Abstract
Increasing demand for safe, efficient, and eco-friendly solutions for pharmaceutical and food industries has led researchers to explore new approaches to bacterial storage. Several advantages make electrospinning (ES) a promising technique for food systems, including simple manufacturing equipment, a relatively low spinning cost, a wide variety of spinnable materials, and a mild process that is easily controlled, which allows continuous fabrication of ultrafine polymeric fibers at submicron or nanoscales without high temperatures or high pressures. This review briefly describes recent advances in the development of electrospun fibers for loading probiotics (PRB) by focusing on ES technology, its efficiency for loading PRB into fibers (viability, digestive stability, growth rate, release, thermal stability, and interactions of fibers with PRB), and the application of PRB-loaded fibers as active packaging (spoilage/microbial control, antioxidant effect, shelf life). Based on the literature reviewed, the incorporation of PRB into electrospun fibers is both feasible and functional. However, several studies have been limited to proof-of-principle experiments and the use of model biological products. It is necessary to conduct further research to establish the industrial applicability of PRB-loaded fibers, particularly in the fields of food and medicine.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing knowledge of the human microbiota has led to a higher awareness of the “microbiom–host” interactions for human health [1,2,3]. Human microbiota imbalances can result in a variety of pathologies known as “dysbioses,” which may cause disorders, including fibrocystic syndrome, diabetes, colon cancer, cardiovascular disease, acne, urinary tract infections, food allergies, obesity, and atopic eczema syndrome [4]. Dysbiosis is characterized by the disruption of the interaction between the microbiota and their metabolic products, as well as the immune system of the host. Adding sufficient quantities of beneficial bacteria, or probiotics (PRB), can restore this microbial balance [5].
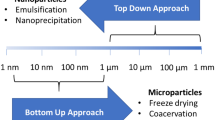
An appropriate delivery system is required to administer sufficient doses of viable PRB. In other words, PRB should be delivered in a way that is patient friendly, provides effective local delivery, incorporates large quantities of viable PRB, and ensures long-term stability, preferably at room temperature. A suitable drying process can be used to enhance long-term stability of PRB by transforming them from aqueous dispersions into dry forms [6]. Microencapsulation is one of the most successful strategies for improving the stability and viability of live PRB strains when exposed to the circumstances of industrial processing, storage, and also for protecting them from the environment of gastrointestinal tract (GIT) [7]. Encapsulation methods for PRB are divided into three main categories: extrusion, emulsification, and drying. Common procedures for the commercial manufacturing of encapsulated PRB include spray drying, freeze drying, lyophilization, lipid-based delivery system, coacervation, and extrusion [8, 9]. On the other hand, electrospinning (ES) offers an alternative method for drying PRB and simultaneously preparing solid dosage forms [10]. In ES, polymeric solutions are electrostatically driven to produce fibers/nanofibers (NFs). The fiber diameter ranges between tens of nanometers (nm), i.e., NFs, and a few micrometers (µm) attributed to microfibers. In addition to functional food delivery systems, ES can be used in beverage filtering, food packaging, and food analysis (e.g., composition analysis, pathogen detection, pesticide residue detection, and antibiotic detection) [11].
It has been reported that ES could be used to incorporate microbial cells into fibers. For example, an agrowaste-based NF was loaded with L. acidophilus [12], whereas PVA electrospun fibers were loaded with B. animalis subsp. lactis Bb12 [13]. Similarly, non-PRB (e.g., E. coli, S. epidermidis) and viruses have been incorporated into PVA-based NFs, although the results indicated low survival following this process [14]. Despite of the potential advantages of ES for PRB incorporation [15], the effects of the process, solutions, and environment parameters on PRB survival remain uncertain due to the lack of literature on this issue. Therefore, the present study was designed to comprehensively review different aspects of ES, as a method to incorporate PRB into fibers by focusing on ES technology, its efficiency, interactions of fibers with PRB, and application of PRB-loaded fibers as active packaging.
Electrospinning Technology: Fundamentals and Process Parameters
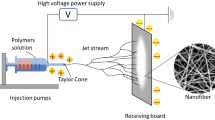
An ES process involves the formation of fibers from polymeric melts or solutions under the influence of an electrostatic field [16]. The conventional ES device involves placing a spinning solution in a syringe, connecting a positive terminal to the solution or metal spinneret, and connecting a negative terminal to the receiving device. In the presence of an external electric field, the polymer droplet held at the tip of the nozzle by the surface tension accumulates charges on its surface and is forced to receive an electric field in the opposite direction from the surface tension. A droplet at the nozzle becomes elongated when the electric field is gradually increased, producing a shape known as a Taylor cone. By increasing the electric field strength to a critical value, the liquid surface tension will be overcome, and the charged solution will be evicted from the Taylor cone to form a jet. Through solvent volatilization and fiber solidification, the jet moves in the air and deposits on the collecting plate to form fibrous films [11]. NFs can be prepared using this method in a convenient and efficient manner. In the ES process, many factors influence the fiber morphology and structure, including the properties of the polymeric solution, environmental conditions, spinning parameters, and the type of ES technique used [16]. In general, ES techniques can be classified into six groups: basic, emulsion, melt, coaxial, blend, and gas jet ES [17].
With different types of spinnerets and collectors, fibers can be generated in various forms and structures to meet various demands. As an example, compared with uniaxial NFs, coaxial NFs are significantly more effective at releasing bioactive compounds encapsulated in the core [18, 19]. By decreasing the recombination of photogenerated cavities and mats, NFs placed side by side can significantly increase catalytic activity [20]; NFs have been applied for the controlled release of drugs, separation devices, sensors, packaging of food, and other fields [11, 21].
In ES, the most basic problem is the formation of fibers under the influence of an electric field force. To generate a jet, the material must have a certain viscosity, conductivity, and surface charge, which can be drawn and refined by an electric field force [22]. ES can be performed with many polymers, including synthetic and natural ones [23]. Suitable organic solvents are required for ES of hydrophobic polymers, whereas water, strong acids, or strong polar organic solvents are used for the ES of hydrophilic polymers. A wide variety of polymers have been used in ES, such as polyacrylonitrile (PAN), polycaprolactone (PCL), poly(ethylene oxide) (PEO), poly vinyl pyrrolidone (PVP), poly vinylidene fluoride (PVDF), poly vinyl alcohol (PVA), and many others.
Electrospinning Efficiency for Loading Probiotics into Fibers
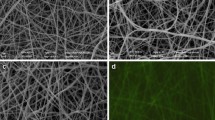
Based on literature, NFs from the mucoadhesive PEO without PRB were uniform, with a mean diameter of 135 nm [10]. Local thickening in the NFs directed effective incorporation of PRB into the PEO NFs. Even though the PRB cells had a larger diameter (492 nm) than the NFs, they were totally coated with polymer and thus enclosed within the NFs. The PRB cells were oriented along NFs, which is consistent with the study conducted by Salalha et al. [24]. The authors demonstrated that random-oriented bacteria in a polymer solution start orienting along streamlines during ES in the Taylor cone and align in a jet that solidifies into NFs at the end. Furthermore, Škrlec et al. [10] demonstrated that PRB incorporated into NFs had a flatter morphology compared with that air dried from dispersions. They found that the diameter of PRB cells incorporated into NFs was 815 nm, which was thicker than the air-dried PRB alone or with an additional theoretical PEO layer. It is possible that the significantly altered morphology of PRB was the result of drying and dehydration, as well as the mechanical stress they had suffered [25]. The change in average diameter with and without probiotics depends on the specific system or material being studied. The presence of probiotics can potentially influence the average diameter of fibers or particles. This change can be attributed to various factors, such as the interaction between probiotics and the fiber/particle matrix, potential swelling or aggregation effects, or the release of bioactive compounds by the probiotics. The exact impact on average diameter will depend on the characteristics of the probiotics, the material, and the experimental conditions [26]. The morphology of the various samples is depicted in Fig. 1. The effect of probiotics on morphology depends on the specific system or material being studied. Probiotics can potentially induce changes in morphology, such as alterations in fiber/particle arrangement, aggregation, or changes in surface roughness. These changes can be attributed to interactions between the probiotics and the material matrix, including physical interactions or bioactive compound release. The extent and nature of the morphological changes will vary depending on the specific probiotic and material characteristics, as well as the experimental conditions [26].
Viability of Probiotics
PRB viability is critical for maintaining our health (recommended daily dose ~ 109 live cells); therefore, studies have been conducted to improve or maintain their survival during food processing and storage. Recently, the incorporation of PRB into edible films or coatings has become relatively common [27, 28]. As discussed in the second section, some parameters can affect the ES process and production of NFs. These factors can be divided into processing (voltage, collector, etc.) solvent (dielectric constant, volatility, etc.), solution (viscosity, percentage of polymer, fluid consistency, etc.), and environmental parameters (humidity, temperature, etc.). The viability of bacterial cells can also be influenced by these parameters. In addition, the type of polymer can affect the viability of PRB [29]. Adding prebiotics can increase the stability of PRB. Commonly explored PRB strains are B. bifidum, B. breve, L. acidophilus, L. rhamnosus, and L. plantarum.
The use of stabilizing excipients is a common practice in both spray drying and freeze drying, but so far, they have been applied only in a few studies using ES. Osmotic stress, high voltage, and dehydration stress are exposed to bacterial cells during ES, but these can be reduced by using stabilizing excipients. For instance, Hirsch et al. [30] successfully encapsulated PRB in PVA-PEO fibers containing different excipients (glucose, lactose, mannitol, sucrose, trehalose, INU, and skim milk) to increase the viability of bacteria during formulation and storage. Over 80% of the bacterial cells survived. In another study, a range of safe lactic acid bacteria (LAB) were incorporated into PEO NFs, several of which were confirmed PRB. Finally, there was no decrease in the viability of PRB, and all LAB remained viable after encapsulation into electrospun NFs (ENFs) [31].
Mojaveri et al. [32] compared the survival of encapsulated PRB inside PVA/INU–CS/PVA–CS/PVA/INU NFs and observed that the PRB L. acidophilus encapsulated in CS/PVA/INU NFs showed higher viability than the others after ES. The survival rate of L. rhamnosus 1.0320 encapsulated by PVA/PEC was higher than those encapsulated by PVA. When the PVA-PEC proportion was 9:1, the survival rate of L. rhamnosus was 89.26%, which was the highest. In addition, after 21 days of storage at 25 °C, the viable count of L. rhamnosus 1.0320 encapsulated in PVA/PEC NFs decreased by 2.16 log CFU/g and the survival rate of L. rhamnosus was 75.84%, but the viable count of encapsulated L. rhamnosus decreased by 3.58 log CFU/g [33].
Table 1 lists the most important recent findings regarding the effect of the ES process on the viability of PRB. Accordingly, it is understandable that by choosing the proper polymer, applying the correct voltage, following the principles of ES, and adding prebiotics, we can improve the survival of PRB during and after the ES process; a result, encapsulating PRB by ES can be a suitable way to improve their viability during the storage of food to increase their shelf life or in digestive conditions to maintain the nutritional value. It is expected that in the future, more studies will be conducted in the field of increasing the growth of PRB in the ES process and its effective factors.
Digestive Stability of Probiotics
At the beginning of the twentieth century, the important function of the intestinal flora was completely unknown. At the end of the century, it was found that the intestinal microflora plays an important role in our body functions including metabolic, trophic, and protective roles. To play their role, probiotics must be able to maintain their stability after passing through the stomach and intestines. This means that they must tolerate the acidic, protease-rich conditions of the stomach and can grow in the presence of bile acids [44]. Recently, encapsulation of PRB in ENFs has been performed to increase their stability in the human digestive system, especially at low pH, digestive enzymes, and bile salts. The most common PRBs used in the food industry are Lactobacillus sp. and Bifidobacterium sp., which are generally recognized as safe (GRAS) by several regulatory agencies [44].
Water-soluble polymers are usually used for the encapsulation of PRB because mild solvents are used in the preparation of these polymer solutions. However, the rapid release of encapsulated cells in high water environments and the poor stability of hydrophilic polymers in the digestive system limit their use. Therefore, polymers with lower solubility in water are used more [43]. In the study conducted on PRB E. coli, the results showed that this bacterium was successfully encapsulated in CA and PVA NFs. Then, the stability of free and encapsulated E. coli in PVA/PVA NFs and PVA/CA NFs were compared under gastric conditions. None of the free cells were viable after 100 min of exposure to simulated gastric fluids (SGF). The PRB cells within the PVA/CA composites had a higher tolerance to the stomach simulation, after 2 h SGF exposure. Therefore, the survival rate of E. coli encapsulated in NFs in SGF was affected by the presence of CA [43]. These results are in agreement with another study, in which L. plantarum was encapsulated in double-layerPVA/SA NFs. accordingly, no significant loss of viability was found for the encapsulated cells after 120-min incubation in simulated intestinal fluids (SIF); however, a significant decrease in the survival of free PRB was observed; also, 2.6 log CFU/mL reduction was observed after exposure to the bile condition [45].
Another study investigated the effect of chitosan and INU on the viability of encapsulated B. animalis subsp. lactis BB12 in NFs. The stability of PRB under GIT conditions in 5 samples was compared, including PVA–PVA/INU–CS/PVA–CS/PVA/INU and free cells. It was clearly observed that the free cells were sensitive to SGF and SIF. PVA/CS NFs showed better protection against PRB than PVA NFs after exposure to SGF. It was also found that adding INU to NFs as a prebiotic can increase the viability of Bifidobacterium under SGF and intestinal fluids [32]. It has been shown that the survival rate of Lactobacilli and Bifidobacteria strains in acidic environment and simulated GI conditions can be increased by encapsulating them in corn starch and SA NFs. In addition, it was revealed that the best SA supplement, in this context, is corn starch, which provides a higher viability rate in gastric and intestinal fluids for PRB. Alginate and starch granules exert a protective effect on each other that can reduce the action of digestive enzymes on the encapsulated PRB [39]. Table 2 refers to the effect of various factors on improving the survival of PRB encapsulated in NFs exposed to acidic environments and simulated GI conditions.
Release of Probiotics
It is also important to note that PRB may be released at the intended location (e.g., the gut) and protected from harmful circumstances in GI system according to their encapsulation, which maximizes our body’s ability to absorb them [46]. Because of the gentle solvents employed in the manufacture of polymeric solutions, water-soluble polymers are frequently selected for PRB encapsulation [13]. Nevertheless, the rapid release of PRB from water-soluble polymers in high-water-content solutions and the poor stability of hydrophilic polymers in GIT limit their application [47, 48]. Because of their excellent durability under digestive circumstances, polymers weakly soluble in water have acquired significance.
Understanding the release behavior and mechanisms is indeed crucial for assessing the effectiveness and controlled delivery of probiotics or any other drug incorporated into the fibers. With controlled drug release, it is expected to decrease amount of drugs and side effects and increase affection treatment [49]. It can also provide insights into optimizing the formulation for specific applications. The observation of burst release behavior in the initial stage of loaded nanofibers aligns with the high surface area-to-volume ratio of nanofibers. This characteristic leads to a larger exposed surface area available for drug release, resulting in an initial rapid release of the drug. The non-uniform distribution of the drug within the fibers contributes to this burst release phenomenon, with the portion of the drug located near the surface being released more rapidly. This is because the drug molecules present near the surface encounter shorter diffusion paths through the fiber matrix, facilitating their release. It is important to note that the release of the drug from the fibers may occur through various mechanisms, including swelling, diffusion, desorption, and matrix degradation. These mechanisms can act individually or in combination, depending on the specific characteristics of the fiber matrix and the drug incorporated [50, 51].
Swelling refers to the absorption of fluids by the fiber matrix, resulting in the expansion of the matrix and subsequent release of the drug. Diffusion involves the movement of drug molecules through the fiber matrix, driven by concentration gradients. Desorption refers to the detachment of drug molecules from the fiber surface [52]. Matrix degradation involves the breakdown of the fiber matrix, which can release the encapsulated drug. Analyzing the release kinetics and employing appropriate mathematical models can provide valuable insights into the release mechanisms and kinetics of the loaded nanofibers. These models, such as zero-order, first-order, Higuchi, Fick’s laws, and Korsmeyer-Peppas models, can help quantify and predict the drug release behavior over time [49]. By fitting experimental release data to these models, parameters such as release rate, release exponent, or diffusion coefficients can be obtained, aiding in the understanding of the release mechanisms and facilitating comparisons between different formulations.
Incorporating discussions on the release behavior, mechanisms, and kinetics in the article will enhance the overall understanding of how probiotics are released from the loaded nanofibers and contribute to optimizing the design and formulation strategies for controlled probiotic delivery. It is important to note that specific experimental conditions, such as pH, temperature, and the surrounding media, may influence the release behavior and mechanisms (Rizwan et al. 2017). Therefore, considering these factors and their potential impact on the release profile should also be addressed in the article.
According to Çanga and Dudak [43], the hydrolytic breakdown rate is correlated with the period at which PRB cells release from NFs. To accomplish this, the hydrolytic decomposition of E. coli Nissle 1917 loaded into CA and PVA NFs (PVA/CA and PVA/PVA) was examined to acquire additional information regarding the release of cells throughout the GI simulation. The populations of E. coli in the PVA/PVA and PVA/CA fibers declined at the same rate in the simulated intestinal fluid at the end of the digestion simulation after the initial losses in SGF, which was probably caused by the entire release of cells from the CA-containing fibers in the intestinal circumstances. In other words, the presence of CA affected the likelihood that encapsulated E. coli in stomach juice would survive. As a result, when nanocomposite mats are used as the encapsulation medium, higher E. coli cells may enter the digestive tract and are totally released there, improving the host’s health.
Škrlec et al. [10] revealed that PEO NFs released virtually all L. plantarum cells over the course of 30 min, which is sufficient for their administration in food matrices. According to a study conducted on L. acidophilus 016 loaded with BCNF, the immobilization release ratio was 71% as measured by the number of viable PRB cells that were released from the BCNF on the 24th day of storage. BCNF immobilized by PRB can therefore be maintained for up to 24 days [38]. After incubation for 2 weeks in phosphate buffered saline (PBS) or media containing E.coli Nissle 1917, PEG-PLA NFs loaded with the bacteria showed < 10% molecular weight loss, leading to minimal E. coli release into the media from NF cores [48]. An innovative method for delivering PRB to biological locales and extending the shelf life of raw and processed foods (whether implemented in a food coating or packaging) that require their controlled and/or continuous release is the integration of PRB into NFs. Because there have been few studies, it may be necessary to conduct additional research to develop NFs with controlled/sustained release of PRB in the digestive tract, controlled release in foods packaged with NFs, enhanced encapsulation efficiency, and highly stable PRB-loaded NFs, as well as to assess the in vivo release and effectiveness of encapsulated PRB.
Thermal Stability of Probiotics
ES techniques are used to improve the resistance of PRB cells to high-temperature conditions. However, several food thermal processing techniques have a negative impact on cell survival, reducing their effectiveness. Given this problem, the encapsulation technique is a desirable way to improve the thermal stability of cells [53]. Studies related to PRB-loaded ENFs and their thermal resistance are presented in Table 3.
According to Table 3, the data were classified into four different temperatures, including freezing (− 20 °C), refrigeration (4 °C), ambient (20 or 25 °C), and hot conditions > 40 °C. At – 20 °C, the number of L. paracasei cells in all formulations containing a stabilizing excipient did not drop significantly. Over a year, L. paracasei in a capsule lost its viability by a maximum of 0.7 log units. This means that it stayed alive at low temperatures when it was in a capsule [30]. At fridge temperature, the viable count of E. coli Nissle 1917 encapsulated with PVA/PVA and PVA/CA decreased [43]. However, an increase was observed in other species of Lactobacillus such as L. acidophilus encapsulated by OPF/OPT/Okara, L. rhamnosus encapsulated by PVA/PEC, L. gasseri encapsulated by PVA, and L. rhamnosus/L. acidophilus/L. plantarum/L. casei encapsulated by GA/PUL. At 20 and 25 °C, the viability of Lactobacilli sp. and Bifidobacteria sp. cells was reduced by > 1.0 log unit. There was no significant difference between the viability of free and encapsulated cells by FOS/PVA and uniaxial fiber/core–shell fiber when the temperature was 45 °C. The results of Feng et al. [45] demonstrate that both the uniaxial and core–shell fiber mat could improve the stability of the encapsulated L. plantarum cells under heat moisture treatment (at 60 and 70 °C). Nevertheless, the core–shell fiber mat seems to provide better protection for the encapsulated PRB cells. Moreover, the vitality of L. plantarum cells encapsulated in FOS/PVA at 60 and 70 °C was considerable, despite the low cell count [53].
It is possible that encapsulation by NFs, which provided a barrier to inhibit the diffusion of hot moisture into NFs, is responsible for the increased viability of the cells. This resulted in an improvement in the thermal stability of PRB cells during the process of moist heat treatment [53]. In addition, Atraki and Azizkhani [39] concluded that ES encapsulation considerably improved acid tolerance and viability rate. The findings of Ma et al. [35] showed that drying and encapsulating Lactobacillus with GA/PUL ENFs was possible, minimizing cell damage by environmental stresses because it has been proven that the survival of microencapsulated PRB is highly dependent on the matrices and species used [54]. Several studies have indicated that the presence of oxygen decreases the survival rate of PRB. The PVA/PEC NFs may considerably increase the survival rate of L. rhamnosus 1.0320 during storage because PVA is a hydrophilic polymer that forms a strong oxygen barrier when dried [13, 55, 56]. Due to the quick evaporation of water during ES, there is a significant shift in the osmotic environment, which is responsible for the reduction in PRB viability after ES. The continued survival of PRB suggests its resistance to high voltage and shear stress during ES [12].
In conclusion, the findings indicate that ES with a variety of polymers may be an effective approach for improving the viability of cells under a variety of heat conditions, particularly at high temperatures. Moreover, using polymers such as uniaxial fiber and core–shell fiber for temperatures > 40 °C and corn starch and SA for 25 °C was recommended.
Interactions of Fibers with Probiotics
The functional groups of the raw material are revealed by FTIR spectroscopy, which also examines changes in its chemical structure. PRB-loaded ENFs are scanned in the mid-IR region between 400 and 4000 cm−1. For example, the development of integrated PEO NFs and a composite PEO/lyoprotectant containing PRB L. plantarum ATCC 8014 was studied using FTIR spectroscopy, and the results showed that the lyophilized L. plantarum cells displayed three main amide bands from proteins: amide A (3291 cm−1), amide I (1651 cm−1), and amide II (1529 cm−1). When lyophilized PRB is compared with L. plantarum-loaded PEO NFs, the amide I peak exhibits a distinct shape [10]. Because lyophilizing L. plantarum in deionized water causes a change in the protein’s structure, ES could stabilize L. plantarum more effectively than lyophilization [57]. Characteristic functional groups in NFs composed of PVA and L. rhamnosus CRL1332 were assessed in another research by Silva et al. [58]. The spectra of NFs containing L. rhamnosus showed a minor band at around 1040 cm−1, which may represent the COH vibrations of the peptidoglycan in the cell wall of immobilized PRB. This band was not observed in NFs made of PVA. Nevertheless, amide I bands at 1600 to 1680 cm−1 were observed, which were most likely connected to the peptide and protein content. When the spectra of all NFs were analyzed, the amplitude of the band at 1333 cm−1 decreased for samples stored at low temperatures, which might be attributable to in-plane O–H bending. Refrigeration can reduce molecular mobility during storage, altering the H-bonded connections among NF constituents [59].
The presence of bacteria inside PUL fibers did not significantly alter the IR spectra, according to a study on the potential use of ES to enclose Bifidobacterium sp. in whey protein concentrate and pullulan. However, a shift of the broad band entirely based around 3300 cm−1, which corresponds to OH and NH vibrations, was observed [34]. It can be observed that the incorporation of bacterial cells into the polymer solution results in fibers having a different intensity ratio of the identified bands when compared with untreated pullulan capsules. The intensity ratio of 995/1022 cm−1 has been utilized as a convenient indicator for assessing the degree of orientation in molecular organization in pullulan. B. animalis Bb12 cells may interact with pullulan because Bifidobacteria can adhere to carbs such as starch [60]. The intensities of peaks linked to CH2 bending and C–H deformation were found to be improved for the PVA/GA blend fiber encasing L. acidophilus ATCC 43.142 in GA-PVA blended ENFs [42].
Using FTIR spectroscopy on PVA and FOS NFs containing PRB L. plantarum, it was discovered that more H-bonds were established between PVA, FOS, and L. plantarum. This was demonstrated by the O–H stretching peak at 3319 cm−1 in the spectrum of the FOS/PVA/L. plantarum NFs. In addition, this area of the FOS/PVA/L. plantarum exhibited a higher intensity and a narrower peak compared with the peaks between 900 and 1300 cm−1 of the FOS/PVA film, which clarified that PRB were encapsulated in the fibers [53]. The L. plantarum spectra also demonstrated an absorption area between 900 and 1300 cm−1, which matched the protein molecules and nucleic acids [61]. Another study by Feng et al. [45] found that the stretching vibrations of the O–H group were responsible for the distinctive absorption peaks for PVA, SA, and L. plantarum at 3471, 3464, and 3418 cm−1, respectively. The presence of bacteria inside PVA NFs did not affect the IR spectra, according to a study that examined the potential molecular changes of samples after the incorporation of B. animalis Bb12 cells [38]. This was probably because of the bacteria’s low weight rate compared with to the encapsulation polymers [62]. However, the pure fibers and the cell-encapsulated fibrous mats showed significant differences. Nevertheless, the bands in the 2800 to 3500 cm−1 area, which were linked to intra- and intermolecular H-bonding, were substantially more intense when INU was added to NFs as a prebiotic [34].
The FTIR spectra of L. plantarum-loaded PVA-based NFs and L. plantarum-loaded PVA and silk fibroin-based NFs indicated peaks at 3412, 2911, and 1087 cm−1. The uneven stretching of C–H was responsible for the peak at 2911 cm−1. The peak at 1087 cm−1 represents the C–O group, whereas the peak at 3412 cm−1 represents the O–H group. Except for the prior, PVA/SF/L. plantarum-based NFs displayed maxima at 500, 700, 1324, 1638, and 3381 cm−1. The peaks given relate to the –CONH2 functional group [40]. In the FTIR spectrum of PVA/SA NF mats loaded with L. paracasei KS-199, peaks were also found in regions 1 (3000–2800 cm−1 as fatty acids in bacterial cell) and 2 (1700–1500 cm−1 as amide I and II bands of proteins and peptides), which are the most valuable sources of information for routine PRB identification [63]. This demonstrates the excellent encapsulation of strain inside the NF mats [41]. Through peak alterations of O–H and C–H groups, which indicate H-bonding contact across particles, FTIR analysis of the synthesized NFs confirmed the presence of cross-linking between polymer constituents [64].
As a result, even though FTIR is an effective technique for spotting functional groups and intermolecular interactions in various component structures, most research on NFs containing PRB has found that it is challenging to spot changes in functional groups in treatment samples compared with the control. PRB cells were integrated with polymers in ENFs in the few studies that were done, and functional groups such as amide I and II, H-bonded, CH2 bending, C–H deformation, and O–H stretching were the variables involved (Fig. 1). It is also advised that functional groups in PRB-loaded ENFs should be subtracted in future studies using the chemometrics method.
Although FTIR is a valuable tool for examining the interactions between fibers and PRB, it may not be solely conclusive for determining these connections, particularly in studies aiming to obtain a thorough knowledge of such interactions (Fig. 2). To gain a more complete comprehension of the interactions between fibers and PRB, it may be imperative to use supplementary methodologies in conjunction with FTIR. Raman spectroscopy is a vibrational spectroscopy method that offers supplementary insights to FTIR spectroscopy, particularly for materials exhibiting a crystalline structure [65]. Attenuated total reflection (ATR)-FTIR spectroscopic imaging is an additional advanced technique that can aid in the comprehension of molecular interactions and the development of new products and processes [66]. On the other hand, scanning electron microscopy (SEM) enables the acquisition of high-resolution images that depict the surface shape and structure of fibers, as well as their interaction with the PRB. It can provide data regarding the adhesion, colonization, and distribution of PRB on fiber surfaces [67, 68]. The distribution of probiotics within the fiber/particle matrix can vary depending on multiple factors. The distribution may be homogeneous, with probiotics uniformly dispersed throughout the matrix, or non-uniform, resulting in localized regions of higher or lower probiotic concentration. The distribution is influenced by factors such as the method of probiotic incorporation, the characteristics of the fiber/particle matrix, and the interactions between probiotics and the matrix material. The average pocket size, referring to void spaces or cavities within the matrix, can also vary depending on the system and experimental conditions. Techniques like microscopy or image analysis can be used to measure and determine the average pocket size. The effect of probiotic incorporation on pockets and average diameter can vary depending on the specific system or material being studied. In some cases, probiotic incorporation may lead to an increase in pocket size due to potential swelling or aggregation effects. Likewise, the average diameter may also increase if probiotics contribute to the growth or enlargement of the fibers or particles. However, the specific impact on pocket size and average diameter will depend on the characteristics of the probiotics, the material, and the experimental conditions. Furthermore, laser scanning confocal microscopy (LSCM) is a significant imaging technology capable of offering three-dimensional viewing of PRB on fibers. It can assist in the evaluation of the localization, attachment, and biofilm creation of PRB, thus offering insights into the interaction of PRB with fibers [68]. Transmission electron microscopy (TEM) employs an electron beam to generate high-resolution photographs of objects, including their interior structures at the nanoscale [68].
By discussing the DSC results reported in the literature, it is possible to compare and contrast the findings with the FTIR analysis, providing a more comprehensive understanding of the interactions between fibers and probiotics. These complementary techniques can shed light on the physical, chemical, and thermal changes occurring within the fiber matrix upon probiotic incorporation [68]. DSC is a thermal analysis technique that measures the heat flows associated with phase transitions, such as melting, crystallization, and glass transitions, in a material as a function of temperature. By examining the thermal behavior of fibers before and after probiotic incorporation, valuable insights can be gained regarding the physical and chemical changes occurring within the fiber matrix. The melting and crystallization behavior of the fibers can be examined to determine if probiotic incorporation affects the thermal properties of the fibers. Changes in the melting temperature, enthalpy of fusion, or crystallinity of the fibers can indicate potential interactions between the fiber material and probiotics [68].
Furthermore, DSC can provide insights into the compatibility between the fiber material and probiotics. The presence of distinct thermal events, such as shifts in melting or crystallization peaks, can suggest possible chemical or physical interactions between the fiber matrix and probiotics. These interactions may involve hydrogen bonding, molecular dispersion, or other intermolecular forces that influence the thermal behavior of the system. In addition, DSC can help elucidate the thermal stability of the fibers and probiotics during processing or storage conditions. By measuring the onset temperature and heat flow associated with degradation or decomposition, the thermal stability of the probiotic-loaded fibers can be evaluated. This information is crucial to ensure that the probiotics remain viable and effective throughout the intended application or product shelf life [12].
It is worth noting that the specific DSC results and their interpretation will depend on the nature of the fiber material, the probiotic strain, and the experimental conditions employed in the studies (Lin et al. 2022). Therefore, a careful examination of the literature and the relevance of the reported DSC results to the specific fiber-probiotic system under investigation is essential for a meaningful discussion and correlation with the FTIR findings.
Optimal Conditions and Suitable Polymers for Loading Probiotics
Natural polymers, including gum Arabic (GA), alginate, pectin, animal proteins, chitosan, xanthan, k-carrageenan, and starch, are commonly employed as carriers for encapsulated cells in food packaging. This is because of their biodegradability, low toxicity, biocompatibility, and neutral effect on certain PRB. These polymers are used in conjunction with coating and ES techniques for PRB delivery [69]. Alginate is now a highly regarded polymeric carrier in the field of delivery systems because of its exceptional qualities, both mechanical and physiochemical. Its moderate processing, simple structure, low toxicity, and ability to easily form a gel or gel coating over bacteria contribute to its attractiveness in this regard [70,71,72].
Several factors must be addressed while selecting the polymer carrier for the ES solution. The intended use of the product and the shelf life of the PRB in the polymer, depending on the type and concentration of the polymers, are probably the most relevant considerations. In rapid release scenarios, a polymer exhibiting fast dissolution and substantial solubility in aqueous solvents, such as polyvinyl pyridone, may be appropriate. Poly (N-isopropylacrylamide), which has thermoresponsive properties, the methacrylate family of polymers and poly(4-vinylpyridine), which are pH sensitive, and rapidly alter their nature, are among the most researched stimuli-responsive polymers [73]. pH-sensitive polymers, such as acrylic acid derivatives, cellulose acetate (CA) trimellitate, hydroxypropyl methyl CA succinate, CA phthalate, and hydroxypropyl methylcellulose phthalate, can deliver PRB to the colon and ileum under elevated pH conditions, while ensuring their viability in the acidic environment of the stomach [74]. The effectiveness of PRB in the intestinal environment may be enhanced by the use of microencapsulation techniques, including chemically modified alginate, thus concurrently improving the efficiency of encapsulation. Certain PRB exhibit pH responsiveness. Alginate has often been used as a means of encapsulating PRB because of its simplicity, non-toxic nature, biocompatibility, and cost-effectiveness [75]. Redox-responsive polymers, which include disulfide and diselenide links, as well as enzyme-responsive polymers such as cellulose, starch, and glycogen, are significant classes of polymers used for PRB delivery targeting. A variety of hydrolytic enzymes, includingglycosidase, dextranase, α-D-xylosidase, amylase, and β-D-galactosidase, are excreted inside the colon where they facilitate the breakdown of glycosidic bonds. While glycosidic linkages exhibit stability in the small intestine and stomach, they undergo degradation in the colon. Several polysaccharides, including dextran, chitosan, pectin, cyclodextrin, amylose, guar gum, chondroitin sulfate, inulin, and locust bean gum, belong to the enzyme-sensitive polymer group. These polymers serve as transporters and facilitate the targeted administration of diverse PRB and pharmaceuticals to the colon [76].
According to a general review of the findings of polymer NFs containing PRB, polymers comprising PVA, CA, and alginate had the greatest survival rate, viability, and resistance under environmental conditions and the simulated GI environment.
Application of Probiotics-Loaded Fibers as Active Packaging
To create active packaging, PRB are introduced to food items as well as integrated into biopolymeric matrices as a substitute approach for reducing foodborne organisms, enhancing shelf life and food safety, and offering health advantages [77]. Polymeric NFs have gained popularity as packaging materials because of their ability to change pore size, surface energy, barrier characteristics, antibacterial activity, and mechanical strength [78, 79]. ES is an efficient process for generating NFs with diverse nanostructures and surface properties, which may match the functional requirements of food packaging materials [80]. Using LAB for bio-preservation improves the quality of food mainly because of the synthesis of the principal metabolite lactic acid. Many LAB may hinder the development of a broad range of food spoilage microorganisms and pathogens [81]. According to studies, nisin produced from LAB strains that guarantee a longer shelf life suppresses the development of certain psychotropic bacteria in cheese and lengthens the shelf life of milk. These experiments also showed that nisin offers extra defense against certain spores in canned foods [82, 83].
Martins et al. [84] found that after 120 h of refrigerated storage at 8 °C, fruit salads containing L. rhamnosus as a PRB strain had at least 2.0 log CFU/g less psychotropic bacteria than the control group. However, in the study by Ceylan et al. [37], this ratio was greater because ENFs containing L. rhamnosus GG had a greater contact surface than the microscale material. Hence, it is evident that nanoencapsulation of L. rhamnosus GG inside PVA/SA-based NFs slowed the rapid development of total psychrophilic bacteria in fish filet. The result of Yilmaz et al. [41] demonstrated that ES is an effective method for the fabrication of alginate-based NFs for encapsulating PRB strains while preserving their integrity. There was, for instance, a rise in the viable count of approximately 0.7 log in yogurt products and kefir samples inoculated with encapsulated strain compared to the viable count in the kefir samples made with free strain [41, 85].
The use of edible films or coatings that include PRB might lead to improvements or the preservation of parameters related to the quality, safety, functioning, and storage stability of fresh fruit and vegetables that have undergone minimum processing [86,87,88]. Due to the lack of published papers in this field, it is recommended that more ES projects be carried out according to the variety of food items.
Conclusion and Future Perspectives
ES is one of the most effective methods for manufacturing fibers for encapsulating PRB strains, while preserving their integrity. In this procedure, cells are encapsulated at ambient temperature and under low pressure conditions, which is extremely advantageous for the encapsulation of sensitive live cells. As a result, PRB achieves high encapsulation efficiency and stability (such as light and thermal stability). Electrospun materials for functional foods should be selected with safety as a priority, and the ingredients and solvents should be GRAS. Findings suggest that depending on the polymer used, the voltage applied, ES principles followed, and the addition of prebiotics, PRB are likely to survive and persist, particularly at high temperatures, during and after ES. In addition to viability of PRB, their delivery to biological sites is of critical importance. Integration of PRB into NFs is an innovative method for delivering PRB and extending the shelf life of raw and processed foods.
In terms of identifying functional groups and intermolecular interactions, although infrared spectroscopy is an effective technique in various component structures, there are some limitations. Therefore, it is recommended that functional groups in PRB-loaded ENFs be subtracted using the chemometrics method in future studies. Furthermore, the production and delivery of functional foods based on ES and PRB are mostly carried out in vitro. Although in vitro simulations can provide valuable results, the in vivo environment is generally more complex; therefore, they cannot directly replace in vivo research. Thus, to obtain reliable data for guiding the business process, further in vivo experiments are required. ES nanoencapsulation has the potential to improve the stability of PRB in functional food packaging; however, it is still important to consider the storage stability of fibrous films for commercial applications. Finally, to ensure the safe and healthy development of NFs in the food field, all governments must establish and improve regulations on NFs used in the industry.
Data Availability
Data sharing not applicable to this article because no datasets were generated or analyzed during the current study.
Abbreviations
- B. :
-
Bifidobacterium
- BCNF:
-
Bacterial cellulose nanofibers
- CA:
-
Cellulose acetate
- CS:
-
Chitosan
- E. :
-
Escherichia
- ENFs:
-
Electrospun nanofibers
- ES:
-
Electrospinning
- FAO:
-
Food and agricultural organization
- FOS:
-
Fructooligosaccharides
- FTIR:
-
Fourier-transform infrared spectroscopy
- GA:
-
Gum Arabic
- GIT:
-
Gastrointestinal tract
- GRAS:
-
Generally recognized as safe
- INU:
-
Inulin
- L. :
-
Lactobacillus
- NFs:
-
Nanofibers
- OPF:
-
Oil palm frond
- OPT:
-
Oil palm trunk
- PAN:
-
Polyacrylonitrile
- PRB:
-
Probiotic bacteria
- PBS:
-
Phosphate-buffered saline
- PCL:
-
Polycaprolactone
- PEC:
-
Pectin
- PEG-PLA:
-
Poly(ethylene glycol)–polylactide
- PEO:
-
Poly(ethylene oxide)
- PUL:
-
Pullulan
- PVA:
-
Poly vinyl alcohol
- PVDF:
-
Poly vinylidene fluoride
- PVP:
-
Poly vinyl pyrrolidone
- S. :
-
Staphylococcus
- SA:
-
Sodium alginate
- SGF:
-
Simulated gastric fluids
- SIF:
-
Simulated intestinal fluids
- WHO:
-
World health organization
- WPC:
-
Whey protein concentrate
References
Hashempour-Baltork F, Hosseini H, Shojaee-Aliabadi S, Torbati M, Mirza Alizadeh A, Alizadeh M (2019) Drug resistance and the prevention strategies in food borne bacteria: an update review. Advanced pharmaceutical bulletin 9(3):335
Mirza Alizadeh A, Hosseini H, Meybodi NM, Hashempour-Baltork F, Alizadeh-Sani M, Tajdar-Oranj B, Khaneghah AM (2022) Mitigation of potentially toxic elements in food products by probiotic bacteria: a comprehensive review. Food Res Int 152:110324
Nejati M, Dehghan P, Hashempour-Baltork F, Mirza Alizadeh A, Farshi P, Khosravi-Darani K (2021) Potential dietary interventions for COVID-19 infection based on the Gut-Immune axis: an update review on bioactive component of macronutrients. Int J Prev Med 12
Hashempour-Baltork F, Sheikh M, Eskandarzadeh S, Tarlak F, Tripathi AD, Khosravi-Darani K, Sadanov A (2021) The Effect of probiotics on various diseases and their therapeutic role: an update review. J Pure Appl Microbiol 15:1042–1059
Hill C, Guarner F, Reid G, Gibs GR, Merenstein DJ, Pot B, Salminen S (2014) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Rev Gastroenterol hepatol
Mirtič J, Rijavec T, Zupančič Š, Pobirk AZ, Lapanje A, Kristl J (2018) Development of probiotic-loaded microcapsules for local delivery: physical properties, cell release and growth. Eur J Pharm Sci 121:178–187
Halimi H, Zighami H, Aminzare M, Hassanzadazar H (2022) Effect of microencapsulation on the viability of Lactobacillus acidophilus and Saccharomycess boulardii probiotics at different storage temperatures in Doogh. J Hum Environ Health Promot (JHEHP) 8(2)
Rajam R, Subramanian P (2022) Encapsulation of probiotics: past, present and future. Beni-Suef University Journal of Basic and Applied Sciences 11(1):46. https://doi.org/10.1186/s43088-022-00228-w
Subramanian P (2021) Mucoadhesive delivery system: a smart way to improve bioavailability of nutraceuticals. Foods 10(6). https://doi.org/10.3390/foods10061362
Škrlec K, Zupančič Š, Mihevc SP, Kocbek P, Kristl J, Berlec A (2019) Development of electrospun nanofibers that enable high loading and long-term viability of probiotics. Eur J Pharm Biopharm 136:108–119
Huang H, Song Y, Zhang Y, Li Y, Li J, Lu X, Wang C (2022) Electrospun nanofibers: current progress and applications in food systems. J Agric Food Chem 70(5):1391–1409
Fung WY, Yuen KH, Liong MT (2011) Agrowaste-based nanofibers as a probiotic encapsulant: Fabrication and characterization. J Agric Food Chem 59(15):8140–8147. https://doi.org/10.1021/jf2009342
López-Rubio A, Sanchez E, Sanz Y, Lagaron JM (2009) Encapsulation of living bifidobacteria in ultrathin PVOH electrospun fibers. Biomacromol 10(10):2823–2829
Salalha W, Kuhn J, Dror Y, Zussman E (2006) Encapsulation of bacteria and viruses in electrospun nanofibres. Nanotechnology 17(18):4675
Nagy ZK, Wagner I, Suhajda Á, Tobak T, Harasztos AH, Vigh T, Marosi G (2014) Nanofibrous solid dosage form of living bacteria prepared by electrospinning
Lu X, Wang C, Wei Y (2009) One-dimensional composite nanomaterials: synthesis by electrospinning and their applications. Small 5(21):2349–2370
Shahriar SS, Mondal J, Hasan MN, Revuri V, Lee DY, Lee Y-K (2019) Electrospinning nanofibers for therapeutics delivery Nanomaterials 9(4):532
Pant B, Park M, Park S-J (2019) Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 11(7):305
Wen P, Wen Y, Zong M-H, Linhardt RJ, Wu H (2017) Encapsulation of bioactive compound in electrospun fibers and its potential application. J Agric Food Chem 65(42):9161–9179
Ji S-M, Tiwari AP, Oh HJ, Kim H-Y (2021) ZnO/Ag nanoparticles incorporated multifunctional parallel side by side nanofibers for air filtration with enhanced removing organic contaminants and antibacterial properties. Colloids Surf, A 621:126564
Xue J, Wu T, Dai Y, Xia Y (2019) Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 119(8):5298–5415
Wang X-X, Yu G-F, Zhang J, Yu M, Ramakrishna S, Long Y-Z (2021) Conductive polymer ultrafine fibers via electrospinning: preparation, physical properties and applications. Prog Mater Sci 115:100704
Aman Mohammadi M, Dakhili S, Mirza Alizadeh A, Kooki S, Hassanzadazar H, Alizadeh-Sani M, McClements DJ (2022) New perspectives on electrospun nanofiber applications in smart and active food packaging materials. Crit Rev Food Sci Nutr 1–17. https://doi.org/10.1080/10408398.2022.2124506
Salalha W, Dror Y, Khalfin RL, Cohen Y, Yarin AL, Zussman E (2004) Single-walled carbon nanotubes embedded in oriented polymeric nanofibers by electrospinning. Langmuir 20(22):9852–9855
Reznik S, Yarin A, Zussman E, Bercovici L (2006) Evolution of a compound droplet attached to a core-shell nozzle under the action of a strong electric field. Phys Fluids 18(6):062101
Yoha K, Nida S, Dutta S, Moses J, Anandharamakrishnan C (2021) Targeted delivery of probiotics: perspectives on research and commercialization. Probiotics and Antimicrobial Proteins 1–34
Pavli F, Tassou C, Nychas G-JE, Chorianopoulos N (2018) Probiotic incorporation in edible films and coatings: bioactive solution for functional foods. Int J Mol Sci 19(1):150
Raji F, Khanzadi S, Hashemi M, Azizzadeh M (2019) Effect of chitosan coating nano-emulsion containing Zataria multiflora and Bunium persicum essential oils on Escherichia coli O157: H7 in vacuum-packed rainbow trout fillet. Journal of Human, Environment, and Health Promotion 5(1):21
Zare M, Dziemidowicz K, Williams GR, Ramakrishna S (2021) Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 11(8):1968
Hirsch E, Pantea E, Vass P, Domján J, Molnár M, Suhajda Á, Nagy ZK (2021) Probiotic bacteria stabilized in orally dissolving nanofibers prepared by high-speed electrospinning. Food Bioprod Proc 128:84–94. https://doi.org/10.1016/j.fbp.2021.04.016
Zupani S, Škrlec K, Kocbek P, Kristl J, Berlec A (2019) Effects of electrospinning on the viability of ten species of lactic acid bacteria in poly(ethylene oxide) nanofibers [Article]. Pharmaceutics 11(9):483. https://doi.org/10.3390/pharmaceutics11090483
Mojaveri SJ, Hosseini SF, Gharsallaoui A (2020) Viability improvement of Bifidobacterium animalis Bb12 by encapsulation in chitosan/poly(vinyl alcohol) hybrid electrospun fiber mats [Article]. Carbohydr Polym 241:116278. https://doi.org/10.1016/j.carbpol.2020.116278
Xu C, Ma J, Wang W, Liu Z, Gu L, Qian S, Jiang Z (2022) Preparation of pectin-based nanofibers encapsulating Lactobacillus rhamnosus 1.0320 by electrospinning [Article]. Food Hydrocoll 124:107216. https://doi.org/10.1016/j.foodhyd.2021.107216
López-Rubio A, Sanchez E, Wilkanowicz S, Sanz Y, Lagaron JM (2012) Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids [Article]. Food Hydrocolloids 28(1):159–167. https://doi.org/10.1016/j.foodhyd.2011.12.008
Ma J, Xu C, Yu H, Feng Z, Yu W, Gu L, Hou J (2021) Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology [Article]. Food Hydrocoll 111:106381. https://doi.org/10.1016/j.foodhyd.2020.106381
Amna T, Hassan MS, Pandeya DR, Khil MS, Hwang IH (2013) Classy non-wovens based on animate L. gasseri-inanimate poly(vinyl alcohol): upstream application in food engineering. Appl Microbiol Biotechnol 97(10):4523–4531. https://doi.org/10.1007/s00253-012-4666-z
Ceylan Z, Meral R, Karakaş CY, Dertli E, Yilmaz MT (2018) A novel strategy for probiotic bacteria: Ensuring microbial stability of fish fillets using characterized probiotic bacteria-loaded nanofibers [Article]. Innov Food Sci Emerg Technol 48:212–218. https://doi.org/10.1016/j.ifset.2018.07.002
Jayani T, Sanjeev B, Marimuthu S, Uthandi S (2020) Bacterial Cellulose Nano Fiber (BCNF) as carrier support for the immobilization of probiotic, Lactobacillus acidophilus 016 [Article]. Carbohydr Polym 250:116965. https://doi.org/10.1016/j.carbpol.2020.116965
Atraki R, Azizkhani M (2021) Survival of probiotic bacteria nanoencapsulated within biopolymers in a simulated gastrointestinal model [Article]. Innov Food Sci Emerg Technol 72:102750. https://doi.org/10.1016/j.ifset.2021.102750
Wei L, Zhou D, Kang X (2021) Electrospinning as a novel strategy for the encapsulation of living probiotics in polyvinyl alcohol/silk fibroin [Article]. Innov Food Sci Emerg Technol 71:102726. https://doi.org/10.1016/j.ifset.2021.102726
Yilmaz MT, Taylan O, Karakas CY, Dertli E (2020) An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydr Polym 244:116447. https://doi.org/10.1016/j.carbpol.2020.116447
Fareed F, Saeed F, Afzaal M, Imran A, Ahmad A, Mahmood K, Ateeq H (2022) Fabrication of electrospun gum Arabic–polyvinyl alcohol blend nanofibers for improved viability of the probiotic. J Food Sci Technol 59(12):4812–4821. https://doi.org/10.1007/s13197-022-05567-1
Çanga EM, Dudak FC (2021) Improved digestive stability of probiotics encapsulated within poly(vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr Polym 264:117990. https://doi.org/10.1016/j.carbpol.2021.117990
Del Piano M, Morelli L, Strozzi G, Allesina S, Barba M, Deidda F, Orsello M (2006) Probiotics: from research to consumer. Dig Liver Dis 38:S248-S255
Feng K, Huang RM, Wu RQ, Wei YS, Zong MH, Linhardt RJ, Wu H (2020) A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem 310:125977. https://doi.org/10.1016/j.foodchem.2019.125977
Ashraf R, Shah NP (2014) Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr 54(7):938–956
Liu H, Cui SW, Chen M, Li Y, Liang R, Xu F, Zhong F (2019) Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review. Crit Rev Food Sci Nutr 59(17):2863–2878
Xie S, Tai S, Song H, Luo X, Zhang H, Li X (2016) Genetically engineering of Escherichia coli and immobilization on electrospun fibers for drug delivery purposes. J Mater Chem B 4(42):6820–6829
Vernosfaderani FR, Semnani D (2018) Manufacturing and optimization the nanofibres tissue of poly (N-vinyl-2-pyrrolidone)-poly (e-caprolactone) shell/poly (N-vinyl-2-pyrrolidone)-amphotericin b core for controlled drug release system. Fibers Polym 19:620–626
Luraghi A, Peri F, Moroni L (2021) Electrospinning for drug delivery applications: A review. J Control Release 334:463–484
Zupančič Š (2019) Core-shell nanofibers as drug-delivery systems. Acta pharmaceutica 69(2):131–153
Stewart SA, Domínguez-Robles J, Donnelly RF, Larrañeta E (2018) Implantable polymeric drug delivery devices: classification, manufacture, materials, and clinical applications. Polymers 10(12):1379
Feng K, Zhai MY, Zhang Y, Linhardt RJ, Zong MH, Li L, Wu H (2018) Improved viability and thermal stability of the probiotics encapsulated in a novel electrospun fiber mat. J Agric Food Chem 66(41):10890–10897. https://doi.org/10.1021/acs.jafc.8b02644
Ashwar BA, Gani A, Gani A, Shah A, Masoodi FA (2018) Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem 239:287–294. https://doi.org/10.1016/j.foodchem.2017.06.110
Cruz AG, Castro WF, Faria JAF, Bolini HMA, Celeghini RMS, Raices RSL, Mársico ET (2013) Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage. Food Res Int 51(2):723–728. https://doi.org/10.1016/j.foodres.2013.01.028
Pimentel TC, Gomes de Oliveira LI, Carvalho de Souza R, Magnani M (2021) Probiotic non-dairy frozen dessert: technological and sensory aspects and industrial challenges. Trends Food Sci Technol 107:381–388. https://doi.org/10.1016/j.tifs.2020.11.008
Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM (1995) Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol 61(10):3592–3597
Silva JA, De Gregorio PR, Rivero G, Abraham GA, Nader-Macías MEF (2021) Immobilization of vaginal Lactobacillus in polymeric nanofibers for its incorporation in vaginal probiotic products. Eur J Pharm Sci 156:105563. https://doi.org/10.1016/j.ejps.2020.105563
Smith B (2018) Infrared spectral interpretation: a systematic approach. CRC Press
Crittenden R, Laitila A, Forssell P, Mättö J, Saarela M, Mattila-Sandholm T, Myllärinen P (2001) Adhesion of bifidobacteria to granular starch and its implications in probiotic technologies. Appl Environ Microbiol 67(8):3469–3475. https://doi.org/10.1128/aem.67.8.3469-3475.2001
Mirza Alizadeh A, Hosseini H, Mohseni M, Eskandari S, Sohrabvandi S, Hosseini MJ, Nahavandi S (2021) Analytic and chemometric assessments of the native probiotic bacteria and inulin effects on bioremediation of lead salts. J Sci Food Agric 101(12):5142–5153
Alavarse AC, de Oliveira Silva FW, Colque JT, da Silva VM, Prieto T, Venancio EC, Bonvent J-J (2017) Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater Sci Eng, C 77:271–281
Davis R, Mauer LJ (2010) Fourier transform infrared (FT-IR) spectroscopy: a rapid tool for detection and analysis of foodborne pathogenic bacteria. Curr Res technol Educ topics Appl Microbiol Microb Biotechnol 2:1582–1594
Fortunati E, Puglia D, Monti M, Santulli C, Maniruzzaman M, Kenny JM (2013) Cellulose nanocrystals extracted from okra fibers in PVA nanocomposites. J Appl Polym Sci 128(5):3220–3230
Roque-Ruiz JH, Martínez-Máynez H, Zalapa-Garibay MA, Arizmendi-Moraquecho A, Farias R, Reyes-López SY (2017) Surface enhanced Raman spectroscopy in nanofibers mats of SiO2-TiO2-Ag. Results Phys 7:2520–2527
Drobota M, Gradinaru LM, Vlad S, Bargan A, Butnaru M, Angheloiu M, Aflori M (2020) Preparation and characterization of electrospun collagen based composites for biomedical applications. Materials 13(18):3961
Medeiros GB, Lima F, d. A., de Almeida, D. S., Guerra, V. G., & Aguiar, M. L. (2022) Modification and functionalization of fibers formed by electrospinning: a review. Membranes 12(9):861
Yu H, Liu W, Li D, Liu C, Feng Z, Jiang B (2020) Targeting delivery system for Lactobacillus plantarum based on functionalized electrospun nanofibers. Polymers 12(7):1565
Pech-Canul A, d. l. C., Ortega, D., García-Triana, A., González-Silva, N., & Solis-Oviedo, R. L. (2020) A brief review of edible coating materials for the microencapsulation of probiotics. Coatings 10(3):197
Kowalska E, Ziarno M, Ekielski A, Żelaziński T (2022) Materials used for the microencapsulation of probiotic bacteria in the food industry. Molecules 27(10):3321
Razavi S, Janfaza S, Tasnim N, Gibson DL, Hoorfar M (2021) Microencapsulating polymers for probiotics delivery systems: preparation, characterization, and applications. Food Hydrocoll 120:106882
Sharma H, Sharma S, Bajwa J, Chugh R, Kumar D (2023) Polymeric carriers in probiotic delivery system. Carbohydr Polym Technol Appl 5:100301. https://doi.org/10.1016/j.carpta.2023.100301
Echeverría C, Aragón-Gutiérrez A, Fernández-García M, Muñoz-Bonilla A, López D (2019) Thermoresponsive Poly(N-Isopropylacrylamide-co-dimethylaminoethyl methacrylate) microgel aqueous dispersions with potential antimicrobial properties. Polymers 11(4):606. https://www.mdpi.com/2073-4360/11/4/606
Wang X, Gao S, Yun S, Zhang M, Peng L, Li Y, Zhou Y (2022) Microencapsulating alginate-based polymers for probiotics delivery systems and their application. Pharmaceuticals 15(5):644
Zhu Y, Wang Z, Bai L, Deng J, Zhou Q (2021) Biomaterial-based encapsulated probiotics for biomedical applications: current status and future perspectives. Mater Des 210:110018
Bidla PD, Panda PK, Verma A, Raikwar S, Jain SK (2022) Nanovesicles for colon-targeted drug delivery. In Applications of nanovesicular drug delivery (pp. 253–266). Elsevier
Espitia PJP, Batista RA, Azeredo HMC, Otoni CG (2016) Probiotics and their potential applications in active edible films and coatings. Food Res Int 90:42–52. https://doi.org/10.1016/j.foodres.2016.10.026
Mardani F, Mohseni M, Aminzare M, Hassanzadazar H (2022) Antibacterial properties of bioactive starch films containing bunium persicum seed’s essential oil nanoemulsion fortified with cinamaldehyde. J Hum Environ Health Promot 8(4):222–231
Soltaninezhad B, Khanzadi S, Hashemi M, Azizzadeh M (2020) The inhibition of Escherichia coli O157: H7 inoculated in hamburger using a chitosan/cellulose nanofiber film containing the nanoemulsion of Trachyspermum ammi and Bunium persicum essential oils. J Hum Environ Health Promot 6(1):30–34
Zhao L, Duan G, Zhang G, Yang H, He S, Jiang S (2020) Electrospun functional materials toward Food packaging applications: a review. Nanomaterials (Basel) 10(1). https://doi.org/10.3390/nano10010150
Zapaśnik A, Sokołowska B, Bryła M (2022) Role of lactic acid bacteria in food preservation and safety. Foods 11(9). https://doi.org/10.3390/foods11091283
Mirza Alizadeh A, Hashempour-Baltork F, Alizadeh-Sani M, Maleki M, Azizi-Lalabadi M, Khosravi-Darani K (2020) Inhibition of Clostridium botulinum and its toxins by probiotic bacteria and their metabolites: an update review. Qual Assur Saf Crops Foods 12(SP1):59–68
Savadogo A, Ouattara AC, Bassole HI, Traore SA (2006) Bacteriocins and lactic acid bacteria-a minireview. African J Biotechnol 5(9)
Martins EMF, Ramos AM, Martins ML, Leite JÚNior BRDC (2016) Fruit salad as a new vehicle for probiotic bacteria. Food Sci Technol 36
Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K (2000) Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int J Food Microbiol 62(1):47–55. https://doi.org/10.1016/S0168-1605(00)00380-9
de Oliveira KÁR, Fernandes KFD, de Souza EL (2021) Current advances on the development and application of probiotic-loaded edible films and coatings for the bioprotection of fresh and minimally processed fruit and vegetables. Foods 10(9):2207. https://www.mdpi.com/2304-8158/10/9/2207
Golabi F, Doudi M, Madani M (2019) Antibacterial effects of agicoat silver crystalline nanofibers on wound infection agents. J Hum Environ Health Promot 5(2):61
Saei R, Khanzadi S, Hashemi M, Azizzadeh M (2021) Antibacterial effects of alginate coating prepared by electrolyzed water on Pseudomonas aeruginosa inoculated on salmon fillets. J Hum Environ Health Promot (JHEHP) 7(4).
Author information
Authors and Affiliations
Contributions
A.M.A. Searching and data collection, Investigation, Data curation, Analyzing, Writing - original draft, prepared all figures. M.M., K.G., H.R., M.G-N. and M.A. Searching and data collection, Analyzing, Writing - original draft. E.A. Review & editing. F.H-B. Project administration, Supervision, Review & editing. S.M.J. Conceptualization, Project administration, Supervision, Review & editing.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alizadeh, A.M., Mohseni, M., Gerami, K. et al. Electrospun Fibers Loaded with Probiotics: Fundamentals, Characterization, and Applications. Probiotics & Antimicro. Prot. 16, 1099–1116 (2024). https://doi.org/10.1007/s12602-023-10174-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10174-3