Abstract
Honey is one of the most valuable food products, which, in addition to its nutritional value, also has therapeutic properties. In our study, the physicochemical (Brix, viscosity, free acid content, pH, moisture, diastase activity, 5-hydroxymethylfurfural (HMF), proline content, sugars content, and reducing sugars content) and microbial (mold and yeast content) characteristics and 15 element contents (As, Cd, K, Al, Pb, Hg, Ba, Ni, Na, Ca, Mg, Fe, Mn, Zn, and Se) of the samples were evaluated. Among the essential elements, the maximum mean was related to K (630 ± 50.8 mg/kg), and the minimum mean was related to Se that was lower than the limit of detection. Also, among all toxic elements, the maximum mean was related to Ni (234 ± 54.7 µg/kg), and the minimum mean was related to Hg that was lower than the limit of detection. Furthermore, the mean of free acidity, pH, °Brix, moisture, diastase content, HMF, and proline content was 35.4 ± 1.27 meq/kg, 4.61 ± 0.21, 82.2 ± 3.08, 16.3 ± 0.33%, 9.10 ± 1.14 DN, 21.1 ± 2.65 mg/kg, and 482 ± 18.1 mg/kg, respectively. Also, the mean percentage of fructose, glucose, and sucrose was 32.4 ± 1.07% (27.5–40.0%), 27.2 ± 0.85% (23.5–31.7%), and 2.28 ± 0.70% (0.72–4.11%), respectively. Finally, the mean of mold and yeast in all samples was 14.2 ± 0.37 CFU/g. Also, the principal component analysis and heat map allowed us to determine a more accurate distinction between the physicochemical characteristics of bee honey. The results of our findings showed that in most cases, the results obtained were within the standard range, which indicates the good quality of Iranian honeys.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Foodstuff such as honey can be polluted with many chemicals in diverse ways, and the existence of these contaminants in foodstuff can cause several sicknesses in people. Chemical pollutants can be categorized based on how they enter foodstuff and their source. Regarding residues of metal in foodstuff, the most pollution happens through activity of human and industrial during storage and processing of foodstuff. Essential elements are obligatory for human beings but, if consumed in excess, may cause poisoning; these elements comprise iron (Fe), chromium (Cr), zinc (Zn), manganese (Mn), copper (Cu), calcium (Ca), cobalt (Co), magnesium (Mg), and sodium (Na). Toxic elements (even in very small amounts) can cause poisoning; these elements comprise nickel (Ni), lead (Pb), cadmium (Cd), aluminum (Al), arsenic (As), and mercury (Hg) [1,2,3,4,5]. These metals can be present in any amount in honey samples.
Honey is a viscous and sweet substance that is prepared from the nectar of plants (by the honeybee). This simple description ignores honeydew honey that is made by the bee from honeydew excreted by several insects of plant-sucking. The honey bee collects, transports, and processes the nectar and turns it into honey and packs and stores it in the comb. Processing comprises of decreasing (simultaneously) the content of moisture from the 30 to 60% common to nectars to the range of self-preserving of 15 to 19%, inverting the sucrose (considerable proportion) by the invertase enzyme addition, maintaining it meantime by adding an enzyme of glucose oxidase that produce very little of hydrogen peroxide and acidity. In the comb open cells, ripening takes place, which when the bee honey reaches full density, are sealed [6,7,8]. Honey is a main food that is applied as a medicine (complementary). From the past to now, it is applied as a supplement of nutritional, and in some beliefs of religious, honey for many diseases is an efficacious medicine, which this is confirmed by many researches. Today, honey is broadly applied in wound treatment, based on a many studies. Honey has the characteristics of antioxidant, antimicrobial, and also anti-cancer. The honey unequaled characteristics are related to its ingredients of complex. Natural honey comprises biomolecules, vitamins, carbohydrate (fructose, glucose, maltose, and saccharose), and water [9,10,11].
Honey should contain at least 60% of the reducing sugars, and its moisture should not be more than 21%. Honey contains 10–60% glucose and fructose as major monosaccharides, 7–10% of sucrose and maltose as the most main disaccharide, and the chief trisaccharide is the methallenestril, as well as other low molecular weights. In natural honey, biological molecules include enzymes (invertase, diastase, acid phosphatase, glucose oxidase, protease, esterase, and catalase), amino acids, proteins, and other substances such as flavonoid and phenolic compounds [12,13,14,15].
Combinations of natural honey are affected by vegetation cover and conditions of climatic and region. Owing to many variation of honey types in all over the world, in 1990, the International Honey Commission (IHC) was established, to create a harmonized world standard of honey that contains many parameters such as biological, chemical, and physical characteristics. In some studies, since the biological parameter assessment needs careful experiments (laboratory), the honey quality types have been solely compared based on parameters of chemical and physical. Although the honey’s nutritional value is mostly owing to the existence of combinations of biological that pull out from honeybees or herbs, this mentioned neglect can leads to mistaken outcomes [16,17,18,19].
With these explanations, since there has been no comprehensive and extensive study of different variables in Iranian honey, and on the other hand, given the high consumption of honey in Iran, this study seemed necessary. The present study investigated the physicochemical (Brix, viscosity, free acid content, pH, moisture, diastase activity, HMF, proline content, sugar content, and reducing sugar content) and microbial (mold and yeast content) characteristics and 15 element concentrations (As, Cd, K, Al, Pb, Hg, Ba, Ni, Na, Ca, Mg, Fe, Mn, Zn, and Se) of the 160 samples of Iranian bee honeys based on international standards of honey.
Materials and Methods
Reagent and Standards
From Sigma-Aldrich Co. (USA), formic acid, hydroxylamine hydrochloride, starch, hydroxymethylfurfural (HMF), glucose, hexamethyldisilazane (HMDS), fructose, pyridine, trifluoroacetic acid (TFA), ethylene glycol monomethyl ether, ninhydrin, iodide, nitric acid (65%), potassium iodide, proline, deionized water (with analytical grade), sucrose, mannitol, and other reagents were purchased.
Sample Collection
One hundred sixty samples of honey (with different brands) were obtained in 2021 from supermarkets in Tehran (provinces of West Azerbaijan, Ardabil, Isfahan, Mazandaran, Gilan, Kohgiluyeh Boyer Ahmad, Ilam, Lorestan, Alborz, and Tehran), Iran. The honey samples were stored in glass jars and kept at room temperature (25 °C) until they were analyzed.
Determination of 15 Element Contents
In this study, 15 element concentrations (As, Cd, K, Al, Pb, Hg, Ba, Ni, Na, Ca, Mg, Fe, Mn, Zn, and Se) were evaluated in all honey samples. To minimalize the impacts of the organic matrix, losses of analytes, and avoid the possibility of sample pollution, in our study, closed vessel and acid decomposition was applied in the system of microwave oven. All steps and conditions were carried out according to previous studies [3,4,5]. In this study, ICP-OES (Spectoro, Arcos, Germany) was used with Torch type of flared end EOP Torch 2.5 mm. All conditions, times, and temperatures used in this study were in accordance with previous studies [3,4,5].
In current research, to assess the validity of the analytical method, the LOD, selectivity, LOQ, linear ranges, reproducibility (precision), and repeatability were evaluated (Table 1S). For this purpose, the mixed standard (mix standard CRM: 92,091 Supelco LOT BCCB9855, TraceCERT®, 33 elements, 10.0 mg/L in nitric acid, Hg standard CRM:28,941 Supelco, LOT BCCB8927, 1000 mg/L, Hg in nitric acid) solutions (200 μL) were added to the original samples. The calibration carves (LOD to 5.00 mg/kg) for the 15 metals were ready from the solutions of standard at 5 points. According to Table 1S, LOD, LOQ, recovery, and r2 value were ranged from 0.043 to 2.128, from 0.146 to 6.498 µg/kg, from 95.0 to 104.7%, and from 0.988 to 0.996, respectively.
Physicochemical Parameters of Honey
Determination of °Brix
By using a Mettler Toledo RM40 refractometer (Schwerzenbach, Switzerland), the °Brix parameter were evaluated. After waiting for equilibration of cell, all the experiments (at 20.0 °C) were performed [10].
Free Acidity Determination
By using an Easy Pro Mettler Toledo Titrator (Schwerzenbach, Switzerland), the honey acidity was evaluated, and the results were reported as meq/kg of honey samples [10]. Free acidity was assessed by the method of titrimetric. Ten grams of sample was dissolved in deionized water (75.0 mL), and the mentioned solution with solution of NaOH 0.10 M was titrated until the pH value reached 8.50.
Determination of Proline Content
The content of honey proline was assessed according to the study of Nabati et al. [20]. The content of proline was assessed by using of the AOAC standard technique. Honey sample (5.00 g) was transferred in a beaker and in distilled water (50.0 mL) was dissolved. After that, the mentioned solution was conveyed (quantitatively) to a volumetric flask (100 mL) and was diluted by water (distilled) and shaken. Next, the solution of sample (0.50 mL) was poured into a tube with solution of ninhydrin (1.00 mL, 3.00% in ethylene glycol monomethylether) and formic acid (1.00 mL, 98–100%). The tubes were capped and vigorously shaken. Also, in a bath of boiling water, they were placed for 15 min and to a bath of water conveyed for 10.0 min at 70.0 ºC. Afterward, to each tube, 2-propanol (5.00 mL) was added. Then, they were removed for 45.0 min, and the absorbance was assessed at the room temperature at 520 nm. The color of honey was modified by assessing the absorbance of the solution comprising 2-prppanol (5.00 mL), distilled water (2.00 mL), and solution of sample (0.50 mL).
Moisture Content
By using the refractometric method, the content of moisture in honey samples was evaluated. In this study, by using refractometer, the honey samples’ indices of refractive were assessed at temperature of ambient, and all experiments were done at 20.0 ºC by adding a factor of correction to achieve the moisture % from the index of refractive by referring to AOAC (1990) standard method [20].
Determination of pH
By using a pH meter (Metrohm 914), the pH value of honey samples (adding 75.0 mL of distilled water in 10 g honey) was evaluated [10, 20].
Determination of Hydroxymethylfurfural (HMF)
In this study, HMF parameter was evaluated according to the previous studies [10, 20]. To assess the HMF, a C18 column of reversed phase and isocratic elution with water/methanol (90:10) was applied, at a rate of flow of 1.00 ml/min and at 280 nm UV detection. The preparation of sample only involved dissolution in water (deionized) and filtration through filter paper of Whatman 42.0 and 0.20 mm.
Diastase Activity Assay
According to the previous studies [10, 20, 21], the parameter of diastase activity (by a buffered soluble starch solution) was evaluated that fulfills the technique requirements, and samples in a bath were incubated at 40.0 ºC. Reducing color of blue absorbance that was made in the presence of solution of iodine was followed by using the spectrophotometer (Human crap XMA 2000 UV/VIS) at 660 nm. By using the regression, lines were fitted to the absorption data, and the number of diastase was calculated from the taken of time for the absorbance to reach 0.235.
Determination of Sugar Content
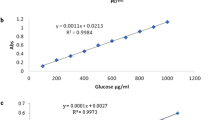
Sugars were determined by gas chromatography (Agilent 6890, USA) as their trimethylsilyl-oxime derivatives. The standards of sugar (0.25 g sucrose, 2.00 g fructose, and 1.50 g glucose) were added in a beaker and, in about 40.0 mL methanol, were dissolved. Then, the solutions were transferred (quantitatively) to a volumetric flask (100 mL) and by water (distilled) were diluted and shaken. Honey sample (0.60 g) was dissolved in water (distilled) and transferred to a volumetric flask. For the next step, 1 mL of mannitol solution (10% (W/V)) (as an internal standard) was added and then with water (distilled) adjusted 100 mL [10, 20]. One hundred microliters of solution was moved to a test tube and dried in an air stream. Afterward, 200 µL oxime reagents (solution of pyridine comprising 12.0 mg/mL hydroxylamine hydrochloride) was added and then sealed and mixed and heated for 30 min at 70.0–75.0 °C. The honey sample was cooled at room temperature, and then 10 μL trifluoroacetic acid was added and the solution maintained for 30.0 min. One microliter of solution was injected into the BP5 column of capillary (30 m and 0.25 mm id). In this study, as a carrier gas, helium gas was applied at a 1.00 mL/min−1 flow rate. Also, detectors and injector were set at 250 °C. The oven temperature was programmed to increase from 70.0 to 140 °C at 50.0 °C/min and to 300 °C at 6.00 °C/min. Finally, the curve calibration was obtained for glucose, fructose, and sucrose and was used for quantification [10, 20]. By the method of dinitrosalicylic acid, reducing sugar was evaluated, according to study of Thi Le et al. [15].
Evaluation of Microbial Parameters of Honey (Measurement of Mold and Yeast)
For this purpose, a selected culture medium was determined, and according to the number of hypothetical colonies, a certain amount of the sample was cultured on the surface of the precast plates. Yeasts and molds were enumerated on standard yeast extract–glucose–chloramphenicol (YGC) agar and incubated for 3 to 5 days at 22.0–25.0 °C. The counts of microbial were reported as cfu/g of honey [21, 22].
Statistical Analysis
The measurement of all data was analyzed by the SPSS statistical packages (version 22.0). The outcomes were indicated in three repetitions and were indicated as mean ± standard deviation (M ± SD). Due to the non-normality of the data, the Mann–Whitney test was employed to evaluate the statistical significance of samples. The significance level was considered at p < 0.05. A heat map was employed to determine a more accurate distinction between the physicochemical of Iranian bee honey [23, 24]. In addition, principal component analysis (PCA) was used to study the relations between the physicochemical variables in the examined Iranian bee honeys by the SPSS statistical packages (version 22.0). Heat map structure (average linkage; Pearson) was employed to explain the relationship between characteristics online at https://biit.cs.ut.ee/clustvis/.
Results and Discussion
Evaluation of 15 Elements of Bee Honey
Organic acids (such as citric acid), amino acids, and minerals present in honey samples form ionic forms in aqueous solutions of honey, which subsequently affect electrical current conductivity and a measurable parameter called electrical conductivity. The content of elements is influenced by the origin of honey. Determining these toxic elements levels in bee honey samples may be useful as a bio-indicator of pollution of environmental [9, 13]. According to Table 1, among all essential elements, the maximum mean was related to K (630 ± 50.8 mg/kg), and the minimum mean was related to Se that was lower than the LOD. Also, the mean of Na, Ca, Mg, Mn, Ba, Fe, and Zn was 72.7, 310, 50.6, 0.07, 0.91, 0.60, and 15.3 mg/kg, respectively. The type and flora of the plants used by the bees, the climate, the geographical area where the honey is harvested, and even the species of bee affect the amount of essential elements in honey [25,26,27,28].
Furthermore, according to Table 2, among all toxic elements, the maximum mean was related to Ni (234 ± 54.7 µg/kg), and the minimum mean was related to Hg that was lower than the LOD. Also, the mean of Al, Pb, Cd, and As was 83.20 17.8, 2.45, and 12.4, µg/kg, respectively. Based on the results of this study, the amount of metals in all samples was lower than the existing standards [3, 5] (Al = 500, As = 140, Cd = 10.0, Hg = 20.0, Pb = 20.0 µg/kg, Fe = 2700 and Mn = 100 µg/kg), which can be due to the good quality of honey produced in Iran and the compliance of the existing standards by the producers [3, 25,26,27,28]. Industrial wastes of geographical origin, environmental pollution (water, air, and soil), chemicals used as pesticides, and incorrect practices in the processing of honey (by producers) are the factors that affect the quality of honey and amount of toxic elements [25,26,27,28].
Mahmoudi et al. analyzed three metals (Pb, Zn, and As) in honey samples and reported the mean of these metals was 0.08 ± 0.04, 4.40 ± 3.40, and 0.11 ± 0.04 mg/kg, respectively [26]. Dhahir et al. assessed elements (Pb, Cd, Ni, Fe, Se, Mg, Cu, Na, K, Ca, and Mn) in honey samples and expressed the mean of these metals ranged from 0.10 ± 0.00 to 0.730 ± 0.0224, from 0.108 ± 0.0084 to 0.8200 ± 0.0158, from 0.210 ± 0.01517 to 0.894 ± 0.0114, from 0.117 ± 0.014 to 0.440 ± 0.0200, from 0.0010 ± 0.0001 to 0.0348 ± 0.0013, from 0.26 to 0.721, ND, from 51.0 to 120.8, from 93.8 to 712.8, from 143.8 to 240.8, and from 0.0116 to 0.0632 mg/kg, respectively [25]. Biluca et al. measured these minerals in honey samples and reported K was ranged from Nd to 449, Na was ranged from 0.78 to 30.5, Ca was ranged from 1.12 to 35.20, Mg was ranged from 0.41 to 13.70, and Mn was ranged from ND to 1.12 mg/100 g, which were somewhat similar to our findings [9]. Saghaei et al. analyzed metal contents (Pb, Co, Mn, Fe, Cr, Zn, As, and Ni) of natural honey samples and stated the mean of these metals were 0.07 ± 0.04 (0.383–0.253), 0.002 ± 0.001 (0.0003–0.005), 0.10 ± 0.05 (0.018–0.305), 0.70 ± 0.20 (0.378–1.935), 8.10 ± 5.30 (3.369–33.765), 19.9 ± 15.1 (0.702–68.49), 0.0011 ± 0.00105 (0.0000264–0.0038), and 0.006 ± 0.01 (0.0015–0.068) mg/kg, respectively [28]. Pisani et al. mesured elements (Na, Mg, K, Ca, Fe, Mn, Zn, Sr, Ba, Cu, Co, Ni, As, Cd, Sb, and Pb) in different honey samples and expressed the mean of these metals in all samples was 96.60, 56.70, 1195, 257, 3.07, 1.54, 1.82, and 1.43 mg/kg and 906, 906, 11.00, 308, 6.96, 3.91, 3.76, and 76.0 µg/kg, respectively [27].
Physicochemical Parameters of Honey
In this study, Table 3 shows the seven parameters of physicochemical (free acidity, Brix, viscosity, pH, moisture, diastase content, HMF, and proline content) results of 160 samples of honey.
Parameters of pH and Free Acidity
The mean of free acidity (Table 3) was 35.4 ± 1.27 meq/kg (10.1–82.2 meq/kg) which the mean level was lower than standard levels (should be lower than 50 meq/kg), and the one sample acidity (82.20 meq/kg) was higher than the standard level. In Brazil, F.C. Biluca et al. analyzed free acidity in honey samples and reported that this parameter ranged from 21.4 to 139 meq/kg, which is higher than our findings [9]. E.-I. Geană et al. measured this parameter in honey and reported that the mean level of free acidity was 19.7 meq/kg, which was lower than our results [10]. According to Table 1, our results showed that the mean of pH was 4.61 ± 0.21 (4.35–4.78), which was in the standard range (≥ 3.50). Our results were somewhat similar to other studies [9, 10, 13, 20]. The amount of acidity in honey is related to the balance of organic acids that is different according to the species of bee and the composition of floral or maybe owing to fermentation (by some bacteria and additional oxidation to carboxylic acids) of sugars to alcohol [7, 9, 12].
°Brix
In this study, the °Brix parameter was ranged from 79.5 to 85.1 with a mean of 82.2 ± 3.08, which is lower than the existing standard levels (70.0–88.0) in all samples (Table 3). In 2016, F.C. Biluca et al. measured °Brix in honey samples, and this value was ranged from 55.2 to 76.1, which was lower than our results [9]. In other study, Geană et al. measured this parameter and reported that the mean of °Brix was 82.4, which was similar to our findings [10]. The soluble solids act as a rate indicator parameter in solution solids like minerals, organic acids, and sugars; nevertheless, it is directly associated to the levels of water and sugars in the samples. In honeys made by Apis mellifera, the °Brix’s value is always higher than the stingless bee honeys and is related to the high content of water, which subsequently reduces the content of sugar.
Moisture
In general, the honey water content belongs on the sources of plant, the ripening degree in the hive, the season of harvesting, climatic conditions, geographical conditions, and the composition of honey. During storage of bee honey, this parameter is important (significantly) for the honey’s shelf life and also for its processing characteristics. In fresh honey, the water content is an indicator of the degree of maturation and its storage method. In general, high levels of moisture in honey cause fermentation, spoilage, loss of taste, and decrease in honey quality [7, 9, 13, 17, 18, 20]. According to Table 3, our results showed that the mean of moisture was 16.3 ± 0.33% (15.8–17.3%) which was in the standard range (≤ 20.0). In 2016, F.C. Biluca et al. (in Brazil) reported the content of honey’s moisture ranged from 23.10 to 43.50%, which was lower than our findings [9]. In Iran, Nabati et al. assessed this parameter in honey and ranged from 14.8 to 16.5% which was somewhat similar to our findings [20].
Diastase Content
Honey contains many enzymes like glucose oxidase, catalase, invertase, and amylase (diastase). The content of enzymatic of bee honey samples proves the conditions of storing, proper heating, and the freshness of honey [9, 13, 17, 20]. The result of diastase contents was shown in Table 3. According this table, the mean of this parameter was 9.10 ± 1.14 DN (2.76 to 16.2 DN), which all samples were in the standard range (≥ 8.00) except 23 samples. Nabati et al. measured that this parameter in Iranian honey and reported that this enzyme was ranged from 0.20 to 12.2 DN, which in some samples were out of standard range [20]. Biluca et al. reported that this enzyme in honey samples was ranged from 4.34 to 49.6 DN, which was higher than our results [9].
Hydroxymethylfurfural (HMF)
HMF content may be affected by climate; therefore, samples of bee honey from tropical regions exposed to high temperatures for a long period of time may provide HMF [9, 13, 20]. In our study (Table 3), the mean of HMF was 21.1 ± 2.65 mg/kg (1.89–34.8 mg/kg), which was in the standard range (≤ 40.0). In Biluca et al. study, this parameter was not detected, which was lower than our results [9]. In study of Nabati et al., HMF parameter was ranged from 1.37 to 74.3 mg/kg that in some samples were out of standard range [20], which can be a sign of poor quality of their honey. Geană et al. also measured this parameter in honey samples and ranged from 1.07 to 17.14 mg/kg, which was somewhat similar to our findings [10].
Proline Content
In bee honey, the major amino acid is proline, constituting 59.0% in honeydew honey and 49.0% in nectar honey of the total content of amino acid. To confirm the authenticity of bee honey, the amount of proline in the samples is determined [8, 13, 20]. According to Table 3, the mean of proline content in this study was 482 ± 18.1 mg/kg, which was in the standard range (≥ 180), which can be a sign of good quality of honey samples in Iran. Nabati et al. measured proline content in Iranian honey, and this parameter was ranged from 177 to 1233 mg/kg, which was higher than our findings [20]. Czipa et al. also measured this parameter in Hungarian honey, and the mean proline content was ranged from 252 to 2283 mg/kg [8].
Analysis of Sugars in Honey
The natural carbohydrate composition of bee honey can be one of the main parameters in determining its plant origin and indirectly its proper classification. By trans-glycosylation and enzymatic sucrose hydrolysis, sugars are formed in bee honey.
Glucose (G) and fructose (F) are the most dominant sugars in honey, and in most honey samples, the amount of F is higher compared to G.
Glucose (G) and fructose (F) are the most predominant sugars in honey, with a high content of F compared to G, for most honey samples [9, 12, 20]. According to Table 4, the mean percentage of fructose, glucose, and sucrose were 32.4 ± 1.07% (27.5–40.0%), 27.2 ± 0.85% (23.5–31.7%), and 2.28 ± 0.70% (0.72–4.11%), respectively. Also, our result showed that the mean ratio of fructose/glucose was 1.27 ± 0.22 (0.90–1.70), which was in the standard range (≥ 0.90), and the mean of reducing sugar in all samples was 76.5 ± 7.18 g/100 g (54.2–99.2 g/100 g), which this parameter was also in the standard range (≥ 65 g/100 g). Geană et al. measured fructose, glucose, and sucrose in samples of bee honey and reported the mean of these sugars was 34.5–41.9%, 25.7–39.1% and ND (non-detected)-0.06%, respectively [10]. Nabati et al. evaluated these sugars in Iranian honey and reported the percentage of fructose, glucose, and sucrose were 26.6–41.9%, 21.1–34.3%, and 0.70–4.70%, respectively, which was also somewhat similar to our findings. Also, they reported the F/G ratio was ranged from 1 to 1.3 [20]. Biluca et al. (in 2016) also reported the percentage of fructose, glucose, and sucrose in Brazilian samples of honey were 30.4–40.5%, 8.21–31.3%, and ND (non-detected), which were lower than our results [9].
Evaluation of Microbial Parameters of Honey (Measurement of Mold and Yeast)
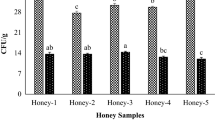
Water content and acidity level are factors that affect the amount of mold and yeast in bee honey samples [13, 21]. According to our results (Table 5), the mean of mold and yeast in all samples was 14.2 ± 0.37 CFU/gr (6.00–27.0 CFU/gr), which was in the standard range (≤ 100 CFU/g). Iurlina et al. assessed mold and yeast in commercial honey samples and reported the mean of these microorganisms (M.O) was 34.0 CFU/gr, which was higher than our findings [22]. Also, Gomes et al. reported these M.O. ranged from < 10.0 to 22.0 CFU/g, which somewhat similar to our results [21].
Multivariate Analysis
Principal component analysis (Fig. 1) in terms of the honey kinds including sugar content, reducing sugar before hydrolyzing sucrose hydroxymethyl furfural, diastase, fructose-to-glucose ratio, proline, Brix, F/G, as functional remarks, was conducted, and the three primary components covering 67.2% of the variability were specified, whereas components 1 and components 2 showed a value of 57.2%. These statistical analyses are displayed in Fig. 1. It can be regarded that hydroxymethyl furfural, proline, and sucrose have similarities in results, meaning that they have a higher value of correlated to the honey than other parameters. In order to supply a better intuitive honey physicochemical among the different samples studied, a heat map was drawn up (Fig. 2). The considerations previously reported are clearly highlighted. To compare composition differences between samples and to achieve a demonstration of the abundance, distribution across samples was carried out using an abundance clustering heatmap on bee honey. The heat map visualizes a very clear picture of the smallest and largest variables according to the clustering of rows and columns of similar variables. The bee honey samples heat map clustered samples into two clusters and four sub-clusters, according to Fig. 2. The first cluster has two sub-clusters (first, fructose and glucose, second, diastase content of bee honey) that express a correlation between diastase content and fructose and glucose. The second cluster comprises two subgroups (first, pH; second, Brix, sucrose, HMF, and proline content), which depicts the existence of a correlation between Brix, sucrose, HMF, and proline content.
Conclusion
Honey is classified as a valuable food, so its quality control is essential. In this research, 15 element contents (including As, Cd, K, Al, Pb, Hg, Ba, Ni, Na, Ca, Mg, Fe, Mn, Zn, and Se) in honey samples consumed in Tehran, Iran, were measured along with the physicochemical (free acidity, pH, Brix, moisture, diastase content, HMF, proline content, and also percentage of fructose, glucose, and sucrose) and microbial properties (mold and yeast) of honey. Our finding outcomes exhibited in most cases the results were within the standard range, which indicates the good quality of Iranian honeys. Also, toxic metals were lower than the existing standards, and the amount of essential elements has an acceptable level for honey, which shows the similarity compared to other studies. The heat map and the principal component analysis allowed us to determine the distinction between the physicochemical of bee honey, representing a worthwhile procedure for the characterization of samples. Finally, it is suggested that, if possible, the honeys of other regions of Iran should also be measured in commercial and wild conditions.
Data Availability
From the corresponding author, the datasets used and/or analyzed during the present research are existing (on reasonable request).
References
Eghbaljoo-Gharehgheshlaghi H, Shariatifar N, Arab A, Alizadeh-Sani M, Sani IK, Asdagh A, Rostami M, Alikord M, Arabameri M (2022) The concentration and probabilistic health risk assessment of trace metals in three type of sesame seeds using ICP-OES in Iran. Int J Environ Anal Chem 102:5936–5950
Karami H, Shariatifar N, Nazmara S, Moazzen M, Mahmoodi B, Mousavi Khaneghah A (2021) The concentration and probabilistic health risk of potentially toxic elements (PTEs) in edible mushrooms (wild and cultivated) samples collected from different cities of Iran. Biol Trace Elem Res 199:389–400
Kargarghomsheh P, Tooryan F, Sharifiarab G, Moazzen M, Shariatifar N,Arabameri M (2023) Evaluation of trace elements in coffee and mixed coffee samples using ICP-OES method. Biol Trace Elem Res pp 1–9
Karimi F, Rezaei M, Shariatifar N, Alikord M, Arabameri M, Moazzen M (2021) Probabilistic health risk assessment and concentration of trace elements in meat, egg, and milk of Iran. Int J Environ Anal Chem 18:6940–6951
Kiani A, Arabameri M, Moazzen M, Shariatifar N, Aeenehvand S, Khaniki GJ, Abdel-Wahhab M, Shahsavari S (2021) Probabilistic health risk assessment of trace elements in baby food and milk powder using ICP-OES method. Biol Trace Elem Res 200:2486–2497
Almasaudi S (2021) The antibacterial activities of honey. Saudi J Biol Sci 28:2188–2196
Bogdanov S, Lüllmann C, Martin P, von der Ohe W, Russmann H, Vorwohl G, Oddo LP, Sabatini A-G, Marcazzan GL, Piro R (1999) Honey quality and international regulatory standards: review by the International Honey Commission. Bee World 80:61–69
Czipa N, Borbély M, Győri Z (2012) Proline content of different honey types. Acta Aliment 41:26–32
Biluca FC, Braghini F, Gonzaga LV, Costa ACO, Fett R (2016) Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J Food Compos Anal 50:61–69
Geană E-I, Ciucure CT, Costinel D, Ionete RE (2020) Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control 109:106919
Machado AM, Miguel MG, Vilas-Boas M, Figueiredo AC (2020) Honey volatiles as a fingerprint for botanical origin—a review on their occurrence on monofloral honeys. Molecules 25:374
Pataca LC, Neto WB, Marcucci MC, Poppi RJ (2007) Determination of apparent reducing sugars, moisture and acidity in honey by attenuated total reflectance-Fourier transform infrared spectrometry. Talanta 71:1926–1931
Puścion-Jakubik A, Borawska MH, Socha K (2020) Modern methods for assessing the quality of bee honey and botanical origin identification. Foods 9:1028
Ranneh Y, Akim AM, Hamid HA, Khazaai H, Fadel A, Zakaria ZA, Albujja M, Bakar MFA (2021) Honey and its nutritional and anti-inflammatory value. BMC Complement Med Ther 21:1–17
Le Thi DH, Chiu C-S, Chan Y-J, Wang C-CR, Liang Z-C, Hsieh C-W, Lu W-C, Mulio AT, Wang Y-J, Li P-H (2021) Bioactive and physicochemical characteristics of natural food: palmyra palm (Borassus flabellifer Linn.) syrup. Biology 10:1028
Ullah A, Gajger IT, Majoros A, Dar SA, Khan S, Shah AH, Khabir MN, Hussain R, Khan HU, Hameed M (2021) Viral impacts on honey bee populations: a review. Saudi J Biol Sci 28:523–530
White JW Jr (1978) Honey. Adv Food Res 24:287–374
Yanniotis S, Skaltsi S, Karaburnioti S (2006) Effect of moisture content on the viscosity of honey at different temperatures. J Food Eng 72:372–377
Zarei M, Fazlara A, Tulabifard N (2019) Effect of thermal treatment on physicochemical and antioxidant properties of honey. Heliyon 5:e01894
Nabati F, Khalighi-Sigaroodi F, Kashefi M, Ghasemi SV, Sadri H, Tajabadi F (2021) Evaluating the quality of commercial Iranian honeys. J Med Plants Stud 20:14–25
Gomes S, Dias LG, Moreira LL, Rodrigues P, Estevinho L (2010) Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem Toxicol 48:544–548
Iurlina MO, Fritz R (2005) Characterization of microorganisms in Argentinean honeys from different sources. Int J Food Microbiol 105:297–304
Kiani A, Ahmadloo M, Moazzen M, Shariatifar N, Shahsavari S, Arabameri M, Hasani MM, Azari A, Abdel-Wahhab MA (2021) Monitoring of polycyclic aromatic hydrocarbons and probabilistic health risk assessment in yogurt and butter in Iran. Food Sci Nutr 9:2114–2128
Moradi M, Bolandi M, Arabameri M, Karimi M, Baghaei H, Nahidi F, Eslami Kanafi M (2021) Semi-volume gluten-free bread: effect of guar gum, sodium caseinate and transglutaminase enzyme on the quality parameters. J Food Meas Charact 15:2344–2351
Dhahir SA, Hemed AH (2015) Determination of heavy metals and trace element levels in honey samples from different regions of Iraq and compared with other kind. Am J Appl Chem 3:83–92
Mahmoudi R, Mardani K, Rahimi B (2018) Analysis of heavy metals in honey from north-western regions of Iran. J Chem Health Risks 5:251–256
Pisani A, Protano G, Riccobono F (2008) Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem 107:1553–1560
Saghaei S, Ekici H, Demirbas M, Yarsan E, Tumer I (2012) Determination of the metal contents of honey samples from Orumieh in Iran. Kafkas Univ Vet Fak Derg 18:281–284
Acknowledgements
The study project was supported by Beheshti University of Medical Sciences and Tehran University of Medical Sciences.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Nabi Shariatifar: Conceptualization, Supervision, Design of study, Writing- Reviewing and Editing. Mojtaba Moazzen: Writing- Original draft, Design of study, Methodology, Writing- Reviewing and Editing. Mahsa Naghashan and Majid Arabameri: Visualization, Investigation, Methodology, Software, Validation, Alireza Gholampour Aliabadi and Mahsa Ahmadloo: Methodology. Software, Validation, Data curation, Writing- Original draft preparation.
Corresponding author
Ethics declarations
Ethical Approval
This study does not involve any human or animal testing.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 13.4 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arabameri, M., Naghashan, M., Ahmadloo, M. et al. Analysis of Elements and Physicochemical and Microbial Properties of Iranian Honeys. Biol Trace Elem Res 202, 4279–4287 (2024). https://doi.org/10.1007/s12011-023-03989-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03989-2