Abstract
Fruit processing wastes contain numerous by products of potential use in food & allied industry. Watermelon seeds represent a major by-product of the processing waste and contain high amount of nutritional proteins. Protein rich cereal based products are in demand due to their health promoting benefits. With this aim, wheat flour was fortified with watermelon seed protein concentrates (2.5 %, 5 %, 7.5 % and 10 % levels) to prepare cookies with desirable physical, nutritional, and textural and sensory properties. Substitution levels of 5 % and 10 % significantly (p ≤ 0.05) increased the dough stability and mixing tolerance index, however pasting properties and dough extensibility decreased considerably above 5 % substitution levels. Cookie fracture force (kg) increased significantly (p ≤ 0.05) above 5 % fortification levels. Cookie spread factor (W/T) increased from 2.5 % to 7.5 % fortification levels, further increase showed negative impact. Sensory scores of the cookies showed that protein concentrate may be added up to 7.5 % fortification levels. This study revealed that watermelon protein concentrates can be fortified with protein concentrates upto 5–7.5 % levels in cookies to improve their protein quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Consumer demand for protein rich food has increased due to due to concerns of diabetes, obesity, coronary heart disease, cancers, hypertension and reduction of LDL cholesterol (Zucco et al. 2011). The other advantage with high protein intake is stronger feeling of satiety as compared to a carbohydrate based food with a similar calorific value. The fabrication of protein rich food has squeezed due to limited availability of inexpensive proteins in a number of developing countries. This has invoked interest to search cheap and nutritional protein sources from the underutilized crops and food processing waste streams (Wani et al. 2008; Firatligil-Durmus and Evranuz 2010). Watermelon seed oil cake, the residual of oil pressing represents poorly utilized industrial by-product mainly used for animal feeding (Wani et al., 2006a; Wani et al. 2011a). It is a rich source of nutritive proteins (45 to 55 %), phenolic components, and dietary fibre. The extraction of watermelon seed cake proteins is promising in developing countries to extract the low cost nutritive proteins (Wang and Ng 2003; Wani et al. 2006a, 2008, 2011b). Alkaline extraction of proteins is receiving criticism due to its adverse effect on essential amino acids. New extraction methods using enzymes and salts are explored to replace the NaOH assisted protein extraction. The watermelon seed proteins mainly comprise of globulins and glutelins, which are readily digestible with high amino acid score (El-Adawy and Taha, 2001; Wani et al. 2011b). These proteins have excellent thermal and functional properties for the production of high quality protein products (Giami and Barber 2004; Wani et al. 2011a).
Cookies are widely accepted and consumed throughout the world and offer a vehicle for the enrichment of proteins for consumers (Zoulias et al. 2002; Arshad et al. 2007; Zucco et al. 2011; Škrbic and Cvejanov 2011). Cereal-based cookies, crackers and breakfast food are cereal based products that represent an important source of energy in human nutrition. Cookies have been suggested as a better use of composite flour than bread due to their wider consumption and relatively long shelf life (Fustier et al. 2009). A number of studies have been performed on the use of composite flours to increase the level of proteins in cookies in different parts of the world. This involve the use of soy flour, pulse flour, sun flower meal, barley flour, melon seed meal, and almond meal (Giami and Barber 2004; Singh and Mohamed 2007; Jia et al. 2011; Zucco et al. 2011; Škrbic and Cvejanov 2011). The fortification with non-wheat proteins is reported to affect the mixing and pasting properties of wheat flour dough (Jia et al. 2011). In order to study the implications of proteins on wheat dough handling, literature has reported the use of farinograph, texture analyzer and rapid visco analyzer to measure these characteristics. These instruments allow measuring the dough handling properties so that they can be correlated with the end product characteristics. The objective of the present study was to investigate the effects of NaCl extracted watermelon seed protein concentrates on composition, dough handling textural and sensory characteristics of cookies.
Materials and methods
Materials
Fruits of two watermelon cultivars cv Sugar baby and Mateera were procured from the Department of Horticulture, Punjab Agricultural University, Ludhiana and Central Institute for Arid Horticulture, Bikaner, India, respectively. Analytical reagent grade chemicals obtained from Sisco Research Laboratories, India were used in this study. Refined wheat flour, hydrogenated vegetable shortening, sugar, eggs and salt were procured from local market of Amritsar, India.
Preparation of protein concentrates
Ripe watermelon fruits were cut by a sharp knife followed by expression of juice using burr mill. The seeds were separated from the pomace according to the methods of Kaur et al. (2006). Wet seeds were dried in a cabinet dryer, dehulled, and grinded using hammer mill according to the methods of Wani et al. (2006a) To prepare oil free meal, meal was extracted 4 times with n- hexane (60–80 °C) using a meal-to-solvent ratio of 1:10 (w/v). The hexane treated meal was desolventized at 40 °C in a vacuum oven and sieved through 212 micron mesh (std mesh no 70) to obtain fine powder termed as defatted seed meal (Wani et al. 2008). For further use the meal was stored at −20 °C.
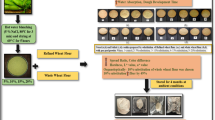
Seed meal was mixed with 0.5 M NaCl in a ratio of 1:20, stirred for 1 h at 40 °C and then centrifuged at 10,000 × g for 15 min. The supernatant (albumin and globulin) was filtered through Whatman No. 1 filter paper, dialysed in a membrane with pore size 204 nm (Hi-Media Laboratories Ltd.; Mumbai, India). After 12 water changes, the protein was recovered from the membrane and dried on a freeze dryer (LL3000 Jouan Nordic, Alleroid Denmark) to obtain a protein concentrate. The protein concentrate was milled and finally sieved through a 60-mesh (Wani et al. 2011a). Figure 1 represents the sequential procedure for the preparation of globulin rich protein concentrates.
Chemical analysis of flour and cookies
Moisture, crude protein (N × 6.25), crude fat, crude fibre and ash were determined according to the methods of AOAC (25). Wheat flour (20 g) was mixed with known amount of water to prepare the dough. The dough was washed under running tap water (flow rate ~2.7 ml/s) to completely remove the starch from the gluten matrix. The end point was determined by squeezing 2–3 drops of water from the gluten network into the container containing clear water. This was considered as wet gluten, and the dry gluten was measured after drying according to AOAC method (1990). Non-protein nitrogen was determined according to the methods of Bhatty and Finlayson (1973) as modified by Naczk et al (1985). One gram of meal was shaken with 40 ml of a 10 % solution of trichroroaceticacid (TCA) at 20 °C for 1 h using incubator shaker (Lab-Ind and Vac Instruments, Lucknow, India). The insoluble residue was removed by centrifugation at 5,000 × g for 10 min and subsequently treated three more times with 15 ml of 10 % (w/v) TCA solution. The supernatant was collected, and volume was made to 100 ml with distilled water. The soluble nitrogen was determined following Kjeldhal protocol (AOAC, 1990) Fig. 2.
Protein in vitro digestibility was measured as per the method of Saunders et al. (1973). Protein suspension (6.25 g /L protein) with pH adjusted to 8.0 was held in a water bath at 37 °C. The pancreatin (Sigma Chemical Co St Louis, USA) solution (5 mL; 100 EU) was added to the protein suspension at 37 °C with constant stirring. The nitrogen contents of the sample and indigestible residue were determined by the Kjeldhal method (AOAC, 1990). Protein digestibility was calculated from the following equation:
Dough handling properties
The dough mixing properties of different wheat flour-watermelon seed protein concentrate blends were examined with Brabender farinograph (Brabender, Duisburg, Germany) according to the constant flour weight procedure (AACC, 1990). All dry ingredients were introduced into the dough mixer maintained at a temperature of 27 °C. Based on the preliminary tests, total water was added within 25 s and the sample run was completed in 25 min duration. The contents were mixed for 30 s at 47.5 rpm by addition of water to sample from a burette based on the pre-estimated values. The parameters of water absorption, arrival time, dough development time, dough stability time, mixing tolerance index and degree of softening were measured for all the samples.
Pasting properties were evaluated with the Starch Master (Newport Scientific Pvt. Ltd.; Warrie-wood, Australia) using a 13 min controlled heating and cooling profile with constant shear. Sample was held for 1 min at 50 °C, heated at 12 °C /min from 50 to 95 °C, held for 2.5 min at 95 °C, cooled at 12 °C /min to 50 °C, and held for 2 min at 50 °C. In each case, 2.5 g (d.b.) wheat flour supplemented with protein concentrates and 25 g accurately weighed distilled water, were added to the sample canister. The analysis used the standard temperature profile and followed idle and hold at 50 °C; 0–1 min at 50 °C, 1–4:45 min a ramp up to 95 °C; 4:45–7:15 min hold at 95 °C; 7:15–11 min cooling (set at 50 °C); hold at 50 °C to 13 min. Parameters recorded were pasting temperature (PT), peak viscosity (PV), hot paste viscosity (HPV) (minimum viscosity at 95 °C); cool paste viscosity (CPV) (final viscosity at 50 °C); breakdown (BD) = PV-HPV; and set back (SB) = CPV-HPV. The average was taken from three replicates.
Dough extensibility was measured on TA-XT2 Texture Analyser (Stable Microsystems Ltd, Godalming, UK) according to the methods of Verbruggen et al. (2001). Small amount of oil was applied to both sides of the dough to avoid sample adhesion. Dough sample (15 g) was clamped onto the grooved base of the form and cut into strips (10 mm × 10 mm × 70 mm strips of ~5.5 ± 0.5 g) by pushing down the upper block of Kieffer Dough of the texture analyser. The dough strips were placed onto the grooved region of the sample plate while, holding down the spring loaded clamp lever and the plate was inserted into the gluten extensibility rig. The handle was released slowly and the tensile test was performed. The pre-test speed was 2 mm/s, test speed 3.3 mm/s, post test speed 10.0 mm/s, distance 75 mm, trigger force 5 g with a data acquisition rate of 200 pps. The extensibility was described as the distance travelled (cm) from the moment that the hook touches the test piece or force (g) required to rupture of the test piece
Cookie preparation
Wheat flour was blended with watermelon seed protein concentrates at 2.5, 5.0, 7.5 and 10.0 % levels (w/w of 14 % wheat flour + protein blend) by gradual mixing in a planery mixer. A sugar cookie recipe and procedure described by McWatters et al. (2003) was used for cookie preparation. The basic ingredients were 300 g of flour blend, 180 g of hydrogenated vegetable shortening, 225 g of granulated cane sugar, 21 g of beaten whole egg, 3.75 g of salt and 1.8 g of baking powder. The dry ingredients (flour, sugar, salt and baking powder) were thoroughly mixed in a bowl by hand for 3–5 min. Vegetable shortening (180 g) was added and mixed until uniform. Egg was added and the mixture was needed in a mixer (Model A 907D, Kenwood, Haven’t, UK) for 3.5 min to get slightly firm dough. The dough was manually rolled on a pastry board into sheets of uniform thickness of 0.4 cm and cut into circular shapes of 5.8 cm diameter using a circular scone cutter. The cut dough pieces were transferred into oil-greased pans and baked at 180 °C for 10 min. The cookies were allowed to cool at room temperature (28 ± 1 °C) for 2 h.
Physical properties of cookies
The spread factor was measured according to the methods of AACC (1990). Spread factor (SF) was determined from the ratio of width and thickness of cookies.
The fracture force test was performed using a Texture Analyzer (TA-XT2, Stable Micro Systems, Surrey, UK). Fracture force was cutting probe (6 cm long and 0.1 mm thick). The analyzer was set at a ‘return to start’ cycle with a speed of 1 mm/s and a distance of 3 mm. The average force was calculated for four cookies and reported as fracture force (kg).
The cookie colour was determined using Hunter colour lab (Hunter Associates, Reston, USA). Calibration with black and white tiles was performed before colour measurement. Total colour difference (ΔE) was calculated as:
where,
- ΔL:
-
(L sample - L standard)
- Δa:
-
(a sample - a standard)
- Δb:
-
(b sample - b standard)
Sensory analysis
A panel of 30 consumers selected randomly were used to evaluate the sensory properties of cookies. The sensory analysis was performed in sensory laboratory with clean sensory cabinets containing fresh water and sinks. The cabinets were separated from each other by opaque glass frames. At each session, samples (20 g) were served on white saucers identified with three-digit code numbers to eliminate bias. Panellists were instructed to evaluate colour first and then to taste each sample to evaluate flavour, texture and overall acceptability. A nine-point hedonic scale with 1-dislike extremely, 5-neither like nor dislike, 9-like extremely was used (Larmond 1977). Samples that obtained 80 % of the scores in the ‘like (6–9 points)’ hedonic region for colour, flavour and texture, were considered acceptable. Water was provided to rinse the mouth between evaluations and covered expectoration cups if they did not wish to swallow the samples.
Statistical analysis
Mean values, standard deviation, and analysis of variance (ANOVA) were computed using a commercial statistical package SPSS 17.0 (SPSS Inc, Chicago, USA). These data were then compared using Duncan’s multiple range tests at 5 % significance level.
Results and discussions
Wheat flour and protein concentrate quality
Table 1 shows the composition and physicochemical properties of wheat flour and watermelon seed protein concentrates. Wheat flour reportedly showed protein content of 10 % whereas wet and dry gluten content of the wheat flour had 21.30 % and 88 % respectively. Mateera protein concentrate had 72.26 % protein content whereas Sugar baby protein concentrate reported slightly lower protein content (71.38 %). The ash content of wheat flour was 0.5 % whereas it was 0.4 % and 0.5 % respectively for Mateera and Sugar baby protein concentrates. SDS, sedimentation value of wheat flour was measured as 30.2 ml while diastatic activity was 265 mg/10 g flour. Protein concentrates reported a digestibility of 93.27 and 96.22 % respectively for Mateera and Sugar baby seed protein concentrates. High protein digestibility rates make them suitable protein ingredient for cookie preparation.
Dough handling properties
The farinogram and dough extensibility data of the wheat flour and watermelon protein concentrate blends is presented in Table 2. Farinograph helps in determining the amount of water to be added to a flour to achieve dough of fixed consistency, mixing characteristics and to predict the baking performance of flours. Water absorption of wheat flour (55.5 %) increased significantly (p ≤ 0.05) until 62.3 % and 62.5 % at 10 % fortification levels with Mateera and Sugar baby protein concentrates, respectively. However differences in protein concentrates did not showed variation in the water absorption. Singh and Mohamed (2007) studied the influence of gluten-soy blends on the cookie quality, reported that with the increase in soy protein isolate levels water absorption showed a significant increase. Likewise trend is reported for wheat flour fortified with tomato, fluted pumpkin, and lupin proteins (Sogi et al. 2002; Doxastakis et al. 2002; Giami and Barber, 2004). Increase in water absorption may be due to high affinity of hydrophilic groups in the mixing environment resulted in limited availability of water for granular form of starch. Differences were noted in arrival time of the wheat flour (1.1 min) and protein concentrate combinations. Arrival time significantly increased from 1.1 to 2.4 min and to 3.3 min respectively with Mateera and Sugar baby protein concentrates. Interestingly the delay in arrival time for Sugar baby protein concentrates-wheat flour blends was significantly (p ≤ 0.05) higher than Mateera protein concentrate blends. Dough development and stability decreased drastically with increasing levels of protein concentrates. Mixing tolerance significantly (p ≤ 0.05) increased with increased levels of protein concentrates in the dough. The drastic decrease in dough development and dough stability time is associated with decrease in gluten network and rapid uptake of water by protein concentrates (HadiNezhad and Butler 2009). Mixing tolerance of wheat flour (30 BU) increased from 45 to 120 BU and 60 to 140 BU respectively with blending of Mateera and Sugar baby protein concentrates. Sugar baby protein concentrate exhibited significantly (p ≤ 0.05) higher impact on mixing tolerance than Mateera protein concentrate. This may be probably due to high hydration index of Sugar baby proteins which offered more resistance to external force on farinograph. The degree of softening increased from 40 to 140 BU for Mateera protein concentrate blends while it was in the range of 50 to 120BU for the Sugar baby protein-wheat flour blends. Doxastakis et al. (2002) reported that substituted flours of lupin, soya and triticale flours delayed the arrival time, and decreased dough stability in wheat flour used for a bread system.
Dough extensibility was measured with texture analyser and is reported in Table 2. Dough extensibility is a measure of force (g) required to break the dough strip (10 mm × 10 mm × 70 mm strips of ~5.5 ± 0.5 g) With the increase in protein concentrate levels, dough extensibility of wheat flour dough (51.1 g) decreased from 38.7 to 37.0 g and 41.1 to 32.9 g respectively for Mateera and Sugar baby protein concentrates. Decrease in extensibility is due to the decrease in gluten network which imparts the visco-elasticity to the dough. Similar findings in dough extensibility were observed for soy-wheat flour blends and lupin, watermelon seed protein isolates, soya, triticale-wheat flour blends (Mishra et al. 1991; Doxastakis et al. 2002). Until 5 % level fluted pumpkin flour substitution, dough extensibility remained unchanged but with further increase in substitution levels extensibility was reported to decrease (Giami 2001). The responsible elastic nature of glutenin in dough extensibility has been elaborated by Khatkar (2004).
Pasting properties represent important quality criteria for performance of wheat flour dough during handling and baking. The pasting temperature did not showed any significant differences with the blending of protein concentrates. However, the peak viscosity, hot paste viscosity, cool paste viscosity, breakdown and set back viscosities decreased with increased levels of protein concentrate (Table 3). Peak viscosity of wheat flour (3234 cP) progressively decreased with increase in the protein concentrate levels. Peak viscosity decreased from 3234 to 2563.3 and 2586.0 cp respectively for the Mateera and Sugar baby protein concentrate blended flours. It is noteworthy to state that the peak viscosity in wheat flour dough is mainly attributed to gelatinization of starch granules. Increase in protein concentrate levels progressively decreased the starch content and thereby affected pasting properties. Similar pattern of results was observed for hot paste viscosity, cool paste viscosity, breakdown viscosity and set back viscosity of the blended dough. It was observed that the protein blends of Sugar baby concentrate exhibited significantly (p ≤ 0.05) higher values for the above said RVA parameters than the Mateera protein blends. Differences in the hydrophilic groups between the two proteins may be responsible for the Lorrenz et al. (1979) reported that with increased levels of fababean protein concentrates in wheat flour blends decreased the visco-amylograph values. Sogi et al. (2002) reported similar results on visco-amylograph properties for tomato seed protein-wheat flour blends.
Composition of cookies
Data on the composition of cookies is shown in the Table 4. Significant (p ≤ 0.05) increase in protein content was observed with fortification of protein concentrates. At 10 % fortification, protein content of cookies significantly increased to from 10.07 % (control) to 17.48 % and 16.17 % respectively with Mateera and Sugar baby protein concentrates. ANOVA showed significant (p ≤ 0.05) differences in the protein contents of cookies which were observed beyond 2.5 % fortification levels. The increase in protein content significantly (p ≤ 0.05) decreased the carbohydrate content of cookies. Giami and Barber (2004) reported that cookies fortified with pumpkin protein concentrates (5–25 %) levels showed an increase in protein content from 12.3 to 25.6 %. Similar increase in protein content has been observed for gluten-soy protein blended cookies, sunflower protein enriched cookies, and pulse flours (Lorrenz et al. 1979; Zucco et al. 201; Jia et al. 2011). In our previous studies, we observed that the watermelon seed proteins are rich source of amino acids with high amino acid score and biological value (Wani et al. 2011a, b). Therefore, protein improvement with watermelon protein concentartes shall result in amino acid balance and reduction of calories in the cookies. Fat, fibre and ash contents being the minor components did not show any specific trend and increase of the cookies.
The surface colour along with the texture of cookies is reported to be primary attributes being judged by the consumers. The total colour difference (∆E) of cookies significantly (p ≤ 0.05) increased with fortification levels (Table 5). The ∆E of control cookies (26.2) progressively increased from 26.8 to 31.4 and 26.5 to 31.4 respectively for cookies prepared from Mateera and Sugar bay protein concentrate bends. Progressive increase in protein concentration of cookies showed high degree of browning in the cookies. This might be probably due to high degree of non-enzymatic browning which occurs between the amino acids and the sugars. Fortification of cookies with soy protein isolate, watermelon seed protein isolate resulted in increased concentration of the reactants along with high baking temperatures mainly responsible for the increase in browning reaction of cookies. Singh and Mohamed (2007) reported that with increase in soy protein isolate concentration of cookies resulted in darker colour of cookies. Studies on effect of pumpkin, pulse and sunflower protein in cookies corroborate with our results on changes in surface colour (Giami and Barber 2004; Zucco et al. 2011; Škrbic and Cvejanov 2011). The cookie spread factor (7.9) progressively increased up to 8.4 with fortification levels of 7.5 %. However, further increase in protein concentration significantly (p ≤ 0.05) decreased cookie spread factor for blends (Table 5). The decrease in spread factor of wheat-watermelon protein fortified cookies is in agreement with the studies on wheat-pulse, wheat-soybean, wheat-sunflower and wheat-cowpea protein enriched cookies (Shrestha and Noomhorm 2002; McWatters et al. 2003; Zucco et al. 2011; Jia et al. 2011). Increase in hydrophilic sites due to watermelon proteins lead to greater water retention thereby affecting the cookie spread factor (Hooda and Jood 2005).
The cookie texture or fracture force measured as a peak force required to snap the cookie is an important measure of the cookies. Data on cookie fracture force (kg) of control (3.8 kg) significantly (p ≤ 0.05) increased from 5.2 to 14.8 kg and 6.2 to 15.2 kg for Mateera and Sugar baby protein concentrate blends (Table 5). Data showed that non significant differences in fracture force of control and protein fortified (2.5 %) cookies was observed. Beyond this protein blending level the cookie fracture force drastically increased with a maximum force of 14.8 and 15.2 kg for cookies blended with 10 % Mateera and Sugar baby protein concentrates. The cookie surface cracks were also observed almost negligible at 10 % protein concentrate levels. Differences in cookie texture may be due to differences in protein carbohydrate interaction or protein-protein interactions.
Sensory analysis
Sensory properties as determined by a random panel judged the appearance, colour, aroma, taste, texture, and overall acceptability of the control and protein blended cookies (Table 6). Sensory scores of control and protein fortified (2.5–10 %) cookies showed non-significant (p > 0.05) differences with regard to different sensory parameters. However, the parameters showed a downfall trend with increasing levels of protein concentrates in the cookies. The cookies fortified beyond 7.5 % protein levels showed decrease in surface cracks which is a disagreeable characteristic in cookies. However, present studies revealed that the incorporation up to 7.5 % level was acceptable. Sensory properties may further be optimised with the addition of flavourings and the dough improvers.
Conclusions
The present study reveals that the nutritive value of cookies could be improved by the addition of inexpensive watermelon seed protein concentrates. Watermelon protein concentrates affected most of the dough handling properties as measured by farinograph, rapid visco analyzer and texture analyzer. The protein concentrate-wheat flour blends showed increase in water absorption, arrival times and mixing tolerance while dough extensibility values decreased. Except pasting temperature, peak viscosity, hot paste viscosity, cool paste viscosity, breakdown viscosity and set back viscosity significantly decreased with increased levels of protein concentrates in wheat flour. Substantial increase in protein content of cookies also resulted in reduction of total carbohydrate value of cookies. The colour values significantly increased as a consequence of non-enzymatic browning in the cookies supplemented with protein concentrates. A decrease in spread factor of protein supplemented cookies was observed with the proportional decrease in fracture force of cookies at different levels of protein fortification. The sensory properties were acceptable with 5 % to 7.5 % fortification levels. Although the differences between the control and protein supplemented cookies our investigation revealed the possible use of inexpensive watermelon seed proteins for partial replacement of gluten in the cookies. Further studies need to be carried on the use of watermelon protein hydrolysates in fruit juices and other liquid products.
References
AACC (1990) Approved methods of the american association of cereal chemists. American Association of Cereal Chemists, USA
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC
Arshad MU, Anjum FM, Zahoor T (2007) Nutritional assessment of cookies supplemented with defatted wheat germ. Food Chem 102:123–128
Bhatty RS, Finlayson AJ (1973) Extraction of non-protein nitrogen from oil seed meals with different solvents. Cereal Chem 50:329–336
Doxastakis G, Zafiriadis I, Irakli M, Marlani THC (2002) Lupin, soya and triticale addition to wheat flour dough’s and their effect on rheological properties. Food Chem 77:219–227
El-Adawy A, Taha KM (2001) Characteristics and composition of watermelon, pumpkin, and paprika seed oils and flours. Food Chem 49:1253–1259
Firatligil-Durmus E, Evranuz O (2010) Response surface methodology for protein extraction optimization of red pepper seed (Capsicum frutescens). LWT - Food Sci Technol 43:226–231
Fustier P, Castaigne F, Turgeon SL, Biliaderis CG (2009) Impact of commercial soft wheat flour streams on dough rheology and quality attributes of cookies. J Food Eng 90:228–237
Giami SY, Barber LI (2004) Utilization of protein concentrates from ungerminated and germinated fluted pumpkin (Telfairia occidentalis Hook) seeds in cookie formulations. J Sci Food Agric 84:1901–1907
Giami SY (2001) Rheological and bread-making properties of wheat fluted pumpkin seed flour blends. J Dairy Foods Home Sci 20:41–45
HadiNezhad M, Butler F (2009) Effect of flour type and dough rheological properties on cookie spread measured dynamically during baking. J Cereal Sci 49:178–183
Hooda S, Jood S (2005) Organoleptic and nutritional evaluation of wheat biscuits supplemented with untreated and treated fenugreek flour. Food Chem 90:427–435
Jia C, Huang W, Abdel-Samie MAS, Huang G, Huang G (2011) Dough rheological, Mixolab mixing, and nutritional characteristics of almond cookies with and without xylanase. J Food Eng 105:227–232
Kaur D, Sogi DS, Wani AA (2006) Degredation kinetics of lycopene and visual color in tomato peel isolated from pomace. Int J Food Prop 9:781–789
Khatkar BS (2004) Dynamic rheological properties and bread-making qualities of wheat gluten: effects of urea and dithiothreitol. J Sci Food Agric 85:337–341
Larmond E (1977) Laboratory methods for sensory evaluation of foods, Canada: Department of Agriculture pp 33–37, 57
Lorrenz K, Dilsaver W, Wolt M (1979) Fababean flour and protein concentrate in baked goods and in pasta products. Baker Digest 53:39–42
McWatters KH, Ouedraogo JB, Resurrection AVA, Hung YC, Philips RD (2003) Physical and sensory characteristics of sugar cookies containing mixtures of wheat, fonnio (Digitaria exilis) and cowpea (Vigna unguiculata) flours. Int J Food Sci Technol 38:403–410
Misra P, Usha MS, Singh S (1991) Soy-wheat flour blends: chemical, rheological and baking characteristics. J Food Sci Technol 56:992–998
Naczk M, Diosady LL, Rubin LJ (1985) Functional properties of canola meals produced by a two-phase solvent extraction system. J Food Sci 50:1685–1692
Saunders RM, Connor MA, Booth AN, Bickoff EM, Kohler GO (1973) Measurement of digestibility of alfalfa protein concentrates by in vivo and in vitro methods. J Nutr 103:530–535
Shrestha AK, Noomhorm A (2002) Comparison of physico-chemical properties of biscuits supplemented with soy and kinema flours. Int J Food Sci Technol 37:361–368
Singh M, Mohamed A (2007) Influence of gluten–soy protein blends on the quality of reduced carbohydrates cookies. LWT - Food Sci Technol 40:353–360
Škrbic B, Cvejanov J (2011) The enrichment of wheat cookies with high-oleic sunflower seed and hull-less barley flour: Impact on nutritional composition, content of heavy elements and physical properties. Food Chem 124:1416–1422
Sogi DS, Sidhu JS, Arora MS, Garg SK, Bawa AS (2002) Effect of tomato seed meal supplementation on dough and bread characteristics of wheat (PBW - 343) flour. Int J Food Prop 5:563–572
Verbruggen IM, Veraverbeke WS, Delcour JA (2001) Significance of LMW-GS and HMW-GS for dough extensibility: addition’ versus ‘Incorporation’ protocols. J Cereal Sci 33:253–260
Wang HX, Ng TB (2003) Isolation of cucurmoschin, a novel antifungal peptide abundant in arginine, glutamate and glycine residues from black pumpkin seeds. Peptide 24:969–972
Wani AA, Kaur D, Ahmed I, Sogi DS (2008) Optimization of watermelon seed protein using response surface methodology. LWT - Food Sci Technol 41:1514–1520
Wani AA, Sogi DS, Singh P, Wani IA, Shivhare US (2011a) Characterisation & functional properties of watermelon (Citrullus lanatus) seed proteins. J Sci Food Agric 91:113–191
Wani AA, Sogi DS, Grover L, Saxena DC (2006a) Effect of temperature alkali concentration, mixing time and meal/solvent ratio on the extraction of watermelon seed proteins-a response surface approach. Biosyst Eng 94:67–73
Wani AA, Sogi DS, Singh P, Shivhare US (2011b) Characterisation and functional properties of Watermelon (Citrullus lanatus) seed protein isolates and salt assisted protein concentrates. Food Sci Biotechnol 20:877–887
Zoulias EI, Oreopoulou V, Tzia C (2002) Textural properties of low-fat cookies containing carbohydrate- or protein-based fat replacers. J Food Eng 55:337–342
Zucco F, Borsuk Y, Arntfield SD (2011) Physical and nutritional evaluation of wheat cookies supplemented with pulse flours of different particle sizes. LWT - Food Sci Technol 44:2070–2076
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wani, A.A., Sogi, D.S., Singh, P. et al. Influence of watermelon seed protein concentrates on dough handling, textural and sensory properties of cookies. J Food Sci Technol 52, 2139–2147 (2015). https://doi.org/10.1007/s13197-013-1224-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1224-3