Abstract
This study was planned to characterize the physicochemical and antioxidant properties, and microbiological quality of honey obtained from the sandbar pumpkin field. In this study, four sugar supplemented and one control (without sugar fed) honey sample was used. Results revealed that all samples exhibited appropriate maturity considering their low moisture content (~ 19%) and high total solids (~ 80%) and TSS (~ 79%). Total acidity (< 40 meq/kg) and pH (~ 4.5) directed the absenteeism of detrimental fermentation. Ash (~ 0.29%) and electrical conductivity (~ 700 µS/cm) were reasonable and distinctive of dark yellowish-brown honey, which is buttressed by color attributes. Reducing sugars, glucose, fructose, and sucrose values ranged from 68.98 to 75.82%, 26.01 to 33.84%, 34.93 to 38.70%, and 1.74 to 5.96%, respectively. Proline (~ 400 mg/kg), HMF (< 40 mg/kg) and diastase action (~ 14° Gothe) were found within accepted limits, and also possesses good antioxidants in terms of total phenol (~ 160 mg GAE/100 g), total flavonoid (4.67–6.25 mg CE/100 g), and DPPH-RSA (30.65–35.97%). The microbial study revealed that the total viable count ranged between 33.33 and 27.66 CFU/g, while yeasts and mold count varied between 14.33 and 12 CFU/g. Principle component analysis (PCA) results revealed that all the studied parameters could be used effectively to discriminate the honey sample. The overall results signpost a new information regarding the quality i.e. processing, maturity, freshness and composition of honey obtained from the sandbar pumpkin field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Honey is a sweet and flavorful fluid substance originated from nectar derived from flowers by honey bees (Apis mellifera), which has been expended as a high nutritive nourishment (Babarinde et al. 2011). Honey is reported to contain about 200 substances with an extraordinary potential to fill in as characteristic nourishment cell reinforcement (Küçük et al. 2007). This wholesome product essentially contains carbohydrate and some other elements like, amino acids, vitamins, minerals (Nayik et al. 2018), along with volatile chemicals, phenolic acids, flavonoids, and carotenoid like substances (Singh and Singh 2018), which have strong antioxidant capacity with beneficial effects against various degenerative diseases viz. cancer, inflammation, cardiovascular disease etc. (Nayik and Nanda 2016). Traditionally, honey is used as medicine to cure asthma, burns, infected wounds, gastrointestinal disorders and skin ulcers and efficient in expanding the total plasma antioxidant and free radical lessening capacity in humans (Küçük et al. 2007). The composition and quality of honey depend on the climatic conditions, beekeeping practices, and the composition of nectar, and place of origin (Nayik et al. 2018). However, the botanical origin of honey is evaluated through the analysis of its physicochemical properties (Küçük et al. 2007; Gomes et al. 2010). Moreover, wholesomeness and contaminant-free product are other factors of great concern for consumer well-being. The main routes for microbial contamination of honey are pollen, nectar, digestive tracts of honeybees and soils and also through honey handlers and processing i.e. lack of following good manufacturing practices (Gomes et al. 2010). Moreover, knowledge of physicochemical and microbiological properties of honey is very important to set up certification marks, expand the beekeeping practices, and also for a probable export.
Pollination is an important step for the monoecious and obligate cross-pollinated crops like pumpkin, where the substitution of pollen to the pistil of the female floret is essential (Rashid et al. 2018). Due to the lack of flora, very few pollinating agents are found in the sandbar areas. Pumpkin growers are typically fertilizing the female flowers by hand pollination, which is a cumbrous, laborious, complex task that hampers the pumpkin production. However, insects, especially bees (Apis mellifera) are the main pollinating agent in the sandbar cropping system that can effectively fertilize the flowers as well as produce honey. Moreover, carbohydrates, proteins, lipids, vitamins, and minerals are the main nutrients required for the growth and development of honey bee (Rashid et al. 2018). They take carbohydrate from nectar and proteins from the pollen. Pumpkin flowers are rich in pollen; however, the amount of nectar is not sufficient to meet up the nutrient requirements as well as honey production. Moreover, due to the lack of diversified crops in the sandbar area, it is difficult to meet up the nectar requirement from other sources to increase honey production. Supplement feeding to honey bee is thus required to supply the nutrients, when the natural food sources are inadequate or not available. Pumpkin flowers are large and contain a huge amount of pollen with a satisfactory level of nectar, which become dried due to the hot environment in the sandbar area during the day time (Rashid et al. 2018). Therefore, supplement feeding to honey bee with sugar syrup can meet up the shortage of nectar, which encourage the bees to increase their population, collection of pollen and honey production (Sahinler et al. 2004).
Sugar is the main commodity to feed honey bees when the availability of natural nectar becomes scared. In the last few decades, a significant number of studies were found that reported the quality characteristics of honey from different origin and geographical locations. Up to date, the literature on honey production by supplementary feeding to the honey bee are few, and no study on the physicochemical and microbial characterization of the honey supported by sugar syrup feeding of bees was reported elsewhere. In the current study, we reported the physicochemical and microbiological characteristics of honey obtained from the sandbar pumpkin cropping system.
Materials and methods
Collection of honey sample and Chemicals
Five honey samples were collected from the northwest sandbar pumpkin field of Bangladesh, where the farmer adopted supplementary feeding technology for the honey bee to pollinate pumpkin flower to produce the pumpkin. These five samples were obtained from five different sugar-syrup feeding treatments, e.g. honey-1 (white sugar: water = 2:1), honey-2 (white sugar: water = 1.5:1), honey-3 (white sugar: water = 1:1), honey-4 (brown sugar: water = 2:1) and honey-5 (without sugar). Samples were stowed in sterilized plastic bottles, transported to the laboratory, and kept in the refrigerator at 4 °C until analysis. Analytical grade chemicals were procured from the Merck chemicals, Germany.
Physical properties of honey
Moisture and ash content
The moisture and ash content of honey was determined following the procedure of AOAC (1990) and expressed as the percentage.
Electrical conductivity (EC)
A conductivity meter (HI86303, Hanna Instruments, Mauritius) was used to measure the EC from a solution containing 10 g honey in a 75 mL of deionized water, and the results were stated as μS/cm.
pH and titrable acidity
Ten gram of honey was mixed properly in a 75 mL distilled water and the pH of that honey slurry was measured using a pH meter (HI 98127, Hanna instruments, Mauritius). Titrable acidity (TA) was determined by titration of a known quantity of a sample (5 mL) against 0.1 N sodium hydroxide and results were expressed as milliequivalents per kilogram using the following equation:
Total soluble solids (TSS) and total solids (TS)
The amount of total soluble solids (°Brix) was determined using a refractometer (Q767-B, Tokyo, Japan) at 20 °C while total solids content (%) was computed following the equation below (Saxena et al. 2010):
Measurements of color parameters
The surface color of the samples was evaluated with a spectrophotometer (CM2500d, Konica, Minolta Optics Inc., Japan) based on the CIE L*a*b* color space, where L* signify brightness, a* for the red–green while b* corresponds to the yellow–blue color gradient. Three measurements were conducted on each sample. The Hue angle (H) and Chroma (C) were computed in relation to the formula below:
where L*, a*, and b* were Hunter L*, a*, and b* values.
Biochemical analysis of honey
Sugar Analysis
The Layne–Enyon technique as explained in AOAC (1990) was used for the estimation of reducing sugar. Briefly, 5 mL of Fehling’s solution A and B were taken in a 250 mL Erlenmeyer flask with 7 mL H2O and 15 mL of honey. With this solution, 1 mL 0.2% methylene blue indicator was added. Thereafter, titration was continued with heating the solution until decolorization of the indicator. Amount of sucrose was determined using the inversion process. In short, 50 mL of honey was taken in a 100 mL volumetric flask in which 10 mL dilute HCl was added followed by heating in a water bath, and volume was made up to the mark. Again, the Layne–Enyon procedure was followed for this solution. Amount of sucrose was calculated using the formula of Saxena et al. (2010):
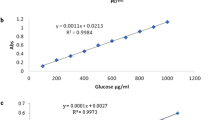
The amount of glucose was determined using the enzymatic oxidation process modified by Buba et al. (2013) while the fructose was determined utilizing the resorcinol reagent method described in AOAC (1990).
Proline content
Proline was measured by applying the protocol used by Meda et al. (2005) with slight alteration. Briefly, 0.5 mL of honey (0.05 g/mL) was assorted with 1 mL formic acid (80%) and 1 mL ninhydrin solution (3% in ethylene glycol monomethyl ether) and shaken vigorously for 15 min. This mixture was heated to boiling for 15 min in a water bath and then heated at 70 °C for 10 min. To this mixture, 5 mL 2-propanol (50%, v/v) was added and cooled. After 45 min, the mixture was removed from the water bath and the absorbance was determined at 510 nm against the water blank. Proline (0.032 mg/mL) was used as the standard solution. Proline concentration (mg/kg) of honey was calculated from the equation below:
where Es and Ea are the absorbances of the sample and proline standard, respectfully; E1 is the amount (mg) of proline required for the standard preparation, and E2 is the amount (g) of honey; 80 is the dilution factor.
Hydroxymethylfurfural (HMF)
Hydroxymethylfurfural was computed following the procedure of AOAC (1990). In short, 5 g honey was liquefied with 25 mL deionized water, treated with 1 mL Carrez I and Carrez II solution (1:1 v/v) and the volume was made up to 50 mL and filtered. Then, first 10 mL of the filtrate was discarded and treated with NaHSO3, and the absorbance was measured at 284 nm and 336 nm. HMF was computed from the equation below:
Diastase content
Diastase activity was calculated using the method of AOAC (1990) as described by Gomes et al. (2010). Briefly, a buffered starch solution (soluble) and honey was heated at 40 °C in a thermostatic bath. From this mixture, 1 mL of the aliquot was taken at 5 min intervals, and absorbance was recorded at 660 nm using UV/VIS spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments LTD.). Utilizing the regression, the absorption data were fitted, and the diastase activity was figured from the time taken for the absorbance to achieve 0.235 and the outcomes were stated in Gothe degrees as mL of 1% starch hydrolyzed by enzyme in 1 g of honey in 1 h.
Total phenolic content
The total phenolic content of the analyzed sample was determined by the Folin–Ciocalteau method (Nayik and Nanda 2016) with slight modification. The extracted solution was obtained using 1 g sample mixed with 40 mL of 100% methanol in a separate glass beaker and stirred for 4–5 min. At that point, the blends were concentrated to 10 mL by heat utilizing hotplate stirrer followed by adding 10 mL of 100% methanol. From these mixtures, an aliquot of 1 mL of each sample was taken in glass test tubes to which 0.2 mL 10% Folin–Ciocalteau reagent was added and vortexed for 3 min. Then, 0.8 mL of 7.5% Na2CO3 was added to that mixture and kept it in a dark place for 1 h before measuring the absorbance at 760 nm using a spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments LTD.) against the blank. Total phenolic content (mg GAE/100 g) was determined using the following formula by comparison of the values obtained with the standard curve of gallic acid (R2 = 0.985).
Total flavonoid content
The total flavonoid content of honey samples was determined following the protocol used by Kim et al. (2003) with slight modifications. Briefly, 15 g honey sample was dissolved in 50 mL of methanol (99%) and mixed properly (Can et al. 2015). Then, 1 mL honey solution was taken in a centrifuge tube to which 4 mL distilled water and 0.3 mL of 5% NaNO2 were added and mixed properly. After 5 min of residence, 0.3 mL of 10% AlCl3 was added to the solution and kept rest for 1 min. Then, 2 mL of 1 M NaOH and 2.4 mL of distilled water was added and the solution was mixed thoroughly following centrifugation at 4000 rpm for 5 min. The solution was then kept in dark for 15 min before taking the absorbance at 510 nm using a UV–Vis Spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments LTD.) against the blank prepared in similar manner without sample (replaced with methanol). The total flavonoid was calculated from the standard curve of catechin and expressed as mg catechin equivalent per 100 g honey (mg CE/100 g).
DPPH radical scavenging activity (DPPH-RSA)
The DPPH radical scavenging activity of honey samples was carried out following the protocol previously described by (Nayik and Nanda 2016) with some modification. Briefly, 0.1 mL of the previously prepared honey solution was taken in a centrifuge tube in which 1.9 mL of 0.3 mM DPPH solution was added and mixed properly. The solution was kept in dark for 30 min before taking the absorbance at 517 nm using a UV–Vis Spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments LTD.). The DPPH-RSA was calculated from the following equation and expressed as % inhibition.
Microbiological analysis
The number of viable bacteria and yeast were computed following the protocol of Babarinde et al. (2011). One milliliter of honey was transferred to a sterile bottle to which 9 mL of sterile deionized H2O was added, and made into a homogeneous suspension. From this 1 mL of 10−1–10−6 dilutions were made on plates holding PCA (plate count agar) and incubated at 37 °C for 24 h. Those plates over-loaded with bacterial colonies were avoided and colonies on each plate were counted having 30–300 colonies using a haemacytometer (Labtronics, Model No. 37, Korea). Finally, the total number of bacteria present was calculated using the following equation:
The same procedure was followed to yeast and mold count using potato dextrose agar (PDA), and PDA plates were kept for 6 days in an incubator at 30 °C. After 6 days, the colonies were counted and calculated for any yeast growth using the above equation.
Statistical analysis
Triplicate analysis was carried out in each case, and results were reported as the mean ± standard error of three replicates. Statistical analysis was accomplished using IBM SPSS statistical software (Version 20). The significant difference (P < 0.05) among the contents of the honey sample was carried out by ANOVA (Analysis of variance) procedure. Principal component analysis (PCA) was performed using XLSTAT 2018 (Addinsoft, New York, USA).
Results and discussion
Physical characteristics of supplement fed honey
Moisture content
Information about the moisture content is important to prevent mold growth in honey, for improving conservation and storage, which are associated with the maturity of honey (Singh and Singh 2018). The moisture content of the analyzed honey was found well below the imposed limit (< 20%) of the European Comission (2002) and ranged from 15.95 to 19.67% (Table 1). These values were analogous to the previous reports (Bath and Singh 1999; Saxena et al. 2010; Silva et al. 2013; Sousa et al. 2013). A high amount of moisture is responsible for the undesirable fermentation of honey during storage, where osmotolerant yeast takes advantage to form C2H6O and CO2. This alcohol further oxidized to CH3COOH and H2O, and gives a bitter taste of honey (Imtara et al. 2018). However, moisture content in honey is strongly correlated to the floral source, climatic conditions, handling techniques, and maturity period etc. (de Sousa et al. 2016; Nayik et al. 2018).
Total solids and total soluble solids
Table 1 indicates a significant difference in percent total solids of investigated honey and was fluctuated from 80.33 to 84.04%. The values of total solids obtained were in line with the reports of Babarinde et al. (2011) and Saxena et al. (2010), they found 72.2–76.5% and 78.4–82.8%, respectively. Sugars i.e. glucose and fructose mainly comprise the total solids present in honey accounting for about 85% (Babarinde et al. 2011). The °Brix or TSS is closely connected to the amount of sugars existing in honey, making it an essential marker of conceivable adulteration. Data presented in Table 1 revealed that the °Brix of the studied honey extended from 76.78 to 79.38. This finding is well corroborated the previous reports for TSS of honey from different sources (Souza et al. 2006; Saxena et al. 2010; de Sousa et al. 2016). As can be seen in Table 1, honey-4 had the highest content of total solids and TSS while it is lowest in honey-1, and fount to be differed significantly (P < 0.05) among the honey samples. This might be due to the variation in the concentration of sugar syrup used to feed the honey bee.
Ash and electrical conductivity (EC)
The botanical source of honey is assessed by its minerals i.e. ash content. The ash content of studied honey extended from 0.17 to 0.31% (Table 1), which was analogous to the range of 0.03–0.43% reported by Saxena et al. (2010), and also corroborated the Codex standard (< 0.6%). However, the data obtained from the analyzed honey were relatively lower than those observed by Baroni et al. (2009). The amount of ash contained in the investigated honey signposted that they could aid as an ample source of dietary minerals. However, variation in the ash content of honey might be due to beekeeping practices, harvesting method, the nectar source and geographical location (Saxena et al. 2010). The EC of honey is strongly associated with the content of minerals, proteins, and organic acids. The EC of investigated honey ranged from 631.95 to 804.54 μS/cm (Table 1), and a significant difference (P < 0.05) exists between examined honey samples. The values for EC of all honey sample was comparable to the standard limit (≤ 800 μS/cm) approved by the Codex Alimentarius Commission (1981) with an exception of honey-1. The high amount of ash contained in the honey sample might have influenced to the intensification of the electrical conductivity, which is corroborated by the previous reports (Downey et al. 2005). However, the ash and electrical conductivity were found to have a linear association between themselves (y = 843.48x + 507.47), which was portrayed by the correlation coefficient (R2 = 0.9881). The EC value of analyzed honey might be governed by its ash, protein, acid content and also by the influence of the geographical region and season of honey production (Azonwade et al. 2018).
Titrable acidity and pH
The acidity level of the analyzed honey ranged from 24.32 to 37.55 meq/kg (Table 1), which was found within the specified limit of the Codex standard (≤ 40 meq/kg). Previous studies stated a higher range of acidity of honey, e.g. 29.5–41.5 meq/kg and 35.7–40.5 meq/kg was reported by Singh and Bath (1997) and Azonwade et al. (2018), respectively. According to Baroni et al. (2009), the acidity of honey varied from 24.4 to 25.4 meq/kg, and changes with the source of nectar (Sahinler et al. 2004). Inappropriate processing, early harvesting, immature honeycombs and broods, the action of microorganisms (e.g. Xerotolerant yeast) can speed up the rate of honey fermentation, which increases the level of total acidity (Sahinler et al. 2004).
From Table 1, it can be seen that the investigated honey was acidic (pH 3.66–4.59) and its pH remained within the recommended limit (pH 3.40–6.10) of the Codex Alimentarius Commission (1981), which ensures honey freshness. pH values of analyzed honey corroborated the values reported elsewhere previously (Saxena et al. 2010). It is clarified that fermentation of honey sugar is largely induced by the acidic environment and contributes to the characteristic honey flavor. This acidic environment provides stability against spoilage of honey caused by microorganisms. Moreover, high acidity is an indicator of the high amount of minerals.
Color attributes of honey
The color attributes of supplement fed honey are shown in the Table 2. Honey with L value over 50 is considered as lighter honey while L value below 50 is dark honey (Saxena et al. 2010). Studied honey exhibited lower L value (27.91–32.15), thus it could be considered as dark honey. From the values of a* (1.86–3.01) and b* (10.64–11.77) of the analyzed honey, it can be seen that the honey had red, yellow and green components, which is corroborated by the hue angle (74.65–80.93) and color saturation (10.93–11.92). Because, hue angle is the quality that differentiates color while chroma determines the strength of the hue i.e. color intensity or saturation. However, color change occurs due to the pigment degradation, enzymatic or non-enzymatic browning reaction (García-Martínez et al. 2013). It might possibly due to the use of sugar syrup to feed honey bees, the reaction between sugar and protein molecule present in honey and sandbar environment might induce the pigment degradation.
Biochemical outlining of sugar fed honey
Sugar profile
The composition sugar in honey mainly depends on different factors as such phyto-geographic source of the honey, and is swayed by beekeeping practices and storage conditions (Nayik et al. 2016). Table 3 illustrated the sugar profile of studied honey. The amount of reducing sugar existing in analyzed honey’s varied between 68.98 and 75.82%, which differed significantly (P < 0.05) among the sample. This range is relatively comparable to the reports of previous studies conducted elsewhere (Saxena et al. 2010; Buba et al. 2013; Silva et al. 2013). Glucose and fructose are the leading reducing sugar in honey. As can be seen in Table 3 that honey-4 had the highest glucose while this value is lowest for honey-1. This might be due to variation in sugar syrup fed to the honey bee. Table 3 exhibited that amount of fructose in the analyzed honey fluctuated between 34.93 and 38.70% while the glucose content was found within a range of 26.01–33.84%. However, the investigated honey had a higher amount of fructose than the glucose. Normally, fructose present in honey dominates slightly over glucose, but has some honey sources (e.g. rape and dandelion), which contained more glucose than fructose (Kirs et al. 2011). Moreover, the sucrose content was found to fluctuate from 1.74 to 5.96% (Table 3). This range is comparatively higher than the range recorded by Küçük et al. (2007). However, all samples had a sucrose level within the approved limits of the Codex standard except honey-4. Generally, a high amount of sucrose present in honey is an indication of an early harvest of honey, and sucrose is unable to transform into glucose and fructose at this stage or excessive feeding of sugar syrup might contribute to this fact (Küçük et al. 2007). Interestingly, fructose and glucose of examined honey covered more than 60% of the honey weight. The sweet taste of honey is greatly affected by the F/G (fructose to glucose) ratio since fructose is much sweeter than glucose (de Sousa et al. 2016). The present study revealed that the ratio of fructose to glucose was fluctuated between 1.14 and 1.34 while the glucose/moisture ratio was found to vary between 1.32 and 2.12 (Table 3). This indicates the floral origin as honey obtained from flowers have a fructose to glucose ratio around 1 and it is about 1.5–2.0 for honeydew honey (Kirs et al. 2011). The early investigation reported that crystallization of honey occurred naturally that depends on the sugar concentration and moisture content, and mostly governed by the two major sugars i.e. glucose and fructose and glucose to moisture ratio (Nayik et al. 2016). Honey having a higher concentration of glucose tends to crystallize faster than fructose (Babarinde et al. 2011) due to the fact that fructose is relatively more water soluble than glucose. Interestingly, the glucose/moisture ratio of investigated honey ranged from 1.32 to 2.12, and had a higher concentration of fructose than glucose, which signposts their less susceptibility to early crystallization. The variation in sugar profile among the honey samples might be because of sugar syrup concentration used in this study to feed up the honey bee.
Proline content
Proline represents almost about 50% of amino acids present in honey (Baroni et al. 2009). Its content in honey is used to assess the quality and adulteration of honey. Some authors reported that the high proline level is characteristic of honeydew honey. The studied honey possessed considerable proline levels that ranged between 390.33 and 453.67 mg/kg (Table 4), which was significantly different (P < 0.05) among the honey sample. These range has well collaborated with the studies of Ouchemoukh et al. (2007) and Meda et al. (2005), they were reported proline content of 202–680 mg/kg and 437.8–2169.4 mg/kg, respectively. Proline is generally used to differentiate floral source honey from that produced from non–floral sources (sucrose, HFCS etc.), and its content should not be less than 180 mg/kg for pure honey (Ouchemoukh et al. 2007). Therefore, examined honey was ripened and not adulterated.
Hydroxymethylfurfural (HMF) content
HMF is a decomposition product of fructose accelerated by heating and is widely accepted parameter for honey freshness, because of its absence in fresh honey and is likely to upsurge during handling and/or ageing (Buba et al. 2013). From the result in Table 4, the HMF content of studied honey obtained from sandbar pumpkin field fluctuated between 28.76 and 37.97 mg/kg and varied significantly (P < 0.05) among the honey sample. This range is identical with the previous studies of Gomes et al. (2010), Babarinde et al. (2011) and Imtara et al. (2018), they reported 18–94 mg/kg, 23.9–27.2 mg/kg and 10.16–81.86 mg/kg, respectively. According to the Codex standard, HMF level should be below 40 mg/kg, and interestingly, studied sugar fed honey had HMF value lower than the approved limit. However, the variation in HMF content in studied honey might be due to the sandbar temperature as well as the concentration of sugar syrup used for the bee feeding purpose. The previous reports supported that climatic conditions of honey production area i.e. mild to warm winter and hot dry summer has significant impact on the HMF content in honey (Imtara et al. 2018). In addition, the acidic condition might also contribute to the formation of HMF the in studied honey (Singh and Singh 2018). Previous literature elucidated that several issues like floral source, temperature, heating time, storage environments, and pH etc. are associated with the HMF formation in the honey (Gomes et al. 2010).
Diastase activity
The enzyme diastase (amylase) is naturally present in honey, and its composition is influenced by phyto-geographic conditions and ripening of flower nectar (Gomes et al. 2010). Diastase activity is globally recognized as a basis for honey freshness or adulteration, and temperature abuse as such in HMF, which lower the quality of honey. Studied honey had diastase number in the range of 12.63° to 16.33° Gothe (Table 4), which was higher than the reports of Buba et al. (2013) who found 8.67–10.57 shade unit. However, Küçük et al. (2007), Babarinde et al. (2011) and Kirs et al. (2011) reported a higher range as 17.7–23, 19.1–21.8, and 16.2–29.1, respectively. All honey analyzed in the present study fall within the recommended level (> 8° Gothe) of international legislation (International Honey Commission 2009).
Antioxidant properties of supplement fed honey
Total phenolic content
The quality and therapeutic properties of food matrix e.g. honey is best evaluated by its content of total phenolics. In the recent year, interest has grown significantly to the researcher for the identification and quantification of natural bioactive polyphenols from foodstuffs like honey. The total phenolic content of supplement fed honey was found in the range of 158.04–174.87 mg GAE/100 g (Table 4). All the examined honey samples were of dark colored (L* ≤ 50), which signposts their possession of high antioxidant activity (Nayik and Nanda 2016). Based on previous studies, a lower range of phenolic content was reported by Imtara et al. (2018) for Palestinian honey (26.96–70.73 mg GAE/100 g), Nayik and Nanda (2016) for different unifloral honey types from Kashmir, India (37–117 mg GAE/100 g), Saxena et al. (2010) for different Indian honey (47–98 mg GAE/100 g), and Al et al. (2009) for Romanian honeydew honey (23–125 mg GAE/100 g). However, a higher range was reported by Bertoncelj et al. (2007) for Slovenian honey (448–2414 mg GAE/100 g). The amount and form of phenolic substances existing in honey rely upon the nectar source, beekeeping practices, climatic conditions and biochemical changes in honey constituents (Küçük et al. 2007; Nayik and Nanda 2016).
Total flavonoid content
Flavonoids are usually low molecular weight substance found in honey, which also contributes to the antioxidant boosts of honey. The total flavonoid content studied honey ranged from 4.67 to 6.25 mg CE/100 g (Table 4), which corroborated to Khalil et al. (2012), they stated 2.7–7.1 mg CEQ/100 g for Algerian honey, Meda et al. (2005) for Burkina Fasan honey (0.17–8.35 mg QE/100 g), and Al et al. (2009) for Acacia honey (0.91–2.42 mg QE/100 g) and Tilia honey (4.70–6.98 mg QE/100 g). Comparatively, higher range of total flavonoid content was reported in the previous studies of Nayik and Nanda (2016) for different unifloral honey (8–17 mg QE/100 g), Nayik et al. (2018) for honey from Kashmir valley (6.10–8.12 mg QE/100 g), and Al et al. (2009) for sunflower honey (11.53–15.33 mg QE/100 g) and honeydew (5.46–28.25 mg QE/100 g). However, the results obtained in the present study for total flavonoid content were high as compared to Malaysian honey (1.1–3.4 mg CEQ/100 g) (Khalil et al. 2011) and Turkish pine honey (1.58 mg QE/100 g) (Can et al. 2015). The variation in total flavonoid content can be attributed due to geographical location, environmental factors, and the treatment used in the present study (Nayik et al. 2018).
DPPH radical scavenging activity (DPPH-RSA)
The free radical scavenging ability of honey was evaluated by DPPH assay and the results were expressed as % inhibition. Results show that DPPH-RSA was found to range between 30.65 and 35.97% (Table 4), which is analogous to the reports of Al et al. (2009) for Acacia honey (35.80–45.27%) and Tilia honey (36.60–40.91%). Our results are far lower than different unifloral honey (55–84%) (Nayik and Nanda 2016) and Indian honey (44–71%) (Saxena et al. 2010). Previous studies of Meda et al. (2005), Bertoncelj et al. (2007), and Imtara et al. (2018) also informed about the of DPPH-RSA (IC50) as 1.63–29.13 mg/mL for Burkina Fasan honey, 7.20–53.8 mg/mL for Slovenian honey, and 9.04–86.90 mg/mL for Palestinian honey, respectively.
Microbiological characteristics
Microbiological contamination of honey results from the fermentation due to the lack of appropriate harvesting method e.g. immature combs and broods, and not following the proper hygienic practice during harvesting and storage, which enhanced the fermentation rate (Babarinde et al. 2011). Total viable count of microorganisms in analyzed honey was found to have significantly different (P < 0.05) and ranged from 33.33 to 27.66 CFU/g (Fig. 1). This amount is comparatively higher than the reports of Babarinde et al. (2011), they found 29–31 CFU/g. On the other hand, total yeasts and molds count of examined honey obtained by supplement feeding to the honey bee ranged between 14.33 CFU/g to 12.0 CFU/g, which were found almost similar except in honey-5 (Fig. 1). All samples possess < 15 CFU/g of yeasts and molds, which was much below the limit specified by MERCOSUR (100 CFU/g) and the previous reports of Gomes et al. (2010). Thus, microbiological counts in our study were comparatively low and lie within the safety limits for use. From the quality viewpoint, a lower amount of microorganism viz. total viable bacteria and yeasts and molds are indicative of an appropriate management of beehives. However, the figure reported for microbial content in the current study might be due to the beekeeping practice applied during harvesting of honey. Also, the environmental condition of the sandbar pumpkin field might contribute to the microbial contamination.
Microbial quality of sugar fed honey. [Mean ± SEM followed by different lowercase letters above each bar are significantly different (P < 0.05); Sugar fed: Honey-1 (white sugar: water = 2:1), honey-2 (white sugar: water = 1.5:1), honey-3 (white sugar: water = 1:1), honey-4 (brown sugar: water = 2:1) and honey-5 (without sugar)]
Principle component analysis (PCA)
Based on the evaluated parameters, principal component analysis (PCA) is generally used to scrutinize the association between data and samples and their distribution. Also, PCA is identified to be a useful tool for the information abstraction from a multivariate matrix and focus it in only a few components (Imtara et al. 2018). The results obtained from the PCA of the studied honey sample was given in Fig. 2. The results depicted that the first three principal components accounted for more than 89.08% of the total variance in the physicochemical and antioxidant properties of examined honey. PC1, PC2 and PC3 were elucidated for 58.11%, 17.79% and 13.19%, respectively of the total variance with Eigen values greater than 1.0 (11.62 for PC1, 3.56 for PC2 and 2.64 for PC3). The first principal component (PC1) mostly controlled by the moisture content, total solids, total soluble solids, pH, reducing sugar, glucose, fructose, HMF, diastase activity, total phenols and flavonoids and L* color coordinates, which were scrutinized more than 58.11% of the variance. However, the second principal component elucidated 17.79% of the variance mostly subjected by ash, electrical conductivity, sucrose, and proline. Furthermore, the third principal component (PC3) clarified more than 13.19% of the variance mostly governed by acidity, DPPH-RSA and b* color coordinates. Therefore, all the assessed parameters in the current study could be used to discriminate the honey sample. Conferring to the PCA biplot, examined honey samples were discriminated successfully.
Principle component analysis (PCA) of the evaluated parameters of sugar fed honey. a PCA of the physicochemical and antioxidant properties. b Distribution of honey samples conferring to PCA: Sugar fed: Honey-1 (white sugar: water = 2:1), honey-2 (white sugar: water = 1.5:1), honey-3 (white sugar: water = 1:1), honey-4 (brown sugar: water = 2:1) and honey-5 (without sugar)]
Conclusion
This study is the first time report on the physicochemical and microbial characteristics of supplement (sugar) fed honey obtained from sandbar pumpkin field. Among the several parameters, moisture content, ash and electrical conductivity, pH and acidity, diastase number and HMF value indicated the honey freshness and good conservation and microbial safety. Moreover, examined honey contained a considerable amount of sugars with adequate total phenolics and flavonoid with sufficient DPPH radical scavenging activity. Conclusively, the results obtained from the supplement (sugar) fed honey were of good quality and in agreement with the legal limits recommended by international honey legislation. This information will help the honey grower to produce honey in adverse condition like sandbar region as well as can able to earn profits by trading the produced honey.
References
Al ML, Daniel D, Moise A et al (2009) Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem 112:863–867
AOAC (1990) Official methods of analysis. Association of Official Agricultural Chemists, Washington, DC
Azonwade FE, Paraiso A, Dossa CPA et al (2018) Physicochemical characteristics and microbiological quality of honey produced in Benin. J Food Qual 2018:1–13
Babarinde GO, Babarinde SA, Adegbola DO, Ajayeoba SI (2011) Effects of harvesting methods on physicochemical and microbial qualities of honey. J Food Sci Technol 48:628–634
Baroni MV, Arrua C, Nores ML et al (2009) Composition of honey from Córdoba (Argentina): assessment of north/south provenance by chemometrics. Food Chem 114:727–733
Bath PK, Singh N (1999) A comparison between Helianthus annuus and Eucalyptus lanceolatus honey. Food Chem 67:389–397
Bertoncelj J, Doberšek U, Jamnik M, Golob T (2007) Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem 105:822–828
Buba F, Gidado A, Sgugaba A (2013) Physicochemical and microbiological properties of honey from North East Nigeria. Biochem Anal Biochem 2:1–7
Can Z, Yildiz O, Sahin H et al (2015) An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem 180:133–141
Codex Alimentarius Commission (1981) Revised codex standard for honey Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001). Codex Standard 12:1–7
de Sousa JMB, de Souza EL, Marques G et al (2016) Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT Food Sci Technol 65:645–651
Downey SG, Hussey K, Daniel Kelly J et al (2005) Preliminary contribution to the characterisation of artisanal honey produced on the island of Ireland by palynological and physico-chemical data. Food Chem 91:347–354
European Comission (2002) European Commission Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off J Eur Communities 10:47
García-Martínez E, Igual M, Martín-Esparza ME, Martínez-Navarrete N (2013) Assessment of the bioactive compounds, color, and mechanical properties of apricots as affected by drying treatment. Food Bioprocess Technol 6:3247–3255
Gomes S, Dias LG, Moreira LL et al (2010) Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem Toxicol 48:544–548
Imtara H, Elamine Y, Lyoussi B (2018) Physicochemical characterization and antioxidant activity of Palestinian honey samples. Food Sci Nutr 6(8):2056–2065
International Honey Commission (2009) Harmonized methods of the international honey commission. Retrieved from http://www.ihc-platform.net/ihcmethods2009.pdf
Khalil MI, Alam N, Moniruzzaman M et al (2011) Phenolic acid composition and antioxidant properties of Malaysian honeys. J Food Sci 76:921–928
Khalil MI, Moniruzzaman M, Boukraâ L et al (2012) Physicochemical and antioxidant properties of algerian honey. Molecules 17:11199–11215
Kim DO, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81:321–326
Kirs E, Pall R, Martverk K, Laos K (2011) Physicochemical and melissopalynological characterization of Estonian summer honeys. Procedia Food Sci 1:616–624
Küçük M, Kolayli S, Karaoǧlu Ş et al (2007) Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem 100:526–534
Meda A, Lamien CE, Romito M et al (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577
Nayik GA, Nanda V (2016) A chemometric approach to evaluate the phenolic compounds, antioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT—Food Sci Technol 74:504–513
Nayik GA, Dar BN, Nanda V (2016) Physico-chemical, rheological and sugar profile of different unifloral honeys from Kashmir valley of India. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.08.017
Nayik GA, Suhag Y, Majid I, Nanda V (2018) Discrimination of high altitude Indian honey by chemometric approach according to their antioxidant properties and macro minerals. J Saudi Soc Agric Sci 17:200–207
Ouchemoukh S, Louaileche H, Schweitzer P (2007) Physicochemical characteristics and pollen spectrum of some Algerian honeys. Food Control 18:52–58
Rashid MH, El Taj HF, Chowdhury NI et al (2018) Supplement feeding to honeybee colony for field crop pollination, pumpkin and honey production in sandbar cropping system. J Apic 33:25–32
Sahinler N, Sahinler S, Gul A (2004) Biochemical composition of honeys produced in Turkey. J Apic Res 43:53–56
Saxena S, Gautam S, Sharma A (2010) Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem 118:391–397
Silva TMS, dos Santos FP, Evangelista-Rodrigues A et al (2013) Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J Food Compos Anal 29:10–18
Singh N, Bath PK (1997) Quality evaluation of different types of Indian honey. Food Chem 58:129–133
Singh I, Singh S (2018) Honey moisture reduction and its quality. J Food Sci Technol 55:3861–3871
Sousa JMB, De Souza AI, Magnani M et al (2013) Aspectos físico-químicos e perfil sensorial de méis de abelhas sem ferrão da região do Seridó, Estado do Rio Grande do Norte, Brasil. Semin Agrar 34:1765–1774
Souza BA, Roubik D, Heard T et al (2006) Composition of stingless bee honey: setting quality standards. Interciencia 31:867–875
Acknowledgement
We thankfully acknowledged the help of the beekeepers for kind supplying of honey samples, Dr. Maruf Ahmed for providing DPPH reagent and helping in PCA analysis, and Md. Saifullah, PhD Fellow, School of Environmental and Life Sciences, University of Newcastle, Central Coast, Ourimbah, NSW 2258, Australia for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamal, M., Rashid, M., Mondal, S.C. et al. Physicochemical and microbiological characteristics of honey obtained through sugar feeding of bees. J Food Sci Technol 56, 2267–2277 (2019). https://doi.org/10.1007/s13197-019-03714-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03714-9