Abstract
Most of antimicrobial peptides interact with food components decreasing their activity, which limit their successful incorporation into packaging material, functional foods and edible films. The aim of this work was to develop a nisin carrier. Nanofibers of amaranth protein and pullulan (50:50) loaded with nisin were obtained by electrospinning. The nanofibers morphology was determined by scanning electron microscopy and fluorescent microscopy. The molecular interactions were characterized by infrared spectroscopy, X-ray diffraction, differential scanning calorimetry, and thermogravimetric analysis. The nisin loading efficiency as well as the antimicrobial activity against Leuconostoc mesenteroides were evaluated. The micrographs of the obtained materials exhibited smooth and continuous fibers with no defects characterized by diameters between 124 and 173 nm. The FTIR analysis showed intermolecular interactions mainly by hydrogen bonding. The electrospinning process improved the thermal properties of the polymeric mixture displacing the Tm peak to higher temperatures and increasing crystallinity. The antimicrobial activity of nisin in broth and agar against L. mesenteroides was maintained after incorporation into fibers. The results presented an outlook for the potential use of protein amaranth nanofibers when incorporating antimicrobials as a food preservation strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of the consumer demand for natural and safe products, the food industry is actively looking for reducing the amount of synthetic antimicrobial substances or replacing them with natural alternatives. The replacement of synthetic antimicrobials is not an easy task since natural antimicrobial compounds are not as effective as synthetic ones, they are more expensive and usually they interact with food components promoting a loss in activity (Blanco-Padilla et al. 2014; Groves and Titoria 2009; Imran et al. 2012). Actually, antimicrobial peptides (AMPs) constitute an attractive alternative for the replacement of synthetic antimicrobials (Joerger 2007). Nisin, a heat-stable 34 amino acid peptide with a molar mass of 3500 Da, is produced by Lactococcus lactis subsp. lactis and belongs to the class I bacteriocins named lantibiotics. It is the only antimicrobial bacteriocin with the status of “General Recognized As Safe” (GRAS) material approved by the Food Drug Administration (Bi et al. 2014) and recognized as a food additive, number E234, in EU (Krivorotova et al. 2016). It shows effective antimicrobial activity against Gram-positive bacteria such as Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus and Leuconostoc mesenteroides (Ollé Resa et al. 2014). The action mechanism of nisin is a multistep process which includes the destabilization of the phospholipid bilayer of the cell producing transient pores followed by leakage of the cellular materials, such as proteins and lipids, rendering the cell´s death (Breukink and de Kruijff 2006). However, nisin loses antimicrobial activity in food matrices due to: (a) enzymatic degradation (Galvez et al. 2008), (b) deactivation by interactions with food components such as divalent cations, fat, phospholipids, emulsifiers and pH (Aasen et al. 2003), and c) emergence of nisin-resistant bacterial strains (Liu et al. 2015). To protect nisin antimicrobial activity, some nanostructure materials have been developed, for example nanoparticles, nanoliposomes and nanofibers (Sadiq et al. 2016). Natural polymers such as zein (Neo et al. 2013a), whey protein (Sullivan et al. 2014), soy-protein (Har-el et al. 2014), and amaranth protein (Aceituno-Medina et al. 2013a) have been used to develop electrospun mats. The mats are composed by fibers with micro or nanoscale diameters and display packaging desirable characteristics such high surface area and porosity, absorbability of liquids, semi permeability for vapors and gases and capability for incorporating heat sensitive bioactive compounds (de Faria et al. 2015). Antimicrobial compounds can be incorporated in electrospun mats to develop antimicrobial food packaging, which can prevent food microbial contamination and extend the product shelf life. Actually, the materials that have been used to produce nisin loaded nanofibers are not food grade materials, so their use in food industry is restricted. The electrospun mats obtained in this study on the other hand, are based on biodegradable food grade biopolymers, protein amaranth and pullulan, therefore, potentially useful as edible films and in the manufacture of active packaging that improves antimicrobial nisin activity.
Materials and methods
Materials
Commercial amaranth protein concentrate (Amaranthus hypochondriacus L. Revancha variety) was supplied by Nutrisol, (Hidalgo, Mexico). The Amaranth Protein Isolate (API) was obtained as previously reported (Aceituno-Medina et al. 2013b). Hydrochloric acid (HCl), sodium hydroxide (NaOH), pullulan (PUL), Tween 80, formic acid, nisin from Lactococcus lactis, dimethylsulfoxide (DMSO) and Fluorescein isothiocyanate isomer I (FTIC) were obtained from Sigma-Aldrich Co. (St. Louis MO, USA) and the Kit bicinchoninic acid (BCA) from Thermo Fisher Scientific (MA, USA). Man, Rogosa and Sharpe (MRS) agar and broth were supplied by Becton–Dickinson (New Jersey, USA). All the other chemicals were purchased from Sigma unless otherwise specified.
Preparation of API/PUL solution for electrospinning
To obtain the electrospun fibers, a polymeric solution of API and PUL at 50:50 proportions with 20%wt. of Tween 80 was prepared in formic acid as described by Aceituno-Medina et al. (2013b). The API:PUL:nisin solutions were prepared by dissolving 10 and 20 mg nisin/mL. Three polymeric solutions were prepared API:PUL (A), API:PUL: 10 mg nisin/mL (B) and API:PUL: 20 mg nisin/mL (C).
Characterization of the polymeric solutions
The apparent viscosity of the polymeric solutions was determined using a two plates rheometer with 0.3 mm gab brand Physica MCR 101 from ISIS (Oxford, USA). The conductivity of the solutions was measured using a conductivity meter ORION SoTAR A212 from Thermo Fisher Scientific (MA, USA). All measurements were performed in triplicate at 25 °C.
Preparation of nanofibers by electrospinning
The electrospinning apparatus was equipped with a variable high voltage 0–30 kV power supply (Acopian P030HPI-230, USA). The anode was attached to a stainless-steel needle with approximately 0.9 mm diameter, which was connected via a PTFE cable to a 5 mL plastic syringe that contained the polymeric solutions. The syringe was connected to the cathode, which in turn was connected to a high voltage source. All experiments were conducted at room temperature. Voltage conditions, the collector distance and the feed flow were set at 22 kV, 10 cm and 0.4 mL/h, respectively.
Characterization of electrospun nanofibers
Fluorescent and scanning electron microscopy (SEM)
The morphology and diameters of electrospun fibers were determined using a scanning electron microscope (EVO-50 of Carl Zeiss; Jena, Germany). The sample was sputter coated with gold under vacuum before imaging and the measurements were carried out at an accelerating voltage of 15 kV. The diameters of the electrospun structures were obtained by measuring at least 100 individual fibers with the software Image J from the SEM micrographs in their original magnification. To obtain the fluorescent images, nisin was labeled with the fluorescent probe FITC according to the procedure described by Heunis et al. (2011), then an API:PUL:FITC-nisin solution was made and electrospun as previously described. Nanofibers were excited at 472 nm and the fluorescent images were captured with a Carl Zeiss (Jena, Germany) microscope (Observer D1 AX10) and then the Zen imaging software was used.
Differential scanning calorimetry (DSC)
Thermal analysis was carried out on a differential scanning calorimeter (Mettler Toledo model DSC822e, Polaris Parkway, USA). The calorimeter was previously calibrated with a pattern of Indio (0.16 mg) and an empty aluminum crucible was used as a control. 2–5 mg of fiber samples and pure components were placed in aluminum crucibles of 40 μL. The samples were heated under nitrogen with a flow rate of 20 mL/min, in a single cycle from 25 to 250 °C and 25 to 200 °C for fiber samples and pure compounds, respectively. Samples were run in triplicate.
Diffuse reflectance infrared spectroscopy (DRIFT)
Infrared absorption spectra of pure compound and fibers were recorded by DRIFT (Spectrum GX spectrophotometer, Perkin Elmer, Massachusetts, USA with a diffuse reflectance accessory, Pike Technology model). Spectra were collected in a controlled chamber at 24 °C and 40% RH.
X ray diffraction
The X-ray pattern of the nanofibers was obtained with an X-ray diffractometer (Dmax 2100 Rigaku Americas, Texas, USA) that has a K α Cu radiation generator (λ = 1.5418 Å). Operating voltage and current were maintained at 30 and 40 mA, respectively. The angle of incidence was varied within a range of 2θ of 5°–40°. Data were collected at intervals of 0.02. The d spacing for a given scattering angle, 2θ, was calculated by application of the Bragg equation (Eq. 1):
where λ is the wavelength of the Cu-Kα radiation.
Thermogravimetric analysis (TG)
TG curves were recorded with a TA Instrument (New Castle, USA) model Q500 TG. The samples (10 mg) were heated from 50 to 800 °C with a heating rate of 5 °C/min under nitrogen atmosphere.
Loading efficiency of nisin in electrospun fibers
The encapsulation efficiency of nisin was obtained using a BCA kit. Briefly, 1 g of nisin-loaded fibers were completely dissolved in DMSO and stirred for 12 h; the resulting solution was taken to pH 9 and centrifuged at 9000g. The pellet was removed and the nisin was quantified using the BCA kit in the supernatant fraction. The loading efficiency (LC) of nisin was calculated using Eq. 2:
In vitro antimicrobial activity
The nisin antimicrobial activity was determined by the disc diffusion method. MRS agar (45 °C) was inoculated with Leuconostoc mesenteroides (103 CFU/mL), homogenated and poured in Petri dishes; then it was incubated for 24 h at 37 °C. Discs of fiber samples (15 mm) were cut, sterilized by UV (15 min) and placed on inoculated MRS agar; plates were incubated for 24 h at 37 °C and the presence of the inhibition zone was assessed. To evaluate the growth of the microorganisms in broth, 25 mg of nanofibers containing nisin were placed in 15 mL of MRS broth previously inoculated with Leuconostoc mesenteroides (103 CFU/mL) and incubated at 30 °C for 48 h. Out of the inoculated tubes, 1 mL aliquots were taken at different times (2, 4, 6, 8, 12, 24 h) and colony counting was carried out.
Results and discussion
Characterization of polymeric solutions
Parameters such as viscosity, electrical conductivity and surface tension of the polymeric solution are known to significantly affect the morphology of the electrospun fibers (Bhardwaj and Kundu 2010). The viscosity and electrical conductivity of the polymeric solutions before the electrospinning process are presented in Table 1. Inspection of these data shows that solution A (neat API/PUL) exhibited a viscosity and conductivity values of 67.30 ± 0.06 mPa s and 7.68 ± 0.12 mS cm−1, respectively. The incorporation of nisin to the API/PUL solution decreased the viscosity values of the solutions, but only solution C showed significant decrease (P < 0.05), from 67.30 to 46.76 mPa s. It can also be observed that the conductivity value was significantly reduced (P < 0.05) as the content of nisin was increased (from 7.68 to 4.42 mS/cm).
Characterization of electrospun fibers
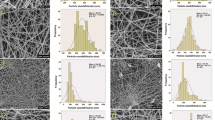
Figure 1 shows the SEM images of the nanofibers. All the polymeric solutions rendered continuous fibers without defects, which indicates a good miscibility between the components of the solution (Aceituno-Medina et al. 2013b). The average diameters of the nisin-loaded fibers ranged from 124 to 173 nm (Table 1). It was found that the average fiber diameter decreased with an increase of nisin content. The decrease of viscosity with the addition of nisin improves the molecular entanglement among the components and the stretching properties of the solution, resulting in a decrease of the electrospun fibers diameter (Neo et al. 2013b). It is interesting to note that the diameters of the fibers obtained in this study were lower than those reported by Ahire and Dicks (2014), who obtained PDLA and PEO electrospun fibers loaded with nisin with diameters between 400 and 450 nm. Lower diameters display more superficial area increasing the distribution of the nisin.
Nisin distribution in fibers was measured by fluorescent microscopy, where nisin needed to be labeled with FITC. Figure 1d shows that nisin is distributed uniformly throughout the fibers. In the image, areas with high fluorescence are attributed to the crossing point of the fibers.
Figure 2 shows the FTIR spectra of API, API/PUL and API:PUL:nisin fibers. In the spectrum of API:PUL fibers, the characteristic bands of the amaranth protein such as the Amide I (650.4 cm−1) and Amide II (1531.2 cm−1) and the pullulan bands (925.45 and 843.19 cm−1) can be observed. As reported by Aceituno-Medina et al. (2013b), the widening of the band at 3500 cm−1 and the displacement of the Amide I band to higher wave number, results from the protein and the carbohydrate polymer interactions through hydrogen bonding. Similar results can be observed in the spectrum of nisin-loaded fibers, as the concentration of nisin was increased a major displacement of Amide I band was observed; this observation is related to a larger formation of hydrogen bonds. Hosseini et al. (2014) reported the formation of hydrogen bonds between nisin and the components of a microparticulated system of alginate and resistant starch.
The X-ray diffractograms of individual polymers, API/PUL fibers and nisin loaded API/PUL fibers are showed in Fig. 3. As expected, Pullulan displayed one broaden peak at 2θ = 18.8 with an interplanar distance of 4.24 Å, indicating a typical non-crystalline structure (Zhu et al. 2014). The protein amaranth isolate also showed one broad peak at 2θ = 20.26 (4.59 Å) attributed to the amorphous zone typical of biopolymers such as zein and soy protein (Diéguez et al. 2015). After the electrospinning process it can be observed that the system becomes crystalline, displaying two sharp peaks at 2θ = 43.44 (2.08 Å) and 50.4 (1.81 Å), respectively. This increase of crystallinity can be attributed to the major order of the protein structure acquired when it is stretched under the electrospinning process and promoted by the hydrogen bonding intermolecular interaction between the components. Similar results have been reported for electrospun nanofibers of zein loaded with tannins (De Oliveira Mori et al. 2014).
To evaluate the thermal stability of the nanofibers, differential scanning calorimetry (DSC) and thermogravimetric analysis (TA) were performed. The melting temperature (Tm) is related to the thermal stability of the polymers and, in the case of proteins, with denaturation by heating. Tm values obtained for API (170.92 °C) and pullulan (165.82 °C) are in agreement with the reported values (Condés et al. 2013). In all fibers, one thermal event was observed corresponding to the melting point of the blends. The Tm values of the fibers are reported in Table 1, the Tm value of API/PUL fibers (174.96 °C) shifted to higher temperatures compared with the pure components, which indicates major thermal stability. This observation correlates with the change of the amorphous phase of the biopolymers to crystalline phase of the fibers (Salas et al. 2014). The electrospun fibers loaded with nisin did not show Tm and ΔH values that are significantly different (P < 0.05).
The loss weight curves obtained by TA for API/PUL and nisin loaded API/PUL fibers from 0 to 600 °C, are shown in Fig. 4. All systems reveal two stages of loss weight. The first stage, observed up to 120 °C, is related to the loss of adsorbed and bound water (Neo et al. 2013a) and the second stage is attributed to the decomposition of the sample and the formation of a carbonaceous residue (Zhu et al. 2014). Similar results have been reported by Aceituno-Medina et al. (2013a). In the case of nisin loaded nanofibers, the first stage of loss weight decreased as the concentration of nisin increased; thus suggesting that nisin contributes to the hydrophobicity of the fibers, rendering less entrapment of water molecules and the need of less energy to release the solvent molecules from the fiber. Electrospun nanofibers with 20 mg of nisin were completely degraded at 600 °C (Table 1).
Loading efficiency of nisin in electrospun fibers
The encapsulation efficiency (EE) is related to factors such as the concentration of the active species, the diameter of the fibers and the intermolecular interactions. In this study the EE increases as the concentration of nisin increases (Table 1), reaching 95.41% at a concentration of 20 mg/mL of nisin. This observation can be attributed to molecular interactions among the bacteriocin and the API and PUL during electrospinning (Ji et al. 2014). The EE values of this study are larger than those reported by Prombutara et al. (2012), who elaborated solid-lipid nanoparticles loaded with nisin reaching efficiencies up to 73.3%, and those published by Bernela et al. (2014) who incorporated nisin in alginate-chitosane-pluronic composite nanoparticles reaching EE of 85%. The main disadvantage of using a nisin carrier in the food industry is low throughput, which can be surpassed by the advantages of high superficial area and efficient encapsulation of the nanofibers like those reported in this study (Khan and Deog-Hwan 2015).
Antimicrobial activity
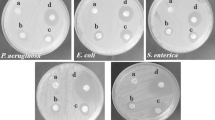
The antibacterial activity against L. mesenteroides in solid and broth medium of nisin, loaded into protein amaranth nanofibers, was maintained (supplementary material). No growth inhibition zone was observed for the API/PUL fibers. Nanofibers containing 10 and 20 mg/mL of nisin showed growth inhibition zones with diameters of 15.51 ± 0.35 and 16.54 ± 0.05 mm, respectively. This finding clearly demonstrates that the observed antibacterial activity was only due to the nisin entrapped into nanofibers and the antibacterial effect was enhanced with the increase of the nisin content. However, the halo of growth inhibition of nisin loaded nanofibers was smaller when compared to the one observed for free nisin (20.00 ± 0.01 mm). The 24 h experiment may not render enough time to allow complete release of nisin from the fibers. A growth inhibition zone of similar diameter was also observed for nisin loaded pectin nanoparticles assayed against Bacillus subtilus and E. coli at pH 6. This system presents a better antimicrobial activity than sodium benzoate (Krivorotova et al. 2016). It is also important to note that Nguyen et al. (2008) developed cellulose films loaded with nisin and ε-polylysine, and that they also observed an increase of antibacterial activity against L. monocytogenes and E. coli as the concentration of nisin increased.
The growth of L. mesenteroides in an MRS broth at different times can be observed in Fig. 5. The fibers of API/PUL did not show antimicrobial activity reaching microorganism concentrations of 108 after 24 h, while the incorporation of nisin (20 mg/mL) to the fibers, reduced 1 Log of initial population in 24 h. Later, the nisin-loaded nanofibers displayed a bacteriostatic effect. Imran et al. (2015) reported similar results for the antibacterial effect against L. monocytogenes of nisin encapsulated in liposomes. The spoilage microorganisms are those that produce characteristic organoleptic changes in food (Alonso and Álvares 2010). Leuconostoc mesenteroides is considered a spoilage microorganism, which is isolated from dairy and meat products, wines, fruits and vegetables, it causes unpleasant odors, mucus and green colors on food products. The development of an active packaging that decreases the contamination of this microorganism is therefore important to maintain the safety of the products. In this sense, the nanofibers developed in this study can be used for this purpose.
Conclusion
The aim of this study was to determine whether the amaranth protein-pullulan nanofibers could incorporate antimicrobial peptides such as nisin. Nisin is important because it has a recognized antimicrobial activity against foodborne pathogenic microorganism; however, its interaction with food components promotes loss of its antimicrobial activity, which limits its use as a food ingredient. In this study, the viscosity and electrical conductivity of the polymer solutions used in the electrospinning process to prepare nisin-loaded nano-fibers are affected by the addition of bacteriocins. These changes in the properties of the solution are reflected in the morphology of the electrospun fibers, notably in the diameter, which decreases to 50% for the nisin concentration of 20 mg/mL. The characterization of the electrospun fibers indicates that there is miscibility between the compounds used for processing, and that the molecular interactions that take place, mainly hydrogen bonds, improve thermal stability. Fibers containing 20 mg/mL of nisin reach a 95.41% of EE. The antibacterial activity against L. mesenteroides of nisin loaded nanofibers in both solid and broth MRS medium was maintained. In broth, API:PUL: 20 mg/mL nisin fibers reduced 1 Log of initial population of L. mesenteroides to further exert a bacteriostatic effect. In this way, it was possible to incorporate bacteriocins into one-dimensional nanostructures such as electrospun nanofibers, maintaining their antimicrobial activity. The advantage of using natural biopolymers which are permitted in the food industry, may allow the development of commercially available nisin loaded electrospun fibers that can work as carrier in edible films to protect food from secondary contaminations; thus increasing food product quality. The potential technology of nisin loaded nanofibers can also be foreseen in other attractive applications such as pharmaceutics and cosmetics.
References
Aasen IM, Markussen S, Møretrø T et al (2003) Interactions of the bacteriocins sakacin P and nisin with food constituents. Int J Food Microbiol 87:35–43. doi:10.1016/S0168-1605(03)00047-3
Aceituno-Medina M, Lopez-Rubio A, Mendoza S, Lagaron JM (2013a) Development of novel ultrathin structures based in amaranth (Amaranthus hypochondriacus) protein isolate through electrospinning. Food Hydrocoll 31:289–298. doi:10.1016/j.foodhyd.2012.11.009
Aceituno-Medina M, Mendoza S, Lagaron JM, López-Rubio A (2013b) Development and characterization of food-grade electrospun fibers from amaranth protein and pullulan blends. Food Res Int 54:667–674. doi:10.1016/j.foodres.2013.07.055
Ahire JJ, Dicks LMT (2014) Probiotics and antimicrobial proteins. Probiotics Antimicrob Proteins 7:52–59. doi:10.1007/s12602-014-9171-5
Alonso C, Álvarez I (2010) Nuevas tecnologías en la conservación y transformación de los alimentos. International Marketing and Communication, S. A., España
Bernela M, Kaur P, Chopra M, Thakur R (2014) LWT—food science and technology synthesis, characterization of nisin loaded alginate e chitosan e pluronic composite nanoparticles and evaluation against microbes. LWT Food Sci Technol 59:1093–1099. doi:10.1016/j.lwt.2014.05.061
Bhardwaj N, Kundu SC (2010) Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 28:325–347. doi:10.1016/j.biotechadv.2010.01.004
Bi X, Wang Y, Zhao F et al (2014) Inactivation of Escherichia coli O157: H7 by high pressure carbon dioxide combined with nisin in physiological saline, phosphate-buffered saline and carrot juice. Food Control 41:139–146. doi:10.1016/j.foodcont.2014.01.012
Blanco-Padilla A, Soto KM, Hernández Iturriaga M, Mendoza S (2014) Food antimicrobials nanocarriers. Sci World J. doi:10.1155/2014/837215
Breukink E, de Kruijff B (2006) Lipid II as a target for antibiotics. Nat Rev Drug Discov 5:321–332. doi:10.1038/nrd2004
Condés MC, Añón MC, Mauri AN (2013) Amaranth protein films from thermally treated proteins. J Food Eng 119:573–579. doi:10.1016/j.jfoodeng.2013.06.006
de Faria AF, Perreault F, Shaulsky E et al (2015) Antimicrobial electrospun biopolymer nanofiber mats functionalized with graphene oxide–silver nanocomposites. ACS Appl Mater Interfaces 7:12751–12759. doi:10.1021/acsami.5b01639
De Oliveira Mori CLS, Dos Passos NA, Oliveira JE et al (2014) Electrospinning of zein/tannin bio-nanofibers. Ind Crops Prod 52:298–304. doi:10.1016/j.indcrop.2013.10.047
Diéguez MCV, Pelissari FM, Sobral PJDA, Menegalli FC (2015) Effect of process conditions on the production of nanocomposite films based on amaranth flour and montmorillonite. LWT Food Sci Technol 61:70–79. doi:10.1016/j.lwt.2014.11.017
Galvez A, Lopez RL, Abriouel H et al (2008) Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit Rev Biotechnol 28:125–152. doi:10.1080/07388550802107202
Groves K, Titoria P (2009) Nanotechnology and the food industry. Food Sci Technol 60:44–51. doi:10.4455/eu.2013.011
Har-el YE, Gerstenhaber JA, Brodsky R et al (2014) Electrospun soy protein scaffolds as wound dressings: enhanced reepithelialization in a porcine model of wound healing. Wound Med 5:9–15. doi:10.1016/j.wndm.2014.04.007
Heunis T, Bshena O, Klumperman B, Dicks L (2011) Release of bacteriocins from nanofibers prepared with combinations of poly(d, l-lactide) (PDLLA) and poly(ethylene oxide) (PEO). Int J Mol Sci 12:2158–2173. doi:10.3390/ijms12042158
Hosseini SM, Hosseini H, Mohammadifar MA et al (2014) Preparation and characterization of alginate and alginate-resistant starch microparticles containing nisin. Carbohydr Polym 103:573–580. doi:10.1016/j.carbpol.2013.12.078
Imran M, Revol-Junelles AM, René N et al. (2012) Microstructure and physico-chemical evaluation of nano-emulsion-based antimicrobial peptides embedded in bioactive packaging films. Food Hydrocoll 29:407–419. doi:10.1016/j.foodhyd.2012.04.010
Imran M, Revol-Junelles AM, Paris C et al (2015) Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT Food Sci Technol 62:341–349. doi:10.1016/j.lwt.2014.12.046
Ji S, Lu J, Liu Z et al (2014) Dynamic encapsulation of hydrophilic nisin in hydrophobic poly (lactic acid) particles with controlled morphology by a single emulsion process. J Colloid Interface Sci 423:85–93. doi:10.1016/j.jcis.2014.02.025
Joerger RD (2007) Antimicrobial films for food applications: a quantitative analysis of their effectiveness. Packag Technol Sci 20:231–273. doi:10.1002/pts.774
Khan I, Deog-Hwan O (2015) Integration of nisin into nanoparticles for application in foods. Innov Food Sci Emerg Technol. doi:10.1016/j.ifset.2015.12.013
Krivorotova T, Cirkovas A, Maciulyte S et al (2016) Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocoll 54:49–56. doi:10.1016/j.foodhyd.2015.09.015
Liu H, Pei H, Han Z et al (2015) The antimicrobial effects and synergistic antibacterial mechanism of the combination of ε-Polylysine and nisin against Bacillus subtilis. Food Control 47:444–450. doi:10.1016/j.foodcont.2014.07.050
Neo YP, Ray S, Jin J et al (2013a) Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: a physicochemical study based on zein-gallic acid system. Food Chem 136:1013–1021. doi:10.1016/j.foodchem.2012.09.010
Neo YP, Swift S, Ray S et al (2013b) Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem 141:3192–3200. doi:10.1016/j.foodchem.2013.06.018
Nguyen VT, Gidley MJ, Dykes GA (2008) Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol 25:471–478. doi:10.1016/j.fm.2008.01.004
Ollé Resa CP, Jagus RJ, Gerschenson LN (2014) Effect of natamycin, nisin and glycerol on the physicochemical properties, roughness and hydrophobicity of tapioca starch edible films. Mater Sci Eng C Mater Biol Appl 40:281–287. doi:10.1016/j.msec.2014.04.005
Prombutara P, Kulwatthanasal Y, Supaka N et al (2012) Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Control 24:184–190. doi:10.1016/j.foodcont.2011.09.025
Sadiq S, Imran M, Habib H et al (2016) Potential of monolaurin based food-grade nano-micelles loaded with nisin Z for synergistic antimicrobial action against Staphylococcus aureus. LWT - Food Sci Technol 71:227–233. doi:10.1016/j.lwt.2016.03.045
Salas C, Ago M, Lucia LA, Rojas OJ (2014) Synthesis of soy protein–lignin nanofibers by solution electrospinning. React Funct Polym 85:221–227. doi:10.1016/j.reactfunctpolym.2014.09.022
Sullivan ST, Tang C, Kennedy A et al (2014) Electrospinning and heat treatment of whey protein nanofibers. Food Hydrocoll 35:36–50. doi:10.1016/j.foodhyd.2013.07.023
Zhu G, Sheng L, Tong Q (2014) Preparation and characterization of carboxymethyl-gellan and pullulan blend films. Food Hydrocoll 35:341–347. doi:10.1016/j.foodhyd.2013.06.009
Acknowledgements
The authors thank the Mexican Project FOMIX-QRO-2011-C02-175350 for financial support and Karen M. Soto thanks the Mexican National Council for Science and Technology (CONACYT) for a graduate fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soto, K.M., Hernández-Iturriaga, M., Loarca-Piña, G. et al. Stable nisin food-grade electrospun fibers. J Food Sci Technol 53, 3787–3794 (2016). https://doi.org/10.1007/s13197-016-2365-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2365-y