Abstract
Prevalence of obesity and type-2-diabetes requires dietary manipulation. It was hypothesized that wheat-legume-composite breads will reduce the spike of blood glucose and increase satiety. Four pan bread samples were prepared: White bread (WB) as standard, Whole-wheat bread (WWB), WWB supplemented with chickpea flour at 25 % (25%ChB) and 35 % (35%ChB) levels. These breads were tested in healthy female subjects for acceptability and for effect on appetite, blood glucose, and physical discomfort in digestion. The breads were rated >5.6 on a 9-point hedonic scale with WB significantly higher than all other breads. No difference in area under the curve (AUC) for appetite was found, but blood glucose AUC was reduced as follows: 35%ChB < WB and WWB, WB >25%ChB = WWB or 35%ChB. We conclude that addition of chickpea flour at 35 % to whole wheat produces a bread that is acceptable to eat, causing no physical discomfort and lowers the glycemic response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbohydrate foods are classified as low or high glycemic index (GI) depending upon the rate of their digestion and absorption and their relative impact on raising blood glucose concentration. GI of a food is measured by comparing the rise in blood glucose to that of a reference food (glucose or white bread) containing the same amount (25 g or 50 g) of carbohydrate consumed by the same individual on two different days (FAO/WHO 1998). This characteristic of carbohydrate foods is determined by the molecular structure of their starch. Starches with high amylose are slowly digested and absorbed (McCrory et al. 2010). Retrogradation, which is faster in amylose, further reduces digestibility of the starch, rendering some starch into resistant starch. Resistant starch does not hydrolyze to produce glucose. Thus carbohydrate foods with high amylose and resistant starch content prevent abrupt increase of blood glucose after consumption and are classified as having a low GI (Alter et al. 2011).
The wheat flour protein, gluten, has a unique structural forming property that confers on it excellent bread-making properties, making wheat bread a popular staple food consumed worldwide on a daily basis. Nutritional limitations of a wheat bread are its low content of essential amino acids (Hefnawy et al. 2012) and its high GI (Foster-Powell et al. 2002). However, GI is not solely responsible for the rise in blood glucose concentration with a carbohydrate food, glycemic response is also influenced by the glycemic load (GL) or the amount of high glycemic carbohydrate in the food (Venn and Green 2007). Habitual consumption of high GI foods at high GL is associated with tissue and organ damage such as microangiopathy, diabetic nephropathy or retinopathy whereas low GI foods consumed at moderate GL are associated with reduced rates of coronary arterial diseases and type-2 diabetes (Brownlee 2003; Liu et al. 2000).
Over the years research to improve bread quality has focused on enhancing the protein nutritional value of bread by supplementation with legumes flours (Ranhotra and Loewe 1974; Onoja et al. 2011). In view of the recent global epidemic of obesity and type 2-diabetes, research is now also needed to improve the glycemic response of breads, again possibly through use of legume supplementation. Chickpeas are a good source of dietary fiber, and of the vitamins, minerals and essential amino acids that are deficient in wheat flour (Onoja et al. 2011; Hefnawy et al. 2012). The starch content of chickpea and wheat flours are 51 % and 64 %, respectively (Idriss et al. 2012). The higher percentage of amylose (30–40 %) in chickpea starch compared to the 20 % in wheat starch (Guillon and Champ 2002) makes chickpea starch more resistant to digestion (Muir and O’Dea 1992). The fiber content of chickpeas is 16.4 % (Berrios et al. 2010) versus 12.2 % in whole-wheat flour and 3 % in white flour (Idriss et al. 2012) and the protein content of raw chickpea flour was reported as 25.5 % compared to 11.9 % in wheat flour (Idriss et al. 2012). Success in developing a legume-supplemented bread with good consumer acceptability is, however, a challenge due to changes in physical and sensory properties of the bread such as development of a tougher texture, reduced loaf volume, darker color, and altered flavor (Doblado-Maldonado et al. 2012; Fenn et al. 2010).
Interest in chickpeas as a nutritional food product has generated valuable data recently, but most research has used the whole product, rather than chickpea flour and little data is available on chickpea flour as a supplement to wheat bread or its effect on bread’s glycemic response. The only study (Johnson et al. 2005) incorporating chickpea flour at a level that should have been sufficient to affect blood glucose (25 % in white bread), did not produce a significant reduction on glycemic or insulinemic responses. Possibly this level is still too low, or the GL of 50 g of available carbohydrate overwhelmed the significant effect of chickpea supplementation, or other components of the breakfast meal in which the chickpea supplemented bread was fed interfered with the glycemic response. The current study was, therefore, designed to test breads with chickpea flour incorporated at 25 or 35 % of total flour, fed at equal weights of bread rather than of available carbohydrate (total carbohydrates minus fiber), and fed alone with only 5 g of butter and a glass of water to ease swallowing. We hypothesized that the addition of 25 or 35 % chickpea flour to whole-wheat flour bread (WWB) would reduce the rapid spike of blood glucose and the overall glycemic response as well as increase satiety in young female healthy subjects, but will not significantly compromise the sensory attributes of the WWB. Thus, the objective of the study was to develop a whole wheat flour bread supplemented with chickpea flour that lowers glycemic response, decreases appetite and is acceptable to consumers. Kuwait being number one among the Arab countries in the prevalence of obesity, with an incidence 3.6 times higher in females than in males (Badr et al. 2012), we chose to conduct the study using female subjects.
Materials and methods
Bread preparation and treatments
Four experimental pan breads, whole-wheat flour bread (WWB), 25 % chickpea flour supplemented WWB (25%ChB), 35 % chickpea flour supplemented WWB (35%ChB) and white flour bread (WB) as the reference standard were prepared under controlled conditions according to the formulation given in Table 1. These breads were made using the optimized straight dough bread making method of AACC (method 10-10B, AACC 1983) as explained elsewhere (Sidhu et al. 2004). Both white flour and whole wheat flour were obtained from the Kuwait Flour Mills and Bakeries Co, Shuwaikh. The chickpea flour (Majdi Food Center, Kuwait) was purchased from the local market.
All four breads were made in triplicate following a procedure given elsewhere (Sidhu et al. 2004). Briefly, all dry ingredients and water were mixed until optimal dough development was achieved and the dough centered around the mixing hook leaving the mixing bowl completely clean. The dough was weighed and kept in the fermentation cabinet (National Manufacturing Co. Nebraska, USA) at a relative humidity of 85–90 %, and temperature of 30 °C for 52 min. Dough was punched and fermented twice for another 25 and 13 min, then molded in bread shape, panned and left for a final proofing for 33 min. It was baked in the oven for 24 min at 220 °C. Bread weight was measured immediately after baking, and percentage loss in fermentation and baking calculated. Bread loaf volume was measured following baking by rapeseed displacement method and specific volume (cc/g) calculated (Table 2). Two separate batches of breads were made: one for sensory evaluation, one to test glycemic and satiety responses. Both batches were made in bulk to minimize loaf differences; they were sliced and portions were weighed out (50 g) for individual servings (around 2–3 slices) before freezing in sandwich bags at −20 °C.
Bread was removed from the freezer and refrigerated 1 day before testing. To prevent an effect of variation in temperature on appetite response, bread was toasted in a toaster for the same time and temperature setting 5 min prior to consumption for each subject. Available carbohydrate content of the WB, a reference bread, was calculated as total carbohydrate minus fiber content of the commercial bread (Kuwait Flour Mills and Bakeries Co), purchased from the local market. The weight of white bread (50 g) supplying 25 g available carbohydrate was used as the weight for all the breads.
Sensory and physical evaluation of bread
Sensory analysis of the bread samples was conducted by young female college students (n = 40), who were blinded to the breads and asked to evaluate them for physical and sensory characteristics: color (desirable or not), texture (lightness or denseness), volume (risen or compact), flavor and mouth feel (sweet or bitter, soft or hard) and overall acceptability. A score below 4 on a 9-point hedonic scale was considered as unacceptable.
Experimental procedures
Subjects
A second set of healthy young females (n = 13) age 17–25 years (mean ± SD, of 21.4 ± 2.3) with a body mass index (BMI in kg/m2) of 23.6 ± 2.4 were recruited through flyers and word of mouth at the College for Women, Kuwait University. Sample size was determined based on power analyses from the results of a previous similar study to detect a difference among the test treatments on glycemic responses with α 0.05 and β 0.08 (Zafar et al. 2011). Diabetics, those taking medication, breakfast skippers, and restrained eaters were excluded from the study (Polivy et al. 1978). Subjects were only scheduled for test sessions during follicular phase of their menstrual cycle to avoid any hormonal effect on appetite and blood glucose. The study protocol was approved by the Humans’ Ethics Committee of the Kuwait University. All subjects had signed the informed consent forms before the study.
Study protocol
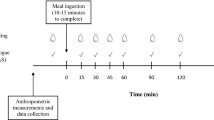
A within subject repeated measures design was used. Subjects came for all the sessions between 8.30 and 10.30 am (2 h after waking up) to the study room at the College for Women, Kuwait University after an overnight fast, water permitted up to 1 h before the scheduled start time. Subjects consumed the test breads in a randomized sequence on four different visits, 6 to 12 days apart accommodating the menstrual cycle. Subjects were advised to finish all the given food within 10 min. They were seated in individual booths for bread consumption.
Blood samples for blood glucose were collected at 0, 15, 30, 45, 60 and 90 min by finger-prick, using Monojector Lancet Devices and analyzed with a portable blood glucose monitoring system (One Touch Ultra, Life Scan Inc and Johnson & Johnson Company, USA). Incremental blood glucose response and area under the blood glucose response curves (AUC), ignoring any area below fasting level, was determined for each test-bread for each subject.
Subjective appetite and physical comfort
Subjective appetite information was collected at baseline, 15, 30, 45, 60 and 90 min using visual analogue scales (VAS) as given elsewhere (Flint et al. 2000). Briefly, each VAS consisted of a 100 mm line anchored at each end with opposing statements for each of the 4 questions : 1) How strong is your desire to eat? (very weak to very strong), 2) How hungry do you feel? (not hungry at all to as hungry as I’ve ever felt), 3) How full do you feel? (not full at all to very full), and 4) How much food do you think you could eat? (nothing at all to a large amount). The subjects were to mark an “X” on the line to indicate their feelings at that given moment. The magnitude of these feelings was determined by measuring the distance (mm) from the left end starting point to the intersection of the “X” on the line. Subjective appetite was the average score for each time point using the formula:
Average appetite = [desire to eat + hunger + (100 − fullness) + prospective consumption]/4 (Flint et al. 2000).
As pulses and legumes may cause discomfort such as stomach pain or bloating in some people, subjects also assessed their physical comfort by VAS at baseline, 30, 60 and 90 min using the question, “How well do you feel?” with options such as “not well at all” or “very well” given at the opposite ends of the line.
Statistical analyses
Statistical analyses were conducted using SPSS (Statistical Package for Social Sciences, IBM Statistics version 20). Physical and sensory parameters of the breads were evaluated by comparing the means using one-way ANOVA followed by Tukey’s post hoc test with significant level at p < 0.05. Data are reported as means ± SD.
Two-way analysis of variance (ANOVA) was conducted on absolute blood glucose concentration at each time point and on change in appetite at each time point and on physical comfort measurements, to assess treatment, time and any time by treatment interaction over 90 min followed by Tukey’s post hoc tests to identify significant mean differences among treatments at each time point of measurements. One-way repeated-measures ANOVA was performed to test for the effect of bread treatments on area under the curve (AUC) for blood glucose and average appetite and tested for significant difference between treatments with Tukey’s post hoc tests. Data were normally distributed and presented as means ± SEM. Correlation analyses were conducted using the Pearson correlation coefficients. Significance level was set at p < 0.05.
Results and discussion
Bread characteristics
The bread characteristics are presented in Table 2. Dough weight of WWB was higher than WB, 25%ChB or 35%ChB. The higher dough weight of WWB compared to WB, 25%ChB or 35%ChB indicates its higher water holding capacity. Its moisture retention during baking was also higher, resulting in its higher weight as compared to the other breads. Addition of chickpea flour imparted additional fiber and protein to the wheat flour (Bojnanska et al. 2012) and thus should have further enhanced water absorption (Tosh and Yada 2010) but this was not observed in the present study perhaps because of partial replacement of insoluble fiber from whole wheat flour with the soluble fiber in chickpea flour. WB had greater absolute and specific volumes than WWB, 25%ChB or 35%ChB (Table 2 and Fig. 1). The higher fiber content of whole wheat flour probably interfered with the action of the gluten that is responsible for structural formation (Doblado-Maldonado et al. 2012). Gluten formation gives bread its special structure by retaining the expanding gases during baking that raises the volume of bread (Tosi et al. 2011). Replacing whole-wheat flour with added chickpea flour reduced the amount of gluten 25–35 % thus progressively reducing loaf and specific volume of breads (Fig. 1, Table 2). A similar decrease in bread volume had been observed with the addition of 40 % or more chickpea flour, whereas bread volume and overall quality was reported to improve with 20 % (Bojnanska et al. 2012) or up to 30 % (Hefnawy et al. 2012).
Physical and sensory evaluation of bread
In all the parameters such as color, texture, flavor and mouth feel, the experimental breads were not significantly different from the WWB except for the 35%ChB that had slightly reduced flavor (Table 3). All WWBs were lower in all the parameters including color, texture, volume and taste compared to WB, however, they were still rated above 5.6 on a 9 point scale reflecting their consumer acceptability. No significant difference in bread palatability was reported earlier for chickpea flour supplementation at 25 % (Johnson et al. 2005) or at 15–30 % (Hefnawy et al. 2012).
Blood glucose response
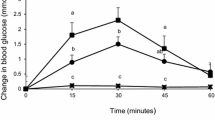
Blood glucose changes over 90 min were affected by both bread treatment (p < 0.0001) and time (p < 0.0001) and a time by treatment interaction was present (p < 0.029) (Fig. 2). The blood glucose concentrations peaked from the baseline at 30 min similarly after WWB, 25%ChB and 35%ChB and at 45 min after WB, then blood glucose started to decline to the baseline (glucose disposal phase). At its peak, blood glucose concentrations were significantly lower after WWB and 25%ChB (p < 0.01) or 35%ChB (p < 0.0001) compared to WB. During the glucose disposal phase, WB showed a faster decline in blood glucose concentration than the other three breads, bringing the glucose level down at 60 min but at 90 min it was still significantly higher than all the whole wheat flour breads, with or without chickpea flour supplementation (p < 0.01) (Fig. 2).
Effect of bread treatment on blood glucose (mmol/L) over 90 min. Values within the same time points are significantly different at p < 0.01, (n = 13). Legends are: WB = white flour bread, WWB = whole wheat flour bread, 25%ChB = whole wheat flour supplemented with 25 % chickpea flour, 35%ChB = whole wheat flour supplemented with 35 % chickpea flour
The blood glucose incremental areas under the curves (AUC) calculated over 90 min showed significant differences among the breads by ANOVA (p < 0.001). The differences are as follows: 25%ChB = WWB WB (p < 0.05), 35%ChB < WB (p < 0.0001) or < WWB (p < 0.03), but = 25%ChB (p = 0.08) (Fig. 3).
Effect of bread treatment on blood glucose incremental area under the curve (AUC) over 90 min. Values with different letter are significantly different at p < 0.05, (n = 13). Legends are the same as given for Fig. 2
Our results demonstrated that replacing 25 % or 35 % of whole wheat flour with chickpea flour substantially reduced the glycemic responses of WWB compared to the WB. Probably, both its soluble fiber and the lower available carbohydrate due to its resistant starch content reduce intestinal glucose absorption resulting in this low glycemic response. However, these results are in contrast with a previous study (Johnson et al. 2005), which showed no difference in the glycemic response when 25 % white wheat flour was replaced by original or extruded chickpea flour.
It is not clear whether the difference in glucose response between these studies was due to the differences in the composition of chickpea flours, or the amount of carbohydrate consumed. In the earlier study, test breads were fed in sufficient quantity (98–114 g) to provide 50 g of available carbohydrate, which is double or more than the carbohydrate and bread used in the current study where 50 g of bread provided 25 g of carbohydrate from the WB and possibly lower amounts from the other breads due to dilution by fiber present in whole wheat (Idriss et al. 2012) and chickpea flour (Berrios et al. 2010). Another difference between the two studies was the addition of chickpea flour to white flour in the previous study versus addition to whole wheat flour in the current study. White flour, high in starch and low in fiber, produces a higher glucose and insulin response than does whole-wheat flour (Alter et al. 2011; Liu 2002). Insulin secretion was reportedly high after the WB or chickpea supplemented white breads, causing excessive removal of glucose from the blood and hypoglycemia at 60 min after all breads, a pattern that is similar to a high GI food (Johnson et al. 2005). Hypoglycemia was, however, not seen with any of the breads in the current study.
Although, chickpea flour has high amylose, resistant starch and dietary fiber contents, our results indicate that the addition of chickpea flour at 25 % level may not be sufficient to affect the glycemic response as no difference was found between the WWB and 25%ChB. Similar negative results in the glycemic response between the WB and 25 % chickpea supplemented WB were reported earlier (Johnson et al. 2005). Also no significant differences have been found between the in vitro carbohydrate digestibility measures of WB and 25 % chickpea flour supplemented WB (Hawkins and Johnson 2005). On the contrary, similar levels of chickpea flour supplementation in pasta have been reported to reduce its in vitro digestibility (Chillo et al. 2010). Thus the difference in glycemic response between WB and WWB in the current study could be due to the fiber content of the WWB that reduced the spike in glucose response, with an additional effect from the chickpea flour seen only at 35 % supplementation.
However, WWB and WB have been reported to have similar GI (Foster-Powell et al. 2002) despite their difference in the fiber content, perhaps because these breads were fed at levels to provide the same amount of available carbohydrate. In the current study, WWB showed a 36 % lower total glycemic effect than WB, which might be because the breads were fed on a bread weight basis rather than on the basis of available carbohydrate. This lower glycemic response of the WWB could be because of the dilution effect of the fiber or of the moisture retained by whole wheat flour bread or both on the starch content. Because of the moisture retention property of the insoluble fiber, bread slices of WWB weighed more than comparable sizes of WB (Table 2).
These results emphasize the impact of glycemic load (GL) of a food on its glycemic response (Venn and Green 2007). Replacing whole-wheat flour with 25 % or 35 % chickpea flour might have reduced the amount of starch and thus the GL resulting in reduced glycemic effect. Chickpea flour supplemented to whole-wheat flour flatbread (roti) has similarly been shown to significantly reduce blood glucose response compared to regular whole-wheat flour flatbread (Radhika et al. 2010).
Subjective appetite, physical comfort and relationship among the dependent variables
The average of appetite responses declined from the baseline after the consumption of all breads up to 15 min then gradually increased over time. The two-way repeated measures ANOVA followed by Tukey’s post hoc analyses suggested a time effect (p < 0.0001) but no treatment effect (P > 0.05), though a time by treatment interaction trend was present (P = 0.07). There was no difference among the breads at any time except at 90 min, when after WB subjects were found to be less hungry than after 35%ChB (Table 4).
No statistically significant physical discomfort was recorded by the subjects for any bread (data not shown). There was no association found between the incremental area under the blood glucose curves and change in appetite curves integrated over 90 min (r = 0.015; p = 0.92).
Although pulses including chickpeas are low GI foods and are considered to promote satiety, subjects were surprisingly hungrier at 90 min after the 35%ChB compared to WB. These results do not support the hypothesis that a reduction in blood glucose spike is associated with increased satiety. The lack of association between the low glycemic effect of the chickpea supplemented breads with satiety could be due to the reduced energy availability from these breads because of their higher amounts of resistant starch and dietary fiber.
A limitation of this study was that we did not analyze the breads for proximate composition or resistant starch content which would have permitted calculation of their resistant starch and thus energy density of the breads. However, earlier studies had shown either no or inverse association (Wong et al. 2009) between energy density and satiety. In a rodent study, rats consumed more food from diets with high amylose resistant starch compared to rats fed the control diet without increase in body weight (Zafar et al. 2009).
Despite higher appetite responses after the 35%ChB at 90 min, one-way ANOVA showed no difference among the appetite AUC for these bread samples. These results are similar to the previous study (Johnson et al. 2005) showing no observed difference in satiety between WB and chickpea supplemented WB. The absence of change in subjective appetite could be due to variability in the subjective appetite measurements. A larger sample size might be needed to demonstrate an appetite effect than that was used in the current study. The sample size determination was based on magnitude of the glycemic response rather than on changes in subjective appetite.
Conclusion
Addition of 35 % chickpea flour to whole wheat bread significantly reduced the blood glucose response compared to WB or WWB, whereas, addition of 25 % chickpea flour reduced the response compared to WB but not to WWB. Apparently 25%ChB contained insufficient chickpea flour to overcome the glycemic effect of WWB. Both the chickpea supplemented breads had substantial effect against WB, which has negligible amount of dietary fiber.
Although its net appetite AUC was not different from the other breads, 35%ChB did not suppress appetite at 90 min as did the other breads. This decreased satiety could have resulted from its reduced energy density due to the higher dietary fiber and resistant starch contents. Sensory and physical quality of the breads decreased dose dependently by the addition of chickpea flour, yet considering the overall quality by the consumers, the breads were acceptable to eat.
Thus chickpea flour supplementation to whole wheat bread at the minimum level of 35 % can improve its glycemic responses without causing any physical discomfort or adverse effect on the organoleptic quality of the pan bread.
References
AACC (1983) American association of cereal chemists. St. Paul MN: American Association Cereal Chemistry.
Alter EEEJG, Abete I, Astrup A, Martinez JA, van Baak MA (2011) Starches, sugars and obesity. Nutrients 3(3):341–369
Badr HE, Shah NM, Shah MA (2012) Obesity among Kuwaitis aged 50 years or older: prevalence, correlates and comorbidities. Gerantologist. doi:10.1093/geront/gns108
Berrios JDJ, Morales P, Camara M, Sanchez-Mata MC (2010) Carbohydrate composition of raw and extruded pulse flours. Food Res Int 43:531–536
Bojnanska T, FrancaKova H, Liskova M, Tokar M (2012) Legumes - The alternative raw materials for bread production. JMBFS 1(February special issue):876–886
Brownlee M (2003) A radical explanation for glucose-induced beta cell dysfunction. J Clin Invest 112:1788–1790
Chillo S, Monro JA, Mishra S, Henry CJ (2010) Effect of incorporating legume flour into semolina spaghetti on its cooking quality and glycemic impact measured in vitro. Int J Food Sci Nutr 61(2):149–160
Doblado-Maldonado AF, Pike OA, Jess C, Sweley JC, Rose DJ (2012) Key issues and challenges in whole wheat flour milling and storage. J Cereal Sci 56:119–126
FAO/WHO (1998) Expert consultation. FAO Food Nutr Pap 66:1–140
Fenn D, Lukow OM, Humphreys G, Fields PG, Boye JI (2010) Wheat-legume composite flour quality. Int J Food Prop 13:381–393
Flint A, Raben A, Blundell JE, Astrup A (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes 24(1):38–48
Foster-Powell K, Holt S, Brand-Miller J (2002) International table of glycemic index and glycemic load values. Am J Clin Nutr 76:5–56
Guillon F, Champ MM (2002) Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr 88(Suppl 3):S293–S306
Hawkins A, Johnson SK (2005) In vitro carbohydrate digestibility of whole-chickpea and chickpea bread products. Int J Food Sci Nutr 56(3):147–155
Hefnawy TM, El-Shourbagy GA, Ramadan MF (2012) Impact of adding chickpea (Cicer arietinum L.) flour to wheat flour on the rheological properties of toast bread. Int Food Res J 19(2):521–525
Idriss M, Ahmed AR, Senge B (2012) Dough rheology and bread quality of wheat–chickpea flour blends. J Food Sci Technol 36:196–202
Johnson SK, Thomas SJ, Hall RS (2005) Palatability and glucose, insulin and satiety responses of chickpea flour and extruded chickpea flour bread eaten as part of a breakfast. Eur J Clin Nutr 59:169–176
Liu S (2002) Intake of refined carbohydrates and whole grain foods in relation to risk of type-2 diabetes mellitus and coronary heart disease. J Am Coll Nutr 21(4):298–306
Liu S, Willet WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE (2000) A prospective study of dietary glycemic load, carbohydrate intake and risk of coronary heart disease in US women. Am J Clin Nutr 71:1455–1461
McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE (2010) Pulse consumption, satiety, and weight management. Adv Nutr 1:17–30
Muir JG, O’Dea K (1992) Measurement of resistant starch: factors affecting the amount of starch escaping digestion in vitro. Am J Clin Nutr 56:123–127
Onoja US, Odo GE, Dibua UME, Eze JI (2011) Physico-chemical properties, energy, mineral, vitamin and sensory evaluation of wheat-based bread supplemented with legume, root, tuber and plantain flour. Glob J Pure Appl Sci 17(3):319–327
Polivy J, Herman CP, Warsh S, Villaume C, Beck B, Rohr R, Pointel JP, Debry G (1978) Internal and external components of emotionality in restrained and unrestrained eaters. J Abnorm Psychol 87:497–504
Radhika G, Sumathi C, Ganesan A, Sudha V, Henry CJK, Mohan V (2010) Glycemic index of Indian flatbreads (rotis) prepared using whole wheat flour and ‘atta mix’-added whole wheat flour. Br J Nutr 103:1642–1647
Ranhotra GS, Loewe RJ (1974) Bread making characteristics of wheat flour fortified with various commercial soy protein products. Cereal Chem 51:629–634
Sidhu JS, Hooti SN, Al-Saqer JM, Al-Amiri HA, Al-Foudari M, Al-Othman A, Hamad A, Al-Haji L, Ahmed N, Mansoor IB, Minal J (2004) Developing functional foods using red palm olein: pilot-scale studies. Int J Food Prop 7(1):1–13
Tosh SM, Yada S (2010) Dietary fibers in seeds and fractions: characterization, functional attributes, and applications. Food Res Int 43:450–460
Tosi P, Gritsch CS, He J, Shewry PR (2011) Distribution of gluten proteins in bread wheat (Triticum aestivum) grain. Ann Bot 108(1):23–35
Venn BJ, Green TJ (2007) Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr 61(1):S122–S131
Wong CL, Mollard RC, Zafar TA, Luhovyy BL, Anderson G (2009) Food intake and satiety following a serving of pulses in young men: effect of processing, recipe, and pulse variety. J Am Coll Nutr 28(5):543–552
Zafar TA, Martin B, Weaver CM (2009) Resistant starches (RS2 and RS3) have variable effects on bone mineral status in rats. Open Nutr J 3:17–22
Zafar TA, Kabir Y, Ghazaii C (2011) Low glycemic index foods suppress glycemic responses, appetite and food intake in young Kuwaiti females. Kuwait J Sci Eng 38(1A):111–123
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zafar, T.A., Al-Hassawi, F., Al-Khulaifi, F. et al. Organoleptic and glycemic properties of chickpea-wheat composite breads. J Food Sci Technol 52, 2256–2263 (2015). https://doi.org/10.1007/s13197-013-1192-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1192-7