a review. Int J Food Sci Technol 44:2365–2376
Castellar R, Obon JM, Alacid M, Fernandez-Lopez JA (2003) Color properties and stability of betacyanins from opuntia fruits. J Agric Food Chem 51:2772–2776
Cejudo-Bastante MJ, Hurtado N, Delgado A, Heredia FJ (2016) Impact of pH and temperature on the colour and betalain content of Colombian yellow pitaya peel (Selenicereus megalanthus). J Food Sci Technol 53(5):2405–2413
Chandran J, Nisha P, Singhal RS, Pandit AB (2014) Degradation of colour in beetroot (Beta vulgaris L.): a kinetics study. J Food Sci Technol 51(10):2678–2684. https://doi.org/10.1007/s13197-012-0741-9
Chuck-Hernández C, Parra-Saldívar R, Sandate-Flores L (2016) Pitaya (Stenocereus spp.). Encyclopeadia of food and health. Elsevier, Amsterdam, pp 385–391
Danisman G, Arslan E, Toklucu A (2015) Kinetic analysis of anthocyanin degradation and polymeric colour formation in grape juice during heating. Czech J Food Sci 33(2):103–108
Delgado-Vargas F, Jimenez AR, Paredes-Lopez O (2000) Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr 40(3):173–289
Elbe JH, Schwartz SJ, Hildenbrand BE (1981) Loss and regeneration of betacyanin pigments during processing of red beets. J Food Sci 46:1713–1715
Franks F (1994) Accelerated stability testing of bioproducts: attractions and pitfalls. Trends Biotechnol 4:114–117
Güneser O (2016) Pigment and color stability of beetroot betalains in cow milk during thermal treatment. Food Chem 196:220–227
Jamilah B, Shu CE, Kharidah M, Dzulkifly MA, Noranizan A (2011) Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int Food Res J 18:279–286
Khan MI (2016) Plant betalains: safety, antioxidant activity, clinical efficacy, and bioavailability. Compr Rev Food Sci Food Saf 15:316–330. https://doi.org/10.1111/1541-4337.12185
King VAE, Lin HJ, Liu CF (1998) Accelerated storage testing of freezed-dried and controlled low-temperature vacuum dehydrated Lactobacillus acidophilus. J Gen Appl Microbiol 44:161–165
Merten S (2003) A review of Hylocereus production in the United States. J Prof Assoc Cactus 5:98–105
Molina GA, Hernández-Martínez AR, Cortez-Valadez M, García-Hernández F, Estevez M (2014) Effect of tetraethyl orthosilicate (TEOS) on the light and temperature stability of a pigment from Beta vulgaris and its potential food industry applications. Molecules 19:17985–18002
Pavokovic D, Krsnik-Rasol M (2011) Complex biochemistry and biotechnological production of betalains. Food Technol Biotechnol 49(2):145–155
Presa-Owen S, Lopez-Sabater MC, Rivero-Urgell M (1995) Shelf-life prediction of an infant formula using an accelerated stability test (Rancimat). J Agric Food Chem 43:2879–2882
Priatni S, Pradita A (2015) Stability study of betacyanin extract from red dragon fruit (Hylocereus polyrhizus) peels. Procedia Chem 16:438–444
Reyes LF, Cisneros-Zevallos L (2007) Degradation kinetics and colour of anthocyanins in aqueous extracts of purple- and red-flesh potatoes (Solanum tuberosum L.). Food Chem 100:885–894
Rodríguez-Sánchez M, Amaya-Guerra CA, Quintero-Ramos A, Perez-Carrillo E, de Ruiz-Anchondo T, Baez-Gonzalez J, Melendez-Pizarro C (2015) Effect of extrusion cooking on bioactive compounds in encapsulated red cactus pear powder. Molecules 20:8875–8892. https://doi.org/10.3390/molecules20058875
Rodriguez-Sanchez JA, Victoria MTC, Barragan-Huerta BE (2017) Betaxanthins and antioxidant capacity in Stenocereus pruinosus: stability and use in food. Food Res Int 91:63–71
Ruiz-Gutiérrez MG, Amaya-Guerra CA, Quintero-Ramos A, Pérez-Carrillo E, de Ruiz-Anchondo T, Báez-González J, Meléndez-Pizarro C (2015) Effect of extrusion cooking on bioactive compounds in encapsulated red cactus pear powder. Molecules 20:8875–8892
Slavov A, Karagyozov V, Denev P, Kratchanova M, Kratchanov C (2013) Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech J Food Sci 31(2):139–147
Stintzing FC, Herbach KM, Mosshammer MR, Carle R, Yi W, Sellappan S, Akoh CC, Bunch R, Felker P (2005) Color, betalain patter, and antioxidant properties of cactus pear (Opuntia spp.) clones. J Agric Food Chem 53:442–451
Sumaya-Martinez MT, Crusz-Jaime S, Madrigal-Santillan E, Garcia-Paredes JD, Carino-Cortes R, Cruz-Cansino N, Valadez-Vega C, Martinez-Cardenas L, Alanis-Garcia E (2011) Betalain, acid ascorbic, phenolic contents and antioxidant properties of purple, red, yellow and white cactus pears. Int J Mol Sci 12:6452–6468
Tesoriere L, Attanzio A, Allegra M, Gentile C, Livrea MA (2014) Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-kB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1b-exposed Caco-2 cells. Br J Nutr 111:415–423. https://doi.org/10.1017/S0007114513002663
Wiset L, Poomsa-ad N, Srilaong V (2012) Comparisons of antioxidant activity and bioactive compounds of dragon fruit peel from various drying methods. Int J Biol Biomol Agric Food Biotechnol Eng 6(10):943–946
Woo KK, Ngou FH, Ngo LS, Soong WK, Tang PY (2011) Stability of betalain pigment from red dragon fruit (Hylocereus polyrhizus). Am J Food Technol 6(2):140–148
Yalçinöz SK, Erçelebi EA (2015) Anthocyanin degradation and colour kinetics of cournelian cherry concentrate. Br J Appl Sci Technol 10(4):1–12
Zhang Q, Pan J, Wang Y, Lubet R, You M (2013) Beetroot red (betanin) inhibits vinyl carbamate- and benzo(a)pyrene-induced lung tumorigenesis through apoptosis. Mol Carcinog 52(9):686–691. https://doi.org/10.1002/mc.21907
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chew, Y.M., Hung, CH. & King, V.AE. Accelerated storage test of betalains extracted from the peel of pitaya (Hylocereus cacti) fruit. J Food Sci Technol 56, 1595–1600 (2019). https://doi.org/10.1007/s13197-019-03673-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03673-1