Abstract
Pleurotus ostreatus is an edible mushroom with significant nutritional properties and highly valuable protein concentrates can be obtained from its fruit bodies. Functional properties of flours and protein concentrates derived from 3 Pleurotus ostreatus strains (PCM, POS and hybrid PCM × POS) were evaluated in this investigation. Fruit bodies were produced on wheat straw substrate, flours were obtained from dried and grinded fruit bodies and protein concentrates were extracted from flours by alkaline solubilization. For all 3 strains, pale yellow flours were obtained and protein concentrates showed a grayish brown color. Flour bulk densities ranged from 0.52 to 0.64 g/mL, a higher value than those for protein concentrates, i.e. 0.30–0.35 g/mL. The highest water absorption capacities (WAC) were observed for flours (300–418.8%) while protein concentrates presented higher oil absorption capacity (OAC) (173.3–214.1%). Flours and protein concentrates presented a minimal gelation concentration of 2%. Protein concentrates showed a higher foam capacity formation (FC) at pH 8. Likewise, flours and protein concentrates presented higher foam stability (FS) at alkaline pH (8 and 10). Emulsion activity index (EAI) for flours ranged from 3.96 to 26.68 m2 g−1 whereas for protein concentrates ranged from 1.55 to 10.28 m2 g−1. These results indicate that flours and protein concentrates from P. ostreatus have remarkable functional properties, valuable in food industry where foaming and emulsifying properties are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agaricus bisporus ranks in the first place among edible cultivated fungi followed by Lentinula edodes and Pleurotus ostreatus (Corrêa et al. 2016). Pleurotus cultivation, however, shows many advantages, it has the shortest cultivation cycle, grows at moderate temperature, 25–30 °C, grows on a broad range of lignocellulosic materials (Sánchez 2004), and Pleurotus cultivation is rather simple and low-cost since sophisticated equipment, complicated technology or resources are not required (Tesfaw et al. 2015). Edible mushrooms are important in human food due to their nutritional value, they present a high protein content with a good balance in essential amino acids, high fibre and low lipid content (Khatun et al. 2012). Proximal chemical analysis of edible cultivated mushrooms shows dry weight protein content in the range of 19 up 35% (Chang and Buswell 1996).
Fruit bodies of the edible fungus Pleurotus sp. are considered a food of high nutritious value of low caloric and lipid content and with a high content of protein, vitamins and minerals of importance for human nutrition (Maftoun et al. 2015). Chemical composition of P. ostreatus fruit bodies, as recently reported by Akyüz and Kirbag (2010) on a dry matter basis, is 6% ashes, 0.5% lipids and 41.6% protein, a higher value than the corresponding for A. bisporus (36.3%). Additionally, proteins from these fungi contain all essential aminoacids, especially abundant in lysine and leucine, aminoacids not found in some basic grains (Kim et al. 2009). Furthermore, 98% digestibility has been reported for Pleurotus proteins (Valencia et al. 2006), a higher value than for Schizophillum commune (53.2%) and for Lentinula edodes (76.3%) (Longvah and Deosthale 1998).
There is an increasing interest for low cost and high protein sources for human diet and usually vegetable proteins have been favored. However, the high quality of fungal proteins has prompted the interest in using them for fortification and enrichment of foods (Akintayo et al. 1999). For an efficient use of fungal flours and acceptance by consumers, it is important to identify their functional properties, i.e. their capacity for water and oil absorption as well as their foaming, emulsifying and gelification capacities (Adebowale and Lawal 2004). Functional properties of proteins are physicochemical properties that determine their yields and behavior in food systems during preparation, processing, storage and consumption (Panyam and Kilara 1996). Functional properties have been established for flours and protein concentrates of Pleurotus tuber-regium sclerotia (Alobo 2003). Flours from fruit bodies of various edible fungi have been used for production of wheat bread (Okafor et al. 2012). It is therefore necessary to study functional properties of cultivated mushrooms in order to gather elements for using flours and protein concentrates in human foods. The aim of this study is to establish functional properties of flours and protein concentrates derived from fruit bodies of three different Pleurotus ostreatus strains.
Materials and methods
Biological material
Three Pleurotus ostreatus strains from the culture collection of the Cellular Cultivations Laboratory of Instituto Politécnico Nacional (UPIBI-IPN) were used: POS (a commercial strain), PCM (a strain from a culture collection) and PCM × POS (an hybrid derived from the 2 previous strains).
Preparation of protein concentrates of Pleurotus ostreatus
Fruit bodies from the three Pleurotus ostreatus strains were produced on pasteurized wheat straw substrate. Fruit bodies were dried at 40 °C and grinded with an homogenizer at 9000 rpm until a flour mash 50 (Montinox, México) was obtained. Flours were defatted using hexane 1:5 (w/v) with continuous agitation for 8 h at 4 °C. Hexane was eliminated by decantation and defatted flours were placed in an extraction cabinet for 24 h, or until solvent was completely evaporated. Flours were then sieved thorough mash 80. Protein concentrates (PC) were obtained by isoelectric precipitation according to Cruz-Solorio et al. (2014).
Determination of physical and functional properties
Color and bulk density
Color Reader CR-10 Konica Minolta was used to evaluate color of flours and protein concentrates. CIE-Lab values (L*, a* and b*) were converted to a system RGB (Red, Green and Blue) with converter ColorMine.org. Hexadecimal values were obtained from RGB values with Abobe Color software and then with Munsell code, the color of flours and protein concentrates were accordingly judged. Whiteness index (WI) and Browning index (BI) (which represents the purity of brown color) were assessed with CIE-Lab values using following equations (Maskan 2001):
where
Volumetric density was assessed by weight difference and indicated as g mL−1.
Water and oil absorption capacity
Water absorption capacity and oil absorption capacity of flours and protein concentrates were measured according to the methods proposed by Alobo (2003), with certain modifications, i.e. 5 g of flour or protein concentrates were placed in either 5 mL of water or oil and agitated for 5 min, the suspension was left standing for 30 min and then centrifuged at 3000 rpm for 30 min, the excess water or oil was decanted. Water absorption capacity (WAC) and oil absorption capacity (OAC) were assessed according to following equations:
Foaming properties
Foaming capacity (FC) and foam stability (FS) was measured according to Alobo (2003). A sample of 1 g of flour or protein concentrate was mixed with 25 mL of deionized water, after adjusting pH at 6.8, the solution was homogenized at 3000 rpm for 5 min and then placed in a 100 mL test tube. Total volume was registered for 0 min to measure foam capacity and in 10 min intervals, until completing 120 min to measure foam stability. Foaming capacity was assessed according to following equation:
where VH1 volume before homogenization, VH2 volume after homogenization.
Foam stability was assessed according to following equation:
where FVt foam volume after time (t), FVi initial foam volume.
To evaluate the effect of pH on foaming properties, the same methodology was followed but adjusting pH at 2, 4, 6, 8 and 10, respectively.
Gelification concentration
The minimum concentration for gelification (MCG) was assessed by the methodology proposed by Alobo (2003) with slight modifications. In 10 mL test tubes, 5 mL solutions of flours and protein concentrates were prepared at 2, 4, 6, 8 and 10% concentrations. After heating test tubes in boiling water for 60 min, they were cooled down in ice and stored at 4 °C for 30 min. Test tubes were placed upside down, the sample not sliding down corresponded to the minimum concentration for gelification.
Emulsifying properties
Emulsifying activity index (EAI) and emulsification stability index (ESI) were assessed according to the methodology proposed by Pearce and Kinsella (1978) with minor modifications. A sample of 0.1 g of flour or protein concentrate was mixed with 10 mL of deionized water, 3.3 mL of corn oil was added and pH was adjusted at 2, 4, 6, 8, and 10, respectively. The mixture was homogenized at 20,000 rpm for 1 min and then, 50 µL of the resulting emulsion was added to a 5 mL solution of sodium dodecyl sulphate (SDS) (0.1%). Absorbance was measured at 500 nm. Emulsifying activity index (EAI) and emulsification stability index (ESI) were assessed according to following equations:
where A0 absorbance at 0 min after homogenization, A10 absorbance at 10 min after homogenization, ∆t 10 min and ∆A = A0 − A10.
Statistical treatment
Data were examined for normal distribution (Shapiro–Wilk and Kolmogorov–Smirnov test) and for homogeneity of variance (Levene test). Thereafter different analysis of variance was performed for 1 or 2 factors and repeated measures. Duncan test was carried out when significant differences were found. All tests were performed with SPPS Ver. 22 software.
Results and discussion
Physical and functional properties
The protein content in protein concentrates produced from the parental and hybrid strains were 48.56, 49.85 and 49.94% for PCM × POS-PC, POS-PC and PCM-PC, respectively. These values were significantly higher than the corresponding values in fungal flours, 26.81, 25.78 and 24.25%, respectively. For the first time, functional and physical properties of flours and protein concentrates were studied using three different P. ostreatus strains, two parental and an hybrid strain. As shown on Table 1, flour bulk density (0.52–0.64 g/mL) were superior to the corresponding protein concentrates (0.30–0.35 g/mL), (F(6,20) = 207.1, p = 0.0001), however, they were lower than wheat flour bulk density (0.78 g/mL). Densities in this range (0.68 g/mL) have been previously reported by Islam et al. (2012) for wheat flour and various studies have reported that flours normally show higher bulk density than the protein concentrates derived from them (Yuliana et al. 2014), suggesting that this property depends upon particle size and the nature of the other components in flour as well as of their interactions. The bulk density value depends on the flour type and the use it is intended for. A large spectrum of values have been reported, as low as 0.179 g/cm3 for defatted pecan flour up to 0.93 g/cm3 for full fat black gram flour. Bulk density is influenced by lipid and moisture content (Joshi et al. 2015). A higher bulk density is desirable since this helps to decrease paste thickness, an important factor for child foods and convalescence patients (Padmashree et al. 1987).
Water and oil absorption capacity
Significant differences were found in water absorption capacity (WAC) (F(6,20) = 140.2, p = 0.0001) and five groups were established by Duncan test. Wheat flour showed the lowest value (109.7%), protein concentrates show intermediate values, in groups “b” and “c” with values from 179.1 to 214.5% while flours from the 3 strains are in groups “d” and “e”, thus showing significant higher WAC values than their corresponding protein concentrates, i.e. 397.8 for protein concentrate of PCM in contrast to 179.1 for PCM flour, 418.8 for protein concentrate of POS while 214.5 for POS flour and 300 for protein concentrate of PCM × POS compared to 182.2 for PCM × POS flour. Although similar, our results showed a clearer tendency than the data previously reported for P. tuber-regium sclerotia, i.e. 337% for flour and 331% for protein concentrates (Alobo 2003). WAC is the ability of proteins to hold water against gravity (Shevkani et al. 2015) and since water molecules are captured by hydroxyl groups in carbohydrates through hydrogen bonds, Chel-Guerrero et al. (2002) pointed out that carbohydrates contribute to the high WAC in legume flours, so the lower WAC of protein concentrates, as observed in Table 1, is a result of the decreased carbohydrates content (around 70%) as previously reported by Cruz-Solorio et al. (2014). Water absorption (WAC) of chickpea protein concentrates was reported to be affected by the procedure for isolation of protein concentrates as well as by protein conformation and the availability of polar amino acids for protein-water interactions (Paredes-López et al. 1991). Therefore, the high WAC values of P. ostreatus flours allow them to be used as ingredients in bakery and meat products (Mao and Hua 2012), noticeably more suitable than wheat flour. Similarly, wheat flour also showed the lowest oil absorption capacity (OAC), 94.9%, and in this case, protein concentrates from the three P. ostreatus strains showed significant higher values than their corresponding flours, i.e. 173.3 for protein concentrate of PCM against 122.2 for PCM flour, 214.1 for protein concentrate of POS while 125.9 for POS flour and finally, 195.8 for protein concentrate of PCM × POS and 104.8 for PCM × POS flour. Oil absorption capacity (OAC) is an important functional property in food technology since higher OAC values promote good flavor retention, better palatability and extended shelf life of foods (Chel-Guerrero et al. 2002) like breads, soups and meat products. The oil absorption capacity of flours is important for the development of new food products, particularly in regards to their storage stability since it promotes flavor binding and prevents development of oxidative rancidity (Siddiq et al. 2010).
Gelification concentration
Pleurotus ostreatus protein concentrates and flours showed a good gelification property with a lower minimum concentration for gelification (MCG), 2%, than wheat flour, 4%, but also much lower than the results previously reported by Alobo (2003) for P. tuber-regium sclerotia, 4% for flour and 6% for protein concentrates. Variations in gelification properties result of the different type of components in flours, i.e. proteins, lipids and carbohydrates, and according to Nithiyanantham et al. (2013) gelification mechanism and gel aspect are controlled by a balance of hydrophobic attractive and electrostatic repulsive interactions. Furthermore, gel properties are influenced by various factors like pH, type and concentrations of electrolytes and type of proteins (Alleoni 2006).
In relation to color of flours and concentrates, one factor variance analyses for browning index (BI) showed significant differences (F(5,17) = 4.52, p = 0.015) and Duncan test indicated that protein concentrates were darker than the corresponding flours, i.e. 74 versus 42.3 for PCM, 67.6 versus 49.4 for POS and 69.4 versus 49.9 for the hybrid strain PCM × POS. The opposite results were found with whiteness index (WI), wheat flour showed the highest value, 65.1, and flours had significant higher WI values than the corresponding protein concentrates, (F(5,17) = 292.48, p = 0.0001). Color determination by Munsell code reflects and takes into account BI and WI values, so fungal flours show a pale yellow color, wheat flour a white color and protein concentrates a brownish-gray color. In some protein concentrates derived cereals, like those obtained from rice bran by Kaewka et al. (2009), brown pigments generated by Maillard reaction are responsible for their brown color, though Mwasaru et al. (1999) associated the brown color of protein concentrates from two legumes, to the recovery methods, i.e. isoelectric precipitation and micellar solubilizing. In this study, color change in protein concentrates are influenced by the method for recovery of protein concentrates and by the Millard reaction due to the presence of carbohydrates, as previously reported (Cruz-Solorio et al. 2014). Has been reported that the variations in the color characteristics of the protein isolates could possibly be due to the presence of different types and concentrations of coloring constituents, for example polyphenols, present in the flours that might have interacted with the proteins and extracted along with them (Shevkani and Singh 2015).
Foaming properties
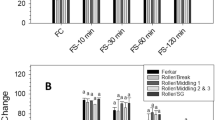
Significant differences were found in foaming capacity of flours and protein concentrates at pH 6.8 (F(6,14) = 46.5, p = 0.0001), it was significantly higher in protein concentrates (109.1–144.5%) than in flours (60.3–69.6%) (Fig. 1). Likewise, Wu et al. (2009) found that peanut protein concentrates showed higher FC (50%) than the corresponding flours (28%) but contrarily, Alobo (2003) reported higher FC values for flours than for protein concentrates of P. tuber-regium sclerotia. Protein concentrates have the double content of proteins than flours (Cruz-Solorio et al. 2014) and this account for differences in FC since proteins unfold and interact among them to form a film or multilayer of proteins in the air/liquid interface protecting air bubbles from collapsing allowing formation of larger volumes of foam (Akintayo et al. 1999). Film formation by proteins may be limited by the hydrophobicity forces and electrostatic interactions that maintain the native state of proteins as well as by intermolecular disulfide covalent bonds (Alleoni 2006). Although protein concentrates showed larger FC values, their foam stability (FS), 36.3–47.5%, was significantly lower (F(6,14) = 12.4, p = 0.0001) than the FS values of the corresponding flours, 45.7 to 66.8% (Fig. 1). Protein concentrates of P. tuber-regium sclerotia showed higher FS values than the corresponding flour as reported by Alobo (2003), however, in this case P. tuber-regium protein concentrate had a lower lipid content (1.5%) than flour (5.95%) while in this study, P. ostreatus protein concentrates had a higher lipid content, 5.6–6.1%, than flours, 1.9–2.0% (Cruz-Solorio et al. 2014). Protein concentrates with higher lipid content have lower FS and, additionally, the high protein concentration favors protein–protein interactions producing foam collapse (Toews and Wang 2013). Foaming properties have been also suggested to be influenced by the presence of other components, like carbohydrates, an important component in flours (Sreerama et al. 2012).
Foaming capacity (FC) and foam stability (FS) at pH 6.8 of flour and protein concentrates of three P. ostreatus strains. Values are expressed as mean ± standard error of means, n = 3. Different letters indicate statistically significant differences (Duncan’s test p < 0.05) for comparisons of treatment means for FC (capital letters) and for FS (lowercase letters)
Foaming properties of flours and protein concentrates were affected by pH, flours generally showing lower FC values than protein concentrates (Fig. 2), but between pH 6 and 8 significant differences were indicated by ANOVA (F(6,14) = 46.56, p > 0.001). As shown in Fig. 2, protein concentrates present the highest FC values at pH 8 (220–276%) decreasing to pH 10 (116–200%). Similar results were reported for protein concentrates of Ginkgo biloba seeds by Deng et al. (2011), i.e. a decrease of FC as pH increased from 8 to 10, and the same behavior was reported by Klompong et al. (2007) for hydrolyzed proteins of fish (Selaroides leptolepis) indicating lower FC values at pH 10. A high FC at a certain pH value is caused by an increased protein flexibility resulting in a faster diffusion to the air–water interface to encapsulate air bubbles, then incrementing foaming capacity (FC) (Kinsella et al. 1985). Configuration of protein molecules is also modified by pH, thus altering foaming capacity and foam stability (Makri and Doxastakis 2006). Contrariwise, foaming capacity of P. ostreatus flours increased from pH 6 to pH 10, from values as low as 18.66–172.00, 158.66 and 133.66% for POS-F, PCM × POS-F and PCM-F, respectively. These results agree with a study of flours from four varieties of Phaseolus vulgarius that showed higher FC values, 103.8–142.0%, at alkaline pH (Wani et al. 2013). Behavior differences of flours and protein concentrates is probably associated with the diversity of proteins present in flours but not in protein concentrates since these were obtained by precipitation at pH 4, resulting in recovery of certain types of proteins. Low molecular weight proteins, like albumins and globulins, might be lost during production of protein concentrates from flours of Pleurotus fruit bodies by isoelectric precipitation (pH 4). Supporting evidence was published by Yalçin and Çelik (2007), they reported that SDS-PAGE patterns of barley flours differed from their corresponding protein isolates, which showed a decrease in albumins and globulins. Similarly, a loss of albumins was found in protein isolates from chickpea and Lathyrus seeds (Pastor-Cavada et al. 2010). The formation of protein aggregates or polymers has been reported by Shevkani and Singh (2015) as a result of covalent and non-covalent interactions, produced between hidrophobic aminoacids during isolation of protein concentrates from legume products.
Foam stability (FS) for flours and protein concentrates of the three P. ostreatus strains markedly decreases, ca. 40%, during the first 10 min in all cases, as shown on Fig. 3. After 60 min, protein concentrates, PCM-PC, POS-PC and PCM × POS-PC, exhibited higher foam stability at pH 8 (73.05, 65.24 and 67.06%, respectively) than the corresponding flours, which presented higher stabilities at pH 10, i.e. 63.49% for PCM-F, 79.86% for POS-F and 67.91% for PCM × POS-F. Both, protein concentrates and flours exhibited better stability at alkaline pH (pH 8 and pH 10) while it was very reduced at pH 2. Yuliana et al. (2014) reported a similar behavior for protein concentrates from cashew shell, i.e. higher foam stabilities at alkaline pH values (8–10), 76.43 and 90.01%, after 60 min. According to these authors, higher foam stability at pH values above pI is related to an increased solubility of proteins causing a viscosity increase and favoring formation of a protein cohesive multilayer at the interface. Furthermore, the negative charge of proteins at alkaline pH also reduces the tendency of foam particles to collapse and hence increases foam stability (Yuliana et al. 2014). Noteworthy, wheat flour displayed an opposite behavior than fungal flours, foam stability was higher at acid pH. Improved foaming properties of flours at acid pH in relation to protein concentrates is attributed to their better flexibility in aqueous solutions and stronger interactions at the air–water interface resulting in stable foams (Aluko et al. 2001). This factor might explain the different behavior of fungal and wheat flours in addition to the differences in regards to their amino acid profile.
Emulsifying properties
Emulsifying properties of P. ostreatus flours and protein concentrates at varying pH are shown on Fig. 4. Flours exhibited higher emulsifying activity index (EAI), 3.96–26.68 m2/g (Fig. 4a), than the corresponding protein concentrates, 1.55–10.28 m2/g (Fig. 4b). Emulsifying capacity of proteins has been reported to diminish with increasing concentration (Kinsella et al. 1985) as observed with protein concentrates of beans (Sathe et al. 1982) and sunflower (Lin et al. 1974). Protein concentration in P. ostreatus protein concentrates doubles that in flours (Cruz-Solorio et al. 2014) explaining their lower EAI values. For protein concentrates, EAI decreased as the protein pI was approached (Fig. 4b) in agreement with a lowering amount of soluble protein (Cruz-Solorio et al. 2014). The highest EAI values for protein concentrates were observed at alkaline pH (8–10) while that of PCM × POS-PC was significantly higher than the corresponding EAI for PCM-PC and POS-PC (F(2,6) = 86.7, p = 0.0001). Similarly, Jamdar et al. (2010) reported for peanut protein hydrolysate, low EAI at the isoelectric point (pH 5.6) and high values at an alkaline pH (pH 9). Likewise, Shevkani et al. (2015) reported that protein concentrates from two different legume products showed a low EAI at pH 4 and 6, which increased at pH 7. Emulsifying activity depends of exposure of hydrophobic amino acids at the oil–water interface, which affects protein solubility and determine emulsifying properties (Du et al. 2014). Emulsion stability index (ESI) for flours were in the range of 10.1–130.0 min (Fig. 4c) and for protein concentrates 3.64 to 180.27 min (Fig. 4d). While stability of protein concentrates tended to improve at alkaline pH (8 and 10), flours from parental strains, PCM-F and POS-F, showed increased stability at acid pH. Similar results were reported by Jamdar et al. (2010) for peanut protein isolates showing increasing emulsion stability index (ESI) at alkaline pH (7 and 9) and acid pH (3).
Emulsifying activity index (EAI) (4A and 4B) and emulsification stability index (ESI) (4C and 4D) at different pH values of flour and protein concentrates of three P. ostreatus strains. Values are expressed as mean ± standard error of means, n = 3. Different letters indicate statistically significant differences (Duncan’s test p < 0.05)
Conclusion
Three different P. ostreatus strains, two parental and an hybrid strain, were used to study functional properties of fungal flours and protein concentrates. These properties were established to vary depending on strain. Both, fungal flours and protein concentrates were found highly suitable for formulation of food products requiring foaming and emulsifying properties or gel formation. Foam formation and stability, as well as emulsion activity and stability were highly dependent on pH, fungal flours and protein concentrates showed higher FC, FS, EAI and ESI at alkaline pH. Protein concentrates showed higher foaming capacity than flours but their foam stability was lower. Gelification concentration was also lower for protein concentrates than for flours. Protein concentrates showed higher browning index than flours. Experimental results indicate that P. ostreatus flours and protein concentrates can be used by the food industry in product formulations of food products when foaming properties, gel formation or emulsifying properties are required.
References
Adebowale K, Lawal O (2004) Comparative study of the functional properties of bambarra groundnut (Voandzeia subterranean) jack bean (Canavalia ensiformis) and Mucuna bean (Mucuna pruriens) flours. Food Res Int 37:355–365. https://doi.org/10.1016/j.foodres.2004.01.009
Akintayo E, Oshodi A, Esuoso K (1999) Effects of NaCl, ionic strength and pH on the foaming and gelation of pigeon pea (Cajanus cajan) protein concentrates. Food Chem 66(1):51–56. https://doi.org/10.1016/S0308-8146(98)00155-1
Akyüz M, Kirbag S (2010) Nutritive value of wild edible and cultured mushrooms. Turk J Biol 34:97–102. https://doi.org/10.3906/biy-0805-17
Alleoni CA (2006) Albumin protein and functional properties of gelation and foaming. Sci Agric 63:291–298. https://doi.org/10.1590/S0103-90162006000300013
Alobo AP (2003) Proximate composition and functional properties of Pleurotus tuberregium sclerotia flour and protein concentrate. Plant Food Hum Nutr 58:1–9. https://doi.org/10.1023/B:QUAL.0000040348.97597.23
Aluko RE, McIntosh T, Reaney M (2001) Comparative study of the emulsifying and foaming properties of defatted coriander (Coriandrum sativum) seed flour and protein concentrate. Food Res Int 34(8):733–738. https://doi.org/10.1016/S0963-9969(01)00095-3
Chang S, Buswell J (1996) Mushroom nutriceuticals. World J Microbiol Biotechnol 12(5):473–476. https://doi.org/10.1007/BF00419460
Chel-Guerrero L, Pérez-Flores V, Betancur-Ancona D, Dávila-Ortiz G (2002) Functional properties of flours and protein isolates from Phaseolus lunatus and Canavalia ensiformis seeds. J Agric Food Chem 50:584–591. https://doi.org/10.1021/jf010778j
Corrêa RCG, Brugnari T, Bracht A, Peralta RM, Ferreira IC (2016) Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: a review on the past decade findings. Trends Food Sci Technol 50:103–117. https://doi.org/10.1016/j.tifs.2016.01.012
Cruz-Solorio A, Garín-Aguilar ME, Leal-Lara H, Ramírez-Sotelo MG, Valencia-del Toro G (2014) Proximate composition of Pleurotus fruit body flour and protein concentrate. J Chem Biol Phys Sci 5:52–60
Deng Q, Wang L, Wei F, Xie B, Huang F, Huang W, Shi J, Huang Q, Tian B, Xue S (2011) Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem 124(4):1458–1465. https://doi.org/10.1016/j.foodchem.2010.07.108
Du S, Jiang H, Yu X, Jane J (2014) Physicochemical and functional properties of whole legume flour. LWT Food Sci Technol 55:308–313. https://doi.org/10.1016/j.lwt.2013.06.001
Islam MZ, Taneya ML, Shams-Ud-Din M, Syduzzaman M, Hoque MM (2012) Physicochemical and functional properties of brown rice (Oryza sativa) and wheat (Triticum aestivum) flour and quality of composite biscuit made thereof. Agriculturists 10:20–28. https://doi.org/10.3329/agric.v10i2.13135
Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Sharma A (2010) Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem 121:178–184. https://doi.org/10.1016/j.foodchem.2009.12.027
Joshi AU, Liu C, Sathe SK (2015) Functional properties of select seed flours. LWT Food Sci Technol 60(1):325–331. https://doi.org/10.1016/j.lwt.2014.08.038
Kaewka K, Therakulkait C, Cadwallader K (2009) Effect of preparation on composition and sensory aroma characteristics of acids hydrolyzed rice bran protein concentrate. J Cereal Sci 50:56–60. https://doi.org/10.1016/j.jcs.2009.02.006
Khatun S, Islam A, Cakilcioglu U, Chatterjee N (2012) Research on mushroom as a potential source of nutraceuticals: a review on Indian perspective. Am J Exp Agric 2:47–73. https://doi.org/10.9734/AJEA/2012/492
Kim MY, Chung M, Lee SJ, Ahn JK, Kim EH, Kim MJ, Kim SL, Moon HI, Ro HM, Kang EY, Seo SH, Song HK (2009) Comparison of free amino acid, carbohydrates concentrations in Korean edible and medicinal mushrooms. Food Chem 113:386–393. https://doi.org/10.1016/j.foodchem.2008.07.045
Kinsella JE, Damodaran S, German B (1985) Physicochemical and functional properties of oil seed proteins with emphasis on soy proteins. New Protein Foods 5:107–179. https://doi.org/10.1016/B978-0-12-054805-7.50012-9
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102(4):1317–1327. https://doi.org/10.1016/j.foodchem.2006.07.016
Lin MJY, Humbert ES, Sosulski FW (1974) Certain functional properties of sunflower meal products. J Food Sci 39:368–370. https://doi.org/10.1111/j.1365-2621.1974.tb02896.x
Longvah T, Deosthale YG (1998) Compositional and nutritional studies on edible wild mushroom from northeast India. Food Chem 63(3):331–334. https://doi.org/10.1016/S0308-8146(98)00026-0
Maftoun P, Johari H, Soltani M, Malik R, Othman NZ, El Enshasy HA (2015) The edible mushroom Pleurotus spp.: I. Biodiversity and nutritional values. Int J Biotechnol Well Ind 4(2):67–83. https://doi.org/10.6000/1927-3037.2015.04.02.4
Makri EA, Doxastakis GI (2006) Emulsifying and foaming properties of Phaseolus vulgaris and coccineus proteins. Food Chem 98(3):558–568. https://doi.org/10.1016/j.foodchem.2005.06.027
Mao X, Hua Y (2012) Composition, structure and functional properties of protein concentrates and isolates produced from walnut (Juglans regia L.). Int J Mol Sci 13:1561–1581. https://doi.org/10.3390/ijms13021561
Maskan M (2001) Kinetics of colour change of kiwifruits during hot air and microwave drying. J Food Eng 48:169–175. https://doi.org/10.1016/S0260-8774(00)00154-0
Mwasaru M, Muhammad K, Bakar J, CheMan YB (1999) Effects of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeon pea (Cajanus cajan L.) and cowpea (Vigna unguiculata) protein isolates. II. Functional properties. Food Chem 67:445–452. https://doi.org/10.1016/S0308-8146(99)00151-X
Nithiyanantham S, Siddhuraju P, Francis G (2013) Proximate composition and functional properties of raw and processed Jatropha curcas L. kernel meal. Int J Res Pharm Biom Sci 4:183–195
Okafor JNC, Okafor GI, Ozumba AU, Elemo GN (2012) Quality characteristics of bread made from wheat and Nigerian oyster mushroom (Pleurotus plumonarius) powder. Pak J Nutr 11:5–10. https://doi.org/10.3923/pjn.2012.5.10
Padmashree TS, Vijayalakshmi L, Puttaraj S (1987) Effect of traditional processing on the functional properties of cowpea (Vigna catjang) flour. J Food Sci Technol 24:221–224
Panyam D, Kilara A (1996) Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci Technol 7:120–125. https://doi.org/10.1016/0924-2244(96)10012-1
Paredes-López O, Ondorica-Falomir C, Olivares-Vázquez M (1991) Chickpea protein isolates: physicochemical, functional and nutritional characterization. J Food Sci 56:726–729. https://doi.org/10.1111/j.1365-2621.1991.tb05367.x
Pastor-Cavada E, Juan R, Pastor JE, Alaiz M, Vioque J (2010) Protein isolates from two Mediterranean legumes: Lathyrus clymenum and Lathyrus annuus. Chemical composition, functional properties and protein characterisation. Food Chem 122(3):533–538. https://doi.org/10.1016/j.foodchem.2010.03.002
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26:716–723. https://doi.org/10.1021/jf60217a041
Sánchez C (2004) Modern aspects of mushroom culture technology. Appl Microbiol Biotechnol 64:756–762. https://doi.org/10.1007/s00253-004-1569-7
Sathe SK, Desphande SS, Salunhkhe DK (1982) Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. J Food Sci 47:491–497. https://doi.org/10.1111/j.1365-2621.1982.tb10110.x
Shevkani K, Singh N (2015) Relationship between protein characteristics and film-forming properties of kidney bean, field pea and amaranth protein isolates. Int J Food Sci Technol 50(4):1033–1043. https://doi.org/10.1111/ijfs.12733
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll 43:679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024
Siddiq M, Ravi R, Harte JB, Dolan KD (2010) Physical and functional characteristics of selected dry bean (Phaseolus vulgaris L.) flours. LWT Food Sci Technol 43:232–237. https://doi.org/10.1016/j.lwt.2009.07.009
Sreerama YN, Sashikala VB, Pratape VM, Singh V (2012) Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: evaluation of their flour functionality. Food Chem 131:462–468. https://doi.org/10.1016/j.foodchem.2011.09.008
Tesfaw A, Tadesse A, Kiros G (2015) Optimization of Oyster (Pleurotus ostreatus) mushroom cultivation using locally available substrates and materials in Debre Berhan, Ethiopia. J Appl Biol Biotechnol 3(1):15–20. https://doi.org/10.7324/JABB.2015.3103
Toews R, Wang N (2013) Physicochemical and functional properties of protein concentrates from pulses. Food Res Int 52:445–451. https://doi.org/10.1016/j.foodres.2012.12.009
Valencia TG, Castelán VR, Garín-Aguilar ME, Leal LH (2006) Biological quality of proteins from three strains of Pleurotus spp. Food Chem 94:494–497. https://doi.org/10.1016/j.foodchem.2004.11.053
Wani IA, Sogi DS, Wani AA, Gill BS (2013) Physico-chemical and functional properties of flours from Indian kidney bean (Phaseolus vulgaris L.) cultivars. LWT Food Sci Technol 53(1):278–284. https://doi.org/10.1016/j.lwt.2013.02.006
Wu H, Wang Q, Ma T, Ren J (2009) Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res Int 42:343–348. https://doi.org/10.1016/j.foodres.2008.12.006
Yalçin E, Çelik S (2007) Solubility properties of barley flour, protein isolates and hydrolysates. Food Chem 104(4):1641–1647. https://doi.org/10.1016/j.foodchem.2007.03.029
Yuliana M, Troung CT, Huynh LH, Ho QP, Ju Y (2014) Isolation and characterization of protein isolated from defatted cashew nut shell: influence of pH and NaCl on solubility and functional properties. LWT Food Sci Technol 55:621–626. https://doi.org/10.1016/j.lwt.2013.10.022
Acknowledgements
The financial support was provided by the Instituto Politécnico Nacional (IPN) with research Project SIP20160240.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cruz-Solorio, A., Villanueva-Arce, R., Garín-Aguilar, M.E. et al. Functional properties of flours and protein concentrates of 3 strains of the edible mushroom Pleurotus ostreatus. J Food Sci Technol 55, 3892–3901 (2018). https://doi.org/10.1007/s13197-018-3312-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3312-x