Abstract
We aimed at investigating oxidative stability and changes in fatty acid and tocopherol composition of extra virgin olive oil (EVOO) in comparison with refined seed oils during short-term deep-frying of French fries, and changes in the composition of the French fries deep-fried in EVOO. EVOO samples from Spain, Brazil, and Portugal, and refined seed oils of soybean and sunflower were studied. Oil samples were used for deep-frying of French fries at 180 °C, for up to 75 min of successive frying. Tocopherol and fatty acid composition were determined in fresh and spent vegetable oils. Tocopherol, fatty acid, and volatile composition (by SPME–GC–MS) were also determined in French fries deep-fried in EVOO. Oil oxidation was monitored by peroxide, acid, and p-anisidine values, and by Rancimat after deep-frying. Differential scanning calorimetry (DSC) analysis was used as a proxy of the quality of the spent oils. EVOOs presented the lowest degree of oleic and linoleic acids losses, low formation of free fatty acids and carbonyl compounds, and were highly stable after deep-frying. In addition, oleic acid, tocopherols, and flavor compounds were transferred from EVOO into the French fries. In conclusion, EVOOs were more stable than refined seed oils during short-term deep-frying of French fries and also contributed to enhance the nutritional value, and possibly improve the flavor, of the fries prepared in EVOO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep-frying is an attractive food preparation method and has been in use for centuries. It is a very popular culinary practice worldwide and can be used both industrially and domestically. Deep-fried foods have unique sensorial properties, such as flavor, texture, and appearance, which are generally highly appreciated by consumers. French fries are a convenient product, highly consumed in industrialized countries [1]. However, the chemical stability of the frying oil can be affected by the high temperatures normally used in deep-frying. Thermal oxidation is an important cause of oil deterioration, especially in the food industry, after consecutive frying. High temperatures promote chemical decomposition resulting in the loss of sensory and nutritional quality. Thermal oxidation of oils leads to the formation of hydroperoxides, known as primary oxidation products, which degrade into hydrocarbons, aldehydes, and ketones, among other classes of compounds, known as the secondary oxidation products. Secondary products tend to be volatile and are responsible for the rancid flavor of oxidized oils. The oxidative degradation indices assess these primary and secondary products and are used as surrogate measures for oil quality and oxidative stability [2].
The heat transferred from oil to food during frying processes promotes water loss from the food and this water is partially replaced by oil that is absorbed from the frying medium by the food. Water availability and the food–oil interface form a favorable medium for chemical decomposition through pathways different from those occurring in heated bulk oil, such as hydrolysis. Deep-frying foods promotes a complex pattern of thermolytic reactions in the frying oils, resulting in increased free fatty acids (FFA), which accelerate the formation of primary and secondary oxidation products, modifying nutritional and sensorial properties of the oils [3]. Changes in chemical composition of deep-frying oils occur even during short-term frying [4], which is of concern for the domestic preparation of French fries, and other deep-fried foods.
Extra virgin olive oil (EVOO) stands out among plant oils because of its nutritional value, especially the high contents of oleic acid (18:1n-9) and of minor components with strong antioxidant activity, and also because of its appreciated flavor [2]. EVOO is typically the major lipid source in the Mediterranean diet, where it is added raw to prepared food products and is also used for cooking, such as by deep-frying. The habitual consumption of EVOO might help prevent cardiovascular diseases, even after deep-frying. Food fried in EVOO reduced insulin and C-peptide responses after intake of a mixed meal, improving postprandial insulin response in adult obese women [5]. Although stability of seed oils and EVOO has been thoroughly studied during long-term deep-frying, short-term deep-frying in EVOO has been scarcely studied [6]. Sánchez-Gimeno et al. [6] studied the formation of polar compounds, and changes in color and viscosity during short-term deep-frying with EVOO. The amounts of primary and secondary oxidation products formed in refined olive oil during short-term deep-frying were lower than those of seed oils [6]; however, the behavior EVOO was not studied. To our knowledge, there is still a lack of information concerning chemical transformations of deep-fried EVOO for short time periods, which is of concern for domestic food preparation.
In the present study we aimed to evaluate changes in the chemical quality of EVOOs, from different olive varieties, during short-term deep-frying of French fries. We also aimed to compare oxidation stability between EVOOs and refined seed oils commonly used for deep-frying foods, and to investigate the composition of tocopherols, fatty acids, and volatile compounds in French fries after frying in EVOOs.
Materials and Methods
Samples
Three commercial samples of European EVOOs were purchased in local markets in Spain (monovarietal Arbequina and Picual) and Portugal. One non-commercial sample of EVOO produced from olives produced in the city of Maria da Fé (Minas Gerais, Brazil) was acquired directly from EPAMIG (Minas Gerais Enterprise for Agricultural Research, Brazil). Two samples of refined oils of sunflower and soybean were purchased at local markets (Rio de Janeiro, Brazil) and used for comparisons with EVOO. Oil samples were aliquoted and stored at −18 °C protected from light and under nitrogen until analysis.
Industrially prefried and frozen French fries (Sadia®, Brazil) were purchased in local markets in Rio de Janeiro, Brazil. Before analysis and experiments, the prefried French fries were completely defrosted and excess water was dried with a clean cloth. Before analysis, the French fries were dried in a convection oven with forced air circulation (65 °C, 24 h) and milled in an analytical mill (A11 Basic mill, IKA®, Brazil) before lipid extraction using petroleum ether in a Soxhlet apparatus ([7], method Ba 3-38). This ether extract, which was the oil absorbed by French fries, was stored at −20 °C until analysis.
Deep-Frying Experiments at 180 °C
EVOOs and seed oils (soybean and sunflower) were used in short-term deep-frying experiments at 180 ± 1 °C with the industrially prefried French fries. After equilibrating 500 mL of each vegetable oil for 10 min at 180 °C in an electric deep-fryer with 850 mL capacity (Vicini®, Brazil), French fries were deep-fried for 5 min in batches of 200 g. Each batch of fries presented acceptable appearance and texture after 5 min. After three consecutive batches of French fries, 50-mL aliquots of the oils were taken at 25, 50, and 75 min of deep-frying tests (Fig. 1). Subsequently, the oil volume was refilled to 500 mL with 60 ± 1 mL, followed by 10 min for reheating the oil back to 180 °C. This sequence was repeated for three times, consisting of frying tests with a total time of 75 min.
A subsample of French fries, deep-fried in Portuguese and Spanish Arbequina EVOOs, was analyzed in order to investigate absorption of oil and chemical compounds from the EVOOs. In these prepared fries, the chemical composition (moisture, and fatty acid, tocopherols, and volatile composition) and oxidative quality (peroxide value) and stability (Rancimat®) were determined. Total lipids in the prepared French fries were extracted from dried and milled samples, as described above. Prefried French fries (non-prepared) were used as controls. All samples were stored at −20 °C under nitrogen until analysis.
Oxidative Stability During Deep-Frying at 180 °C
Quality indices of the oils were determined in fresh samples and during deep-frying at 180 °C. The acid value (AV) of the samples was determined by titrating 1–2 g of sample in ethyl ether/ethanol (2:1, v/v) with 0.01 N NaOH, and the results were expressed as percentage of oleic acid ([7], method Ca 5-40). The peroxide value (PV) was determined by iodometric titration ([7], method Cd 8-53). Briefly, 0.5 mL of saturated KI solution in water was added to 0.5 g of sample dissolved in 10 mL of acetic acid/chloroform (3:2, v:v). After incubation in the dark for 5 min, and addition of 10 mL of distilled water, the I2 liberated was titrated with 0.01 N Na2S2O3. A starch solution was used to help visualize the titration endpoint. PV was expressed as mEq O2/kg. The p-anisidine value (p-Av) was determined in approximately 2–4 g of oil, depending on the samples’ degree of oxidation, and analyzed as described previously ([7], method Cd 18–90). Briefly, 5 mL of oil solution in n-hexane was mixed with the p-anisidine reagent in glacial acetic acid and incubated in the dark for 10 min. The absorbance of samples and blank solutions were measured at 350 nm in a double beam spectrophotometer (Shimadzu 1800, Kyoto, Japan).
In a subsample of olive oils, Portuguese and Spanish Arbequina EVOOs, oxidative stability was monitored through the induction period (hours) determined by Rancimat® equipment (Metrohm 743; Metrohm Co., Basel, Switzerland), both in the bulk oil from the fryer and in the oil absorbed by the French fries. Oil samples (3 g) were heated at 110 °C with a 20 L/h air flow rate and the time required for a sharp increase in water conductivity was calculated by the instrument’s software package and corresponds to the induction period, in hours. All analyses were done in duplicate.
Analysis of Fatty Acids and Calculation of Iodine Value (IV)
Fatty acid methyl esters (FAMEs) from EVOOs and the oil absorbed by French fries during deep-frying were prepared as described previously [8]. The FAMEs were analyzed in a Varian CP 3800 GC (Varian Co., USA) equipped with a split/splitless injector, a fused silica polyethylene glycol capillary column (30 m × 0.53 mm ID and 1.0 µm film; Carbowax 20 M; Supelco, USA), and a flame ionization detector. The column oven temperature was programmed as follows: 150 °C kept for 1 min, followed by gradient heating of 10 °C/min up to 180 °C through a second gradient of 3 °C/min to reach the final column temperature of 230 °C. The FAMEs were dissolved in n-hexane, and 1.0 μL was injected at a 1:10 split ratio. The injector and detector temperatures were 220 and 260 °C, respectively. Peaks were identified on the basis of the retention times of FAME commercial standards (Sigma-Aldrich, Brazil). Fatty acid contents were calculated by internal normalization and the results expressed as grams per 100 g of fatty acids, and presented for the fatty acids with contents higher than 0.5 g/100 g. Iodine value (IV) was calculated on the basis of the samples’ fatty acid composition, for all EVOOs and seed oils, and all time intervals of deep-frying, as described in AOCS method 1c-85 [7].
Analysis of Tocopherols
Tocopherols (α, β, γ, and δ) were analyzed by normal-phase HPLC [9] with fluorescence detection [10] in an LC-10AVp chromatograph equipped with an RF-10AXL detector, a photodiode array detector (PDA), and a CBM-20A communication module (all from Shimadzu, Tokyo, Japan). Commercial standards (Sigma-Aldrich, Brazil) of α-, β-, γ-, and δ-tocopherols were used to identify chromatographic peaks and for quantitative analyses of tocopherols in the samples. Analytes were eluted from a normal-phase silica column (ZORBAX Rx-Sil; Agilent Technologies, USA) with an isocratic binary solvent system of n-hexane/2-propanol (99:1, v/v) at 1.0 mL/min. Samples were injected through a Rheodyne valve with a 20-μL volumetric loop. Peak assignment in the samples’ chromatograms was based on relative retention time, standard spiking, and UV spectra (240–400 nm) from the PDA. Quantitative analysis was based on peak areas from the fluorescence detector operated at 290 nm for excitation and 330 nm for emission. Analyses were performed in duplicate and average values were reported.
Differential Scanning Calorimetry (DSC) Analysis
The DSC analyses were performed according to Che Man and Tan [11] with adaptations. Before analysis, the equipment was purged with dry nitrogen at a flow rate of 30 mL/min. Samples of oil (4–7 mg) were weighed in an specific analytical balance AD6® (Perkin Elmer, USA) into aluminum pans and analyzed in a Pyris Diamond® DSC equipment (Perkin Elmer, USA) from −50 °C, and heated at 10 °C/min to 250 °C, and holding for 2 min at the final temperature. An inert atmosphere was maintained during analysis, with dry nitrogen at 20 mL/min. Thermograms were analyzed with the Pyris Diamond® DSC software (Perkin Elmer, USA), and the transition enthalpies (ΔH, J g−1) were calculated.
Determination of Moisture in French Fries
Moisture was determined by heating 2-g samples of French fries in an oven at 110 °C until constant weight [12]. Non-prepared prefried French fries were also analyzed as controls.
Analysis of Volatile Compounds
Volatile compounds were analyzed in a subsample of EVOO, Portuguese and Spanish Arbequina, both in fresh oils and in oils absorbed by French fries. Oils extracted from non-prepared French fries were used as control.
Qualitative Analysis of Volatile Compounds
Volatile compounds from EVOOs and French fries were extracted by solid phase microextraction (SPME) using a divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, PA, USA) [13]. Extraction fiber was conditioned for 60 min in the GC injection port at 260 °C. An aliquot (1 g) of dehydrated French fries or EVOO was weighed in a headspace vial. Vials were sealed with a PTFE-lined septum and placed in a glycerol bath (40 °C) until equilibrium (30 min). The septum was pierced and the fiber was exposed to the sample headspace for 10 min.
Qualitative analysis was performed by GC–MS on a GC-17A gas chromatograph coupled to a QP5050A mass spectrometer (Shimadzu, Japan) equipped with a split/splitless injector and a fused silica column 5 % phenyl/95 % methylpolysiloxane (30 m × 0.32 mm ID, 3 µm film thickness; 007-5; Quadrex, USA). Volatile compounds were desorbed from the SPME fiber in the injection port for 5 min at 260 °C, in splitless mode, and after 5 min sampling the split purge valve was open at 3.0 mL/min. Helium was used as the carrier gas and the column pressure was set to attain a carrier speed of 25.0 cm/s. The column oven temperature was held at 30 °C for 10 min, then increased at 3 °C/min to 200 °C and held for 10 min. The mass spectrometer was operated in electron impact mode at 70 eV. The interface and ion source temperatures were 260 °C. Analyses were performed in full scan acquisition mode, in the mass range 40–500 m/z at 0.5 scan/s. A mixture of C7–C30 hydrocarbons was run under the same conditions to allow calculation of linear retention index (LRI) values for the volatile compounds [14]. Data were collected by Lab Solutions GC–MS software package (Shimadzu, Japan). Compounds were identified first by comparison of mass spectra with those of the National Institute of Standards (NIST) library and calculation of similarity indices by the instrument’s software (Lab Solutions GC–MS; Shimadzu, Japan), and also by comparing LRI values with published data [15].
Quantitative Analysis of Volatile Compounds
Volatiles were analyzed with a GC-2010 (Shimadzu, Japan) gas chromatograph equipped with a FID detector, split/splitless injector, and the same capillary column used for qualitative analysis. An aliquot (1 g) of dehydrated French fries or EVOOs was weighed in a headspace vial and 20 µL of internal standard (0.1 mg/mL of bromobenzene in methanol) was added. Chromatographic conditions used were similar to those described in the section “Qualitative analysis of volatile compounds”. During analysis, injector and detector temperatures were 260 and 280 °C, respectively. A mixture of C7–C30 hydrocarbons was run under the same conditions to allow calculation of LRI values for the volatile compounds, and comparison with GC–MS data. Exclusively volatile compounds from EVOO transferred to the French fries were targeted in this analysis. To aid the comparison of the contents of these volatile compounds between the fresh EVOOs and the French fries, results were expressed in micrograms of volatile compound per gram of oil. The contents of volatiles in the French fries absorbed oil was corrected by their respective contents in the non-prepared fries and expressed as fold-increase relative to contents in the respective fresh EVOO used for deep-frying.
Statistical Analysis
Descriptive statistics was performed for all variables in order to calculate means, medians, standard deviation, and to estimate data normality. Results were expressed as mean ± standard deviation considering at least two independent replicates. Multifactor analysis of variance (MANOVA) was used for comparisons between means; significant differences between pairs of means were determined by the Fisher’s test. Paired t test was used for comparisons of volatile compounds in French fries. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using the Statistica 8.0 software (StatSoft®, Oklahoma, USA).
Results and Discussion
Fresh Oils: Fatty Acids, Tocopherols, and Quality Indices of EVOOs and Seed Oils
Olive oil production is highly concentrated in the Mediterranean region, and Spain contributes 50 % of the world’s olive oil production. However, olive tree cultivation and olive oil consumption are expanding in other countries, such as in the USA, Argentina, Chile, Asia, and Australia [1]; only recently EVOO started to be produced industrially in Brazil, although its quality was not yet reported. The quality of EVOOs is primarily determined by genetic and climatic factors, as well as by agricultural practices. Olives of good quality are used for obtaining monovarietal EVOOs, especially of the Picual, Arbequina, and Hojiblanca varieties. Monovarietal EVOOs stand out not only for their chemical, sensory, and nutritional properties, but also for their oxidative stability [16]. In this section, the composition and quality indices of oil samples are compared to the corresponding legislation limits and Codex Alimentarius recommendations. Comparisons between samples were not performed (Table 1) because this was not our objective in the present work, although in some cases there were significant differences between oils.
All EVOO samples studied presented high contents of oleic acid (18:1n-9) and of total monounsatured fatty acids (MUFA; Table 1), which might provide protection against cardiovascular diseases [17]. Fatty acid compositions were within the ranges presented by the Codex Alimentarius Commission [18].
Tocopherols protect oil from oxidation and consequently prevent the formation of free radicals from unsaturated lipids. Contents of total tocopherols and the distribution of tocopherol homologues were consistent with previous reports [19]. α-Tocopherol was the most concentrated tocol in the EVOOs, and the contents of β, γ, and δ-tocopherol were low (Table 1). Sunflower and soybean oils showed the highest contents of α-tocopherol and γ-tocopherol, respectively. Concerning the action of vitamin E, α-tocopherol, the major tocol of EVOOs, is the most effective, because of the specificity of absorption and transport systems in the human body [20].
The acid value is a quality index associated with the contents of FFAs, indicating the degree of hydrolysis in a triacylglycerol (TAG) mixture. Therefore, high acid values indicate elevated FFA contents. FFAs accelerate oil oxidation and therefore promote oil fuming and rapid flavor depreciation [2]. Besides, AV is the main criterion for classification of olive oils as extra virgin, and EVOOs should present AV below 0.8 % [18]. Higher AV for cold-pressed olive oils might indicate poor agricultural, post-harvesting, and/or processing practices. All fresh samples of EVOO were in accordance with this upper limit of AV, except the Brazilian EVOO (Table 1), indicating that in this case the adopted production practices need improvement.
Oxidation of fatty acids leads to formation of hydroperoxides, which are often assessed by PV, another important oil quality index. The PV and p-Av are commonly used to estimate the degree of oxidative degradation in heated oils and indicate the levels of primary and secondary oxidation products, respectively. All samples showed initial PV (Table 1) below their respective maximum limits allowed in legislation for EVOOs (20 mEq O2/kg) [18]. The p-Av is a proxy of secondary oxidation products formed by decomposition of the so-called primary oxidation products (hydroperoxides) and therefore it is a valuable measure of oxidation in heated oils [21]. For all samples of fresh oils (Table 1), the p-Av was lower than the recommended upper limit of 10.0 [22].
Deep-Fried EVOOs: Time Course of Quality Indices
The AV increased significantly from 0 to 75 min of short-term deep-frying in all samples of EVOOs and refined oils (Fig. 2a). The Brazilian EVOO presented the smallest AV increase (19 %) after 75 min of short-term deep-frying. Conversely, the Spanish Picual EVOO presented the highest AV increase (147 %) after 75 min of short-term deep-frying, half of which (76 %) occurred from 0 to 50 min. Similarly, AV of Spanish Arbequina EVOO increased by 30 % after deep-frying for 25 min. In contrast, it has been reported that the AV of olive oils increased roughly by 50 % in the first 3 h of a deep-frying test at 170 °C with potato slices [23]. The earlier changes in AV observed in the present study were possibly caused by temperature differences in the adopted frying protocols, differences in the oils’ composition, residual oil in the French fries of the present study, and differences in the surface area and in the rate of water transfer into the frying oil, because of the potato cuts used. Although there is not a rejection limit of AV for frying oils, short-term deep-frying in conditions similar to domestic preparation of French fries appears to possibly influence oil quality. All EVOOs presented lower rates of increase in AV compared to refined soybean and sunflower oils, indicating higher stability of EVOO during deep-frying and suggesting better nutritional and sensory quality of food deep-fried in EVOO.
Evolution of acid value (a), peroxide value (b), and p-anisidine value (c) in the EVOOs and seed oil samples after short-term deep-frying of French fries (180 °C) for 25, 50, and 75 min. Symbols:  Spanish Arbequina,
Spanish Arbequina,  Spanish Picual,
Spanish Picual,  Portuguese,
Portuguese,  Brazilian,
Brazilian,  soybean,
soybean,  sunflower. Quality indices changed significantly (P < 0.05; repeated-measures ANOVA, with Fisher’s post-test) during oxidation for all samples, with the exception of a acid value,
sunflower. Quality indices changed significantly (P < 0.05; repeated-measures ANOVA, with Fisher’s post-test) during oxidation for all samples, with the exception of a acid value,  deep-frying 50 min vs. 75 min; b peroxide value,
deep-frying 50 min vs. 75 min; b peroxide value,  deep-frying 50 min vs. 75 min,
deep-frying 50 min vs. 75 min,  deep-frying 50 min vs. 75 min; c
p-anisidine value,
deep-frying 50 min vs. 75 min; c
p-anisidine value,  deep-frying 25 min vs. 50 min,
deep-frying 25 min vs. 50 min,  deep-frying 25 min vs. 50 min,
deep-frying 25 min vs. 50 min,  deep-frying 25 min vs. 50 min
deep-frying 25 min vs. 50 min
The PV and p-Av increased significantly in all samples during the short-term deep-frying tests (Fig. 2b, c). Peroxide value increased sharply for all EVOO during the first 25 min of deep-frying and plateaued after 50 min, except for Brazilian EVOO. The two varieties of Spanish EVOO showed the steepest increase in this time interval. In contrast, Brazilian EVOO showed a linear increase (r 2 = 0.99, P < 0.0001) in PV, between 0 and 75 min. These results suggest that this was the only sample in which the rate of formation of hydroperoxides was higher than the rate of decomposition for the whole time interval tested. The Portuguese EVOO sample was the only one that showed a high rate of degradation of hydroperoxides from 50 min of deep-frying, suggesting lower oxidative stability for this sample. Although EVOOs presented an average increase of 90 % in PV from 0 to 25 min of oxidation, these oils remained more stable than both the soybean and sunflower oils which showed the highest PV increase (0–50 min) and degradation (50–75 min), respectively. Formation of hydroperoxides varied among oils even between EVOO, because Spanish Arbequina and Picual behaved similarly to the seed oils.
EVOOs behaved similarly concerning p-Av during the short-term deep-frying tests, with increasing values until 75 min, indicating that on average the rate of formation of carbonyls exceeded the rate of loss of these secondary oxidation products. Additionally, all EVOOs presented p-Av lower than the seed oils, for all time intervals of the frying tests (25–75 min; Fig. 2c). These results indicate that formation of rancid flavor by deep-frying in EVOO might occur later than for seed oils commonly used for frying foods. For the Spanish Arbequina and Portuguese EVOOs, p-Av plateaued between 25 and 50 min of deep-frying. During this interval p-Av was probably at a steady state, and the rate of formation of carbonyls from degradation of hydroperoxides was similar to the rate of loss of carbonyls by volatilization or chemical reactions [20]. Higher rates of increase in p-Av were observed in oven-heated oils than in fried oils at 185 °C, consistent with accelerated partial removal of aldehydes by steaming during frying [24]. p-Av was elevated (>10) for virtually all EVOO samples after 75 min of deep-frying French fries. In contrast to previous results with deep-fried olive oil [25], we show that short-term deep-frying similar to domestic preparation promotes oil deterioration.
Deep-Fried EVOOs: Time Course of Losses in Unsaturated Fatty Acids and Tocopherols
Oleic acid (18:1n-9) was the major fatty acid in EVOOs (Table 1) and was relatively stable during the short-term deep-frying tests in all olive oil samples. The contents of 18:1n-9 in EVOOs did not change significantly or changed only slightly after deep-frying for 75 min (from −0.71 to −3.5 %; Fig. 3). However, the contents of 18:1n-9 changed significantly in soybean oil. The apparent stability of 18:1n-9 was probably due to the low rate of oxidation of monounsaturated fatty acids, which is roughly 100-fold lower than that of polyunsaturated fatty acids (PUFA) [21]. According to fatty acid composition, EVOOs might be considered more stable than seed oils to oxidation during deep-frying. However, susceptibility to oxidation depends on antioxidants and other minor components, on chemical micro-environments in the medium, and on the positional distribution of the glycerol backbone of fatty acids [26], as well as on oxidizable fatty acids [20].
Percent changes in the contents of polyunsaturated fatty acids (18:2n-6 and 18:3n-3), iodine value (IV), α-tocopherol (α-Toc), and total tocopherols (Total-Toc) in each EVOO and in refined seed oils after 25, 50, and 75 min of short-term deep-frying (180 °C), and degradation rate of Total-Toc (µmol/h) after 75 min. Legend: white bars frying (25 min), gray bars frying (50 min), black bars frying (75 min); a Brazilian EVOO, b Portuguese EVOO, c Spanish Arbequina EVOO, d Spanish Picual EVOO, e soybean oil, f sunflower oil, g degradation rate of Total-tocopherols
In contrast to 18:1n-9, the contents of polyunsaturated linoleic (18:2n-6) and α-linolenic (18:3n-3) acids reduced appreciably, especially at the end (75 min) of the short-term deep-frying, reaching the maximum losses of 22 % for 18:2n-6 and 26 % for 18:3n-3 in Spanish Picual (Fig. 3). Soybean and sunflower oils lost, respectively, 62 and 38 % of the original 18:3n-3 contents after 75 min of deep-frying showing the highest losses of PUFA. Losses of double bonds in unsaturated lipids might also be observed through changes in IV (Fig. 3), which might be a sensitive measure of oxidative changes in PUFA during thermal oxidation [25]. Accordingly, seed oils presented the highest changes in IV from 0 to 75 min, indicating highest losses in the degree of unsaturation of fatty acids. Consequently, it seems that the losses of unsaturated lipids due to oxidation during short-term deep-frying were higher in refined seed oils than in EVOO.
Cumulative α-tocopherol degradation at the end of deep-frying experiments varied from 18 % (sunflower oil) to 74 % (Spanish Picual EVOO). The degradation rate of tocopherols was higher in EVOO compared to seed oils (Fig. 3g). In heated oils with low PUFA contents, such as EVOO, tocopherols react faster than PUFA with oxidizing species [27]. Additionally, hydroperoxides formed in the highly unsaturated vegetable oils decompose rapidly before reacting with tocopherols [21]. Despite these differences in the degradation rate of tocopherols, α-tocopherol contents in oil after deep-frying for 75 min were similar between EVOO and soybean oil, because the former presented higher initial levels of α-tocopherol. Losses of total tocopherol strongly correlated (r 2 = 0.99; P < 0.05) with α-tocopherol losses, except in soybean oil that correlated strongly (r 2 = 0.99; P < 0.05) with γ-tocopherol losses. These correlations show that the major tocopherol homologue that is acting as an antioxidant in the deep-fried vegetable oils seems to depend at least partly on the initial tocopherol composition in the oil.
Differential Scanning Calorimetry (DSC) of EVOO and Seed Oils: Fresh Samples and Time Course During Short-Term Deep-Frying of French Fries
There is increasing need for fast and reliable methods for monitoring oil quality that produce low levels of chemical waste. Thermoanalytical techniques are increasing in importance in analytical chemistry and other interdisciplinary areas. These methods have been used in quality control of oils because they provide data on oil stability according to its thermal behavior [11] and were compared with Rancimat® for the assessment of olive oil oxidation [28]. DSC monitors changes on the physical and chemical properties of a material as a function of temperature by monitoring heat transfer during heating. This technique is used in lipid chemistry to assess the melting and crystallization characteristics of edible oils, heat of fusion, and polymorphism. Furthermore, DSC has been successfully applied for the assessment of the identity of vegetable oils and changes during oxidation [29]. To our knowledge, this is the first report of DSC heating thermograms of deep-fried EVOO, and soybean and sunflower oils.
Thermal events occurred below 20 °C, for all samples, and heating thermograms showed no events from 20 °C up to 250 °C (data not shown). The heating thermograms of all the EVOOs exhibited one distinguishable exothermic event between −40 and −20 °C (Fig. 4). Similar profiles of DSC thermograms were previously reported for fresh EVOO [11, 30]. This exothermic event represents crystallization and rearrangement of TAG in EVOOs and was not distinguishable in seed oils. In thermograms of deep-fried EVOO this exothermic event was shifted to the right, indicating that thermal oxidation might have changed the crystallization of TAG in EVOO [31]. This shift was possibly promoted by the accumulation of TAG hydrolysis products, such as diacylglycerols, monoacylglycerols, and FFAs and in the present study was paralleled by changes in acid value.
Additionally, two endothermal events were observed in the fresh EVOO and seed oil samples, but the second event was smaller in the seed oils compared to EVOO. In the EVOO, these endothermal peaks occurred between −20 and 14 °C. In contrast, in the seed oils, these endothermal events occurred at a lower temperature interval, from −35 to −18 °C (Fig. 4). These differences are associated with the samples’ fatty acid composition, because endothermal bands represent TAG melting [30]. As the more unsaturated samples (Table 1), the seed oils present endothermal events at lower temperatures (Fig. 4).
Deep-frying with EVOOs and seed oils led to appreciable changes in the endothermal events. The major endothermal signal showed peak broadening and decreased height, and the minor endothermal peak disappeared, or possibly became embedded into the major one. Similar changes in endothermal events were observed in heated EVOO [29]. TAG hydrolysis products and secondary lipid oxidation products in thermo-oxidized EVOOs might have affected the crystals’ stability, making them less stable than in samples of fresh oils. Furthermore, these molecules possibly hindered the rearrangement of TAG polymorphic crystals, changing the phase transition profile at lower temperatures [2].
Transition enthalpies (Table 2) summarize the changes observed in Fig. 4 and described above. Overall, the transition enthalpies of EVOOs, soybean, and sunflower oils decreased during deep-frying, with differences observable from the first 25 min. Portuguese EVOO presented the lowest transition enthalpy among olive oils, and also the smaller changes during deep-frying (7 % decrease from 0 to 75 min). In contrast, among EVOO, Arbequina and Picual presented the most pronounced changes (29 and 24 %, respectively) in transition enthalpy during deep-frying. These changes might be explained by the accumulation of lipid hydrolysis and oxidation products, promoting the formation of mixed and/or less stable polymorphic crystals than in pure oil, melting at lower temperatures [30] or at a broadened temperature range, compared to the fresh oils. These composition changes might have promoted heterogeneous structures, which were more easily disrupted in heating, as previously observed [30, 31].
Deep-Frying in EVOOs (Subsample): Chemical Compounds Transferred into French Fries and EVOO Stability During Deep-Frying at 180 °C
During deep-frying there is heat and mass transfer between the food and the frying medium. Briefly, the heat transferred from the frying medium (at 180 °C in the present work) promotes water vaporization and food dehydration. During dehydration, some of the evaporated water from the fried food is replaced by oil that is absorbed by food from the frying medium [3]. Therefore, transferred oil might carry minor components, such as bioactive and flavor compounds, from the EVOO that would expectedly enhance nutritional, functional, and sensorial value of the prepared food. In this context, we observed that the French fries changed their chemical composition after deep-frying in Portuguese and Spanish Arbequina EVOOs (Table 3, Fig. 6).
As expected, deep-frying promoted a reduction in moisture content of similar magnitude, 27 % on average, for all experimental conditions, with only slight differences between Portuguese and Spanish Arbequina EVOO. Concurrently, there was a significant increase (on average 40 %; dry basis) in fat content in the French fries deep-fried in EVOO. These results are consistent with replacement of the water lost by evaporation with the oil used as frying medium.
Although French fries showed an increase in fat content, there were beneficial changes to the quality of fat after deep-frying in EVOO (Table 1). Deep-frying with both EVOOs significantly, and by several fold, increased the content of 18:1n-9 in French fries. These changes are consistent with the mass transfer of TAGs from EVOO into the French fries. The typically high contents of 18:1n-9 in EVOO promoted beneficial changes in the lipid composition in the French fries, when the health of the consumers is of concern. Similarly, deep-frying in EVOO significantly enriched French fries with α-tocopherol, the main tocol in Portuguese and Spanish Arbequina EVOOs. Alpha-tocopherol was not detected in non-prepared prefried French fries, but high contents were detected in the prepared French fries. In conclusion, French fries deep-fried in EVOO might present added nutritional value, from absorbed EVOO components.
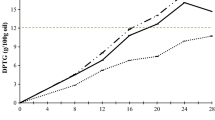
These changes in chemical composition of French fries were paralleled by increased oxidative stability of the fries (Fig. 5a). Oxidative stability of the bulk frying oil during deep-frying was stable for Portuguese EVOO, but for the Spanish Arbequina showed a significant decrease in the first 25 min and stabilized thereafter (Fig. 5b). Possibly this was due to a lower induction period (Fig. 5b) and to a higher peroxide value (Table 3) in the fresh Portuguese EVOO. In both EVOO, the induction period stabilized at approximately 20 h and was not significantly different from 50 min of deep-frying.
Oxidative stability by Rancimat® during short-term deep-frying of French fries at 180 °C. a Induction period of oil absorbed by French fries: Control (non-prepared prefried French fries); and oil absorbed in deep-fried French fries (pooled sample from 25, 50, and 75 min of deep-frying). b Induction period of bulk EVOOs used as deep-frying media for 25, 50, and 75 min:  Spanish Arbequina (*Significantly different from 25 to 75 min; P < 0.05) and
Spanish Arbequina (*Significantly different from 25 to 75 min; P < 0.05) and  Portuguese EVOO
Portuguese EVOO
Considering flavor compounds, we also observed that some volatile compounds of EVOOs were transferred to the French fries when deep-fried in these oils (Fig. 6). Volatile compounds produced enzymatically by the lipoxygenase pathway in olives, such as hexanal, hexanol, hexyl acetate, (Z)-3-hexenal, (E)-2-hexenal, (E)-2-hexenol, (Z)-3-hexenol, and (Z)-3-hexenyl acetate confer green notes to the flavor of EVOO [15]. Volatile compounds in the frying oil might be transferred to French fries, changing its flavor [32]. In the present work, we investigated absorption of volatile compounds from EVOO into the French fries (Fig. 6). The major volatile compounds observed in both EVOOs were (Z)-3-hexenal, (E)-2-hexenal, (E)-2-hexenol, and (Z)-3-hexenyl acetate. Four flavor compounds were transferred from the EVOOs to the French fries (Fig. 6) and were therefore quantified (Fig. 6a). Almost all of these compounds were more concentrated in the French fries than in the fresh EVOO (Fig. 6b), with the exception of hexane. Except for 2-heptenal, absorption of volatiles in the French fries was not different between the two EVOO tested (Fig. 6b). Unlike other vegetable oils, EVOO is not refined and keeps most of its original components, such as volatiles, thus conserving its flavor [33]. Although hexanal is considered an off-flavor in refined oils, it is an important flavor component in EVOOs [15, 34], to which it is attributed a “green” odor. The green odor in EVOO is attributed to low contents of hexanal, whereas a fatty odor is observed in high contents. In oils, contents higher than 3-fold the odor threshold of hexanal (0.9 µg/g) presented a fatty odor [35]. In the present study, we observed that hexanal contents in the French fries were below this threshold: 0.61 ± 0.1 µg/g and 0.32 ± 0.01 µg/g of French fries, when deep-fried in Portuguese and Spanish Arbequina EVOO, respectively. Therefore, the absorption of hexanal by fries to levels below the off-flavor threshold could positively impact in the flavor of French fries deep-fried in EVOO.
Volatile compounds transferred to French fries during deep-frying in EVOO at 180 °C. Other volatiles were identified in the EVOO, but were not transferred to the fries (data not shown). a Contents of volatile compounds absorbed by the French fries before frying, in EVOO and non-prepared prefried French fries (Control1). Different superscript letters within the same column indicate significant differences (P < 0.05, ANOVA). b Fold-increase in contents of volatile compounds, relative to fresh extra virgin olive oils used for frying:  , Portuguese;
, Portuguese;  , Spanish Arbequina EVOOs. *Significantly different between French fries (P < 0.05, T test)
, Spanish Arbequina EVOOs. *Significantly different between French fries (P < 0.05, T test)
Conclusion
EVOO is a highly attractive medium for short-term deep-frying of French fries. EVOO used as a frying medium showed high oxidative stability, preservation of unsaturated fatty acids, and low formation of FFAs and carbonyl compounds. Additionally, deep-frying in EVOO added value to the French fries. Transfer of compounds from the EVOO frying medium into the French fries added nutritional value to the fries, and increased oxidative stability. Transfer of flavor compounds possibly improved the flavor of the French fries. Additionally, differential scanning calorimetry seemed useful to investigate the quality of EVOO during deep-frying. Future investigations concerning associations between sensorial attributes and absorption of minor compounds from EVOO used as the frying medium for French fries are of interest.
Abbreviations
- AV:
-
Acid value
- DAG:
-
Diacylglycerol
- DSC:
-
Differential scanning calorimetry
- EVOO:
-
Extra virgin olive oil
- FAMEs:
-
Fatty acid methyl esters
- FFA:
-
Free fatty acid
- GC:
-
Gas chromatography
- HPLC:
-
High performance liquid chromatography
- IP:
-
Induction period
- IV:
-
Iodine value
- MAG:
-
Monoacylglicerol
- MUFA:
-
Monounsatured fatty acids
- PUFA:
-
Polyunsaturated fatty acid
- p-Av:
-
p-Anisidine value
- PV:
-
Peroxide value
- TAG:
-
Triacylglycerol
- Total-Toc:
-
Total tocopherols
References
Santos CSP, Cruz R, Cunha SC, Casal S (2013) Effect of cooking on olive oil quality attributes. Food Res Int 54:2016–2024
Frankel E (2010) Chemistry of extra virgin olive oil: adulteration, oxidative stability, and antioxidants. J Agric Food Chem 58:5991–6006
Dana D, Saguy S (2001) Frying of nutritious food: obstacles and feasibility. Food Sci Technol Res 7:265–279
Karoui IJ, Dhifi W, Jemia MB, Marzouk B (2011) Thermal stability of corn oil flavoured with Thymus capitatus under heating and deep-frying conditions. J Sci Food Agric 91:927–933
Farnetti S, Malandrino N, Luciani D, Gasbarrini G, Capristo E (2011) Food fried in extra-virgin olive oil improves postprandial insulin response in obese, insulin resistant women. J Med Food 14:316–321
Sanchez-Gimeno AC, Benito M, Vercet A, Oria R (2008) Aceite de oliva virgen extra del Somontano: evaluación de las modificaciones fisico-químicas tras la fritura doméstica de patatas prefritas congeladas. Grasas Aceites 59:57–61
American Oil Chemists’ Society (2004) Official methods and recommended practices of the AOCS, 4th ed. Boulder, USA
Hartman L, Lago R (1973) A rapid preparation of fatty acid methyl esters from lipids. Lab Pract 22:475–476
Tan B, Brzuskiewicz L (1989) Separation of tocopherol and tocotrienol isomers using normal- and reverse-phase liquid chromatography. Anal Biochem 180:368–373
Cunha S, Amaral J, Fernandes J, Oliveira MB (2006) Quantification of tocopherols and tocotrienols in Portuguese olive oils using HPLC with three different detection systems. J Agric Food Chem 54:3351–3356
Che Man YB, Tan CP (2002) Comparative differential scanning calorimetric analysis of vegetable oils: II. Effects of cooling rate variation. Phytochem Anal 151:142–151
Instituto Adolfo Lutz (2008) Physical and chemical methods in food analysis [Métodos físicos-químicos para a análise de alimentos], 4th ed. São Paulo, Brazil
Larick DK, Parker JD (2001) Chromatographic analysis of secondary lipid oxidation products. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ et al (eds) Current protocols in food analytical chemistry. Wiley, Weinheim, pp D2.2.1–D2.2.9
Viegas MC, Bassoli DG (2007) Linear retention index for characterization of volatile compounds in soluble coffee using GC-MS and HP-innowax column [Utilização do índice de retenção linear para caracterização de compostos voláteis em café solúvel utilizando GC-MS e coluna HP-Innowax]. Quim Nova 30:2031–2034
Vichi S, Pizzale L, Conte L, Buxaderas S, López-Tamames E (2003) Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: characterization of virgin olive oils from two distinct geographical areas of northern Italy. J Agric Food Chem 51:6572–6577
Cerretani L, Bendini A, Caro A, Piga A, Vacca V, Carboni M et al (2006) Preliminary characterisation of virgin olive oils obtained from different cultivars in Sardinia. Eur Food Res Technol 222:354–361
López-Miranda J, Badimon L (2006) Monounsaturated fat and cardiovascular risk. Nutr Rev 64:S2–S12
FAO/WHO Alimentarius Codex (2009) Codex standard for olive oil, virgin and refined and for refined olive-pomacem oil. Food Stand Progr 8:25–39
Silva L, Pinto J, Carrola J, Paiva-Martins F (2010) Oxidative stability of olive oil after food processing and comparison with other vegetable oils. Food Chem 121:1177–1187
Farhoosh R, Khodaparast MHH, Sharif A, Rafiee SA (2012) Olive oil oxidation: rejection points in terms of polar, conjugated diene, and carbonyl values. Food Chem 131:1385–1390
Frankel E (2005) Lipid oxidation. Oily Press/Woodhead, Cambridge
Brasil (1999) Technical regulation for fixation of identity and quality of vegetable oils and fats [Regulamento técnico para fixação de identidade e qualidade de óleos e gorduras vegetais]. ANVISA (Brazil), RDC nº 482. http://portal.anvisa.gov.br/wps/wcm/connect/a2190900474588939242d63fbc4c6735/RDC_482_1999.pdf?MOD=AJPERES. Accessed 25 Jan 2015
Casal S, Malheiro R, Sendas A, Oliveira BPP, Pereira JA (2010) Olive oil stability under deep-frying conditions. Food Chem Toxicol 48:2972–2979
Houhoula DP, Oreopoulou V, Tzia C (2002) A kinetic study of oil deterioration during frying and a comparison with heating. J Am Oil Chem Soc 79:133–137
Naz S, Siddiqi R, Sheikh H, Sayeed SA (2005) Deterioration of olive, corn and soybean oils due to air, light, heat and deep-frying. Food Res Int 38:127–134
Kamal-Eldin A (2006) Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur J Lipid Sci Technol 108:1051–1061
Barrera-Arellano D, Ruiz-Méndez V, Ruiz G, Dobarganes C (1999) Loss of tocopherols and formation of degradation compounds in triacylglycerol model systems heated at high temperature. J Sci Food Agric 1928:1923–1928
Ostrowska-Ligeza E, Bekas W, Kowalska D, Lobacz M, Wroniak M, Kowalski B (2010) Kinetics of commercial olive oil oxidation: dynamic differential scanning calorimetry and Rancimat studies. Eur J Lipid Sci Technol 112:268–274
Toro-Vazquez J, Herrera-Coronado V, Dibildox-Alvarado E, Charo-Alonso C, Gomez-Aldapa C (2002) Induction time of crystallization in vegetable oils, comparative measurements by differential scanning calorimetry and diffusive light scattering. Food Eng Phys Prop 67:1057–1065
Chiavaro E, Barnaba C, Vittadini E, Rodriguez-Estrada MT, Cerretani L, Bendini A (2009) Microwave heating of different commercial categories of olive oil: part II. Effect on thermal properties. Food Chem 115:1393–1400
Ferrari C, Angiuli M, Tombari E, Righetti MC, Matteoli E, Salvetti G (2007) Promoting calorimetry for olive oil authentication. Thermochim Acta 459:58–63
van Loon WAM, Linssen JPH, Legger A, Posthumus MA, Voragen AGJ (2005) Identification and olfactometry of French fries flavour extracted at mouth conditions. Food Chem 90:417–25
Vichi S, Castellote AI, Pizzale L, Conte LS, Buxaderas S, López-Tamames E (2003) Analysis of virgin olive oil volatile compounds by headspace solid-phase microextraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J Chromatogr A 983:19–33
Morales MT, Rios JJ, Aparicio R (1997) Changes in the volatile composition of virgin olive oil during oxidation: flavors and off-flavors. J Agric Food Chem 45:2666–2673
Dierkes G, Bongartz A, Guth H, Hayen H (2012) Quality evaluation of olive oil by statistical analysis of multicomponent stable isotope dilution assay data of aroma active compounds. J Agric Food Chem 60:394–401
Acknowledgments
We thank Alma Brasilis® (Rio de Janeiro, Brazil), which kindly provided the three commercial samples of European EVOOs. The financial support of FAPERJ, CAPES, and CNPq (Brazil) is greatly acknowledged. E. Akil was a recipient of an MSc scholarship, A.M.M. Costa was a recipient of a DSc scholarship, and V. Calado was a recipient of a research fellowship, from CNPq (Brazil). V.N.Castelo-Branco was a recipient of a DSc scholarship from CAPES (Brazil).
Conflicts of interest
The authors declare that there are no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Akil, E., Castelo-Branco, V.N., Costa, A.M.M. et al. Oxidative Stability and Changes in Chemical Composition of Extra Virgin Olive Oils After Short-Term Deep-Frying of French Fries. J Am Oil Chem Soc 92, 409–421 (2015). https://doi.org/10.1007/s11746-015-2599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2599-2